Abstract
Vitamin D deficiency has been recognized as an environmental risk factor for Crohn's disease since the early 80s. Initially, this finding was correlated with metabolic bone disease. Low serum 25-hydroxyvitamin D levels have been repeatedly reported in inflammatory bowel diseases together with a relationship between vitamin D status and disease activity. Subsequently, low serum vitamin D levels have been reported in various immune-related diseases pointing to an immunoregulatory role. Indeed, vitamin D and its receptor (VDR) are known to interact with different players of the immune homeostasis by controlling cell proliferation, antigen receptor signalling, and intestinal barrier function. Moreover, 1,25-dihydroxyvitamin D is implicated in NOD2-mediated expression of defensin-β2, the latter known to play a crucial role in the pathogenesis of Crohn's disease (IBD1 gene), and several genetic variants of the vitamin D receptor have been identified as Crohn's disease candidate susceptibility genes. From animal models we have learned that deletion of the VDR gene was associated with a more severe disease. There is a growing body of evidence concerning the therapeutic role of vitamin D/synthetic vitamin D receptor agonists in clinical and experimental models of inflammatory bowel disease far beyond the role of calcium homeostasis and bone metabolism.
1. Introduction
Vitamin D is a fat-soluble vitamin whose active form, calcitriol or 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), regulates bone, calcium, and phosphorus metabolism [1]. However, vitamin D also influences immune system function, and deficiency has been recognized as an environmental risk factor for autoimmune diseases like Crohn's disease (CD) [2].
In humans, vitamin D may be obtained from two sources: diet (as fat-soluble vitamin) and by ultraviolet- (UV-) mediated synthesis in the epidermal layer of the skin where UV-rays promote photolytic cleavage of 7-dihydrocholesterol (7-HDC) into vitamin D3 [3]. The latter is the most important source of this metabolite and, at this point, vitamin D can be considered as a hormone [4]. After production, vitamin D is activated by a two-step hydroxylation, first in the carbon 5-position by 25-hydroxylase in the liver then by 1α-hydroxylase in the kidney: this active metabolite exerts its functions by interacting with the vitamin D receptor (VDR), a receptor that belongs to the superfamily of nuclear hormone receptors [1]. Binding to VDR leads to the transcription of several vitamin D-response genes, located on single loci [5]. Various tissues and, especially, immune-related cells express VDRs and are able to produce 1,25(OH)2D3. This implies that the vitamin exerts its action beyond its classic hormonal-endocrine function tending towards an autocrine role [6].
2. Vitamin D and Its Role in Immune Regulation
Vitamin D affects the immune system acting at various levels, such as antibacterial response, antigen presentation, and regulation of adaptive and innate immunity. Genome-wide analysis has revealed that a large number of genes are influenced by vitamin D levels [7]. VDRs have been discovered in almost all immune cells as activated or naïve CD4+ and CD8+ T cells, B cells, neutrophils, and antigen-presenting cells (APCs) such as dendritic cells and macrophages. In particular, vitamin D3 enhances the chemotactic and phagocytic responses of macrophages and production of antimicrobial proteins, such as cathelicidin, inhibits the surface expression of the MHC-II-complex antigen and costimulatory molecules and downregulates the production of many proinflammatory cytokines, such as interleukin- (IL-) 1, IL-6, IL-8, and TNF-α [4, 8]. An experimental study demonstrated that transferring CD8+ T cells isolated from the spleen of wild type (WT) and IL-10 KO mice into immunodeficient Rag KO recipients, that is, mice with no mature B or T cells, did not induce colitis, whereas transferring CD8+ T cells from VDR KO mice led to colonic inflammation, and transferring CD8+ T cells from IL-10/VDR KO mice led to fulminant colitis. These data indicate that expression of VDR is required to prevent replication of quiescent CD8+ T cells and that the lack of VDR induced the formation of more aggressive T cells [9]. Another study evaluated the difference of protein expression in the small intestinal mucosa between WT mice and VDR KO mice identifying a higher expression of proteins involved in cell adhesion, proliferation, and migration and stress response in VDR KO mice. The authors conclude that vitamin D and VDR play a direct, or indirect role, in balancing these functions [10].
Vitamin D/VDR status regulates development, function, and balance of T-lymphocytes dampening T-helper- (Th-) 1 cell function and cytokine patterns (IL-2 and interferon-γ (IFN-γ)) by enhancing the Th-2 cell response (IL-4, IL-5, and IL-10) [11]; moreover 1,25(OH)2D3 promotes a regulatory outcome through the inhibition of Th-17 cells and their related cytokines, and the induction of regulatory T cells (Treg) that are protective against autoimmunity, stimulating the expression of the cytotoxic T-associated protein 4 (CTLA-4) and forkhead box P3 (Foxp-3), together with the induction of IL-10 [12, 13]. In addition, 1,25(OH)2D3 appears to have a chemopreventive role through an antiproliferative action, for example, through VDR-mediated inhibition of the Wnt/beta-catenin pathway [8, 14, 15], inhibiting growth without inducing apoptosis and inducing differentiation in colon cancer cell lines [16, 17].
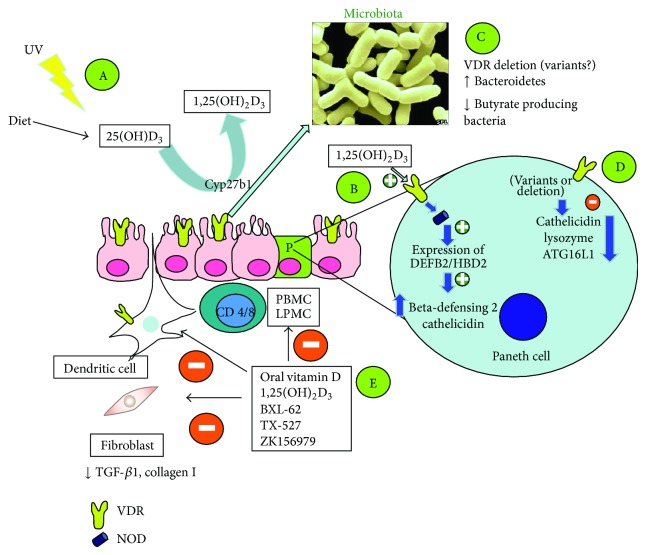
The molecular and genetic link between CD and the vitamin D/immune system axis may be in part explained by the NOD2 gene (Figure 1). The precise etiology of the inflammatory bowel disease CD is unknown. Like many chronic diseases, there are environmental factors that act on a polygenic background. Variants of the NOD2/CARD15 gene are associated with the development and phenotypic patterns of CD. This gene encodes for a protein of the family of intracellular pattern recognition receptors for bacterial components that play an important role in the innate immune system [18, 19]. Transcription of the NOD2 gene is stimulated by 1,25(OH)2D3/VDR and signaling through NOD2 induces expression of DEFB2/HBD2 which stands for the antimicrobial peptide beta-defensin 2, and of CAMP which codifies for cathelicidin [20]. In a study on a VDR KO model, a downregulation of the ATG16L1 gene, together with a reduced expression of lysozyme by Paneth cells was reported [21]. These mice had an increased susceptibility to dextran sulfate sodium (DSS) colitis, whereas in human colon samples of low VDR expression correlate with ATG16L1 and a reduction of Bacteroides species. This finding implies that alterations of the vitamin D status might interfere with autophagy and alter the antimicrobial barrier of the intestinal mucosa and, consequently, the control of the microbiota [22].
Figure 1.
Potential involvement of vitamin D in the pathogenesis of inflammatory bowel disease and immunologic effects of vitamin-D-related therapeutic approaches. Scenario A: reduced UV exposure as risk factor for CD and for hospitalizations and surgery [86]; Scenario B: NOD2 gene transcription is stimulated by 1,25(OH)2D3/VDR and signaling through NOD2 induces expression of DEFB2/HBD2 which stands for beta-defensin 2 and cathelicidin [20]; Scenarios C and D: variants or loss of function of VDR may lead to changes of the microbiota and reduce host defense by reducing production of cathelicidin, lysozyme, and ATG16L1 protein (autophagy) [21, 22]; Scenario E: experimental studies with vitamin D or its analogues showing inhibitory effects on PBMC, LPMC, dendritic cells, and fibroblasts in terms of cytokine production and differentiation (Table 3). VDR: vitamin D receptor; NOD: nucleotide-binding oligomerization domain.
3. VDR Polymorphisms in IBD
From the above, it appears that variants of VDR interfere with the immune system and, thus, may contribute to susceptibility to inflammatory bowel disease (IBD) [23, 24]. In fact, VDR polymorphisms have been identified in various diseases, such as cancer [25] or cancer risk [26], asthma [27], and kidney diseases [28]. The best-studied polymorphisms include BsmI (rs1544410), FokI (rs2228570), TaqI (rs731236), and ApaI (rs7975232). However, the results of these still few studies in IBD patients are contradictory (Table 1): for example, no statistical significance compared to controls was found in two studies on IBD patients for BsmI, FokI, TaqI, and ApaI [29, 30] with a borderline significance for heterozygous carriage of the FokI allele [29]. In three Chinese studies on ulcerative colitis (UC) patients [31, 32] and on CD patients, no difference [32] or an association of the Bb genotype of the BsmI variant with UC [31] was reported; whereas no association was found for ApaI, TaqI, and BsmI with CD [33].
Table 1.
Genetic polymorphisms and IBD (chronological order).
| Author | Year | Population | Investigated gene polymorphisms | Main findings |
|---|---|---|---|---|
| Single- or multicenter studies | ||||
|
| ||||
| Simmons et al. [23] | 2000 | England 158 UC, 245 CD, 164 CRADC |
VDR: TaqI, ApaI, FokI | TaqI polymorphism (“tt” genotype) more frequent in CD compared to UC or controls |
|
| ||||
| Martin et al. [24] | 2002 | Germany, 95 CD, 93 UC, 119 HC | VDR: TaqI | TaqI (“tt” genotype) significantly more frequent in fistulizing and stenosing CD |
|
| ||||
| Dresner-Pollak et al. [36] | 2004 | Israel, 228 CD (129 Ashkenazi and 99 non-Ashkenazi), 151 UC (72 Ashkenazi, 79 non-Ashkenazi), 495 HC (352 non-Ashkenazi and 143 Ashkenazi) | VDR: BsmI | BB genotype more frequent in Ashkenazi UC compared to Ashkenazi HC |
|
| ||||
| Noble et al. [34] | 2008 | United Kingdom, 286 CD, 154 UC, 240 HC | VDR: TaqI, ApaI | Overall no differences between CD, UC, and HC for TaqI and ApaI. TaqI variants more frequent in male IBD patients compared to (male) HC |
|
| ||||
| Naderi et al. [37] | 2008 | Iran, 150 UC, 80 CD, 150 HC | VDR: ApaI, TaqI, BsmI, FokI | FokI polymorphism significantly higher in UC and CD. Frequency of polymorphic “f” allele and f/f genotype higher in UC and CD comparing with HC |
|
| ||||
| Pluskiewicz et al. [30] | 2009 | Poland, 47 UC, 47 HC | VDR: TaqI, BsmI, ApaI | No differences between UC and HC |
|
| ||||
| Hughes et al. [29] | 2011 | Ireland, 660 IBD, 699 HC | VDR: ApaI, TaqI, BsmI, FokI | Borderline significance for heterozygous carriage of the FokI allele |
|
| ||||
| Pei et al. [31] | 2011 | China, 218 UC, 251 HC | VDR: ApaI, TaqI, BsmI, FokI | Only Bb genotype of the BsmI variant associated with UC; frequency of the BsmI polymorphic allele (B) increased in UC |
|
| ||||
| Eloranta et al. [42] | 2011 | Switzerland, 404 CD, 232 UC, 248 HC | DBP: rs 7041, rs 4588 | Significantly reduced frequency of the 420 variant Lys in IBD compared to controls |
|
| ||||
| Bentley et al. [35] | 2011 | New Zealand, 449 CD, 448 UC, 482 HC | VDR: FokI, TaqI | No overall differences, only a higher minor allele frequency for TaqI, in male CD and UC compared to HC |
|
| ||||
| Luo et al. [33]* | 2013 | China, 19 CD, 122 HC | VDR: ApaI, TaqI, BsmI | No significant differences in the frequencies of TaqI, BsmI, and ApaI polymorphisms |
|
| ||||
| Xia et al. [32] | 2014 | China 382 UC, 489 HC | VDR: ApaI, TaqI, BsmI, FokI | No difference between UC and HC. The mutant allele C and genotype TC + CC of FokI were significantly increased in patients with mild and moderate UC compared to severe UC. The frequency of AAC haplotype was statistically lower in UC than HC (AAC haplotype formed by the VDR BsmI, ApaI, and TaqI gene might engender a reduced risk of UC attack) |
|
| ||||
| Meta-analyses | ||||
|
| ||||
| Xue et al. [38] | 2013 | ApaI: 1024 CD, 974 UC, 1551 HC FokI: 1187 CD, 1221 UC, 1746 HC BsmI: 721 CD, 813 UC, 1642 HC TaqI: 1568 CD, 1515 UC, 2152 HC |
VDR: ApaI, TaqI, BsmI, FokI | FokI “ff” genotype associated with a significant risk for UC in Asians; TaqI “tt” genotype associated with an increased risk for CD in Europeans and with an increased risk for CD and UC in Asian males. ApaI “a” allele confers protection from CD |
|
| ||||
| Wang et al. [39] | 2014 | ApaI: 940 CD, 962 UC, 1468 HC FokI: 1098 CD, 1217 UC, 1676 HC BsmI: 713 CD, 799 UC, 1616 HC TaqI: 1553 CD, 1500 UC, 2145 HC |
VDR: ApaI, TaqI, BsmI, FokI | ApaI, BsmI, and FokI are not significantly associated with IBD. Significant association between TaqI polymorphism and IBD risk. In subgroups, ApaI increases the overall CD risk and BsmI increases this CD risk only in East Asians, whereas TaqI reduces the risk for UC especially in Caucasians |
CD: Crohn's disease; UC: ulcerative colitis; CRADC: cadaveric renal allograft donor controls; PCR: polymerase chain reaction; IBD: inflammatory bowel disease; HC: healthy controls; DBP: vitamin-D-binding protein; VDR: vitamin D receptor.
*Article in Chinese.
In another study on European Caucasian patients, a significantly higher frequency of the TaqI polymorphism (genotype “tt”) was reported in CD compared to UC or HC [23]. This finding was replicated in German IBD patients where the “tt” genotype was significantly more frequent in fistulizing and stenosing CD [24]. Subsequently, always in Caucasians, the finding of a lack of association of ApaI but a more frequent presence of TaqI in male IBD patients was reported [34] and confirmed 3 years later [35].
Concerning BsmI polymorphisms, the BB genotype was more frequent in Ashkenazi UC patients compared to Ashkenazi controls [36]. Finally, in a mixed IBD population investigating all 4 VDR variants, only the Fok I variant (“ff” genotype) was significantly more frequent in IBD patients [37].
Two recent meta-analyses including the same 9 studies with slightly different patient numbers (Table 1) yielded different results [38, 39]; Xue et al. [38] found that the “ff” genotype of FokI was associated with a significant risk for UC in Asians, whereas the “tt” genotype of TaqI was associated with an increased risk for CD in Europeans, but with an increased risk for both diseases, CD and UC, in Asian males. Carriage of the “a” allele (ApaI) resulted protective from CD. In contrast, Wang et al. [39] concluded that there was no association between ApaI, BsmI, and FokI and IBD, whereas subgroup analysis evidenced an increased risk for CD for ApaI and limited to East Asians, for BsmI. Conversely, TaqI variants reduced the risk for UC in Caucasians.
One study examined the influence of VDR polymorphisms on serum vitamin D levels [40] (not included in Table 1) showing a significant association of variants of the TaqI and the signal peptide, CUB domain, and EGF-like 3 (SCUBE3, rs732594) genes, the latter encodes for a protein involved in the VDR pathway, in CD patients, whereas ApaI and SCUBE3 and two variants of PHD finger protein-11 (PHF-11) gene, namely, rs2980 and rs2981, showed a significant association with serum vitamin D levels in CD patients. PHF-11 variants have been shown to be involved with vitamin D levels in other pathologies, such as asthma [41].
Besides investigations on VDR variants, 2 SNPs of the vitamin D-binding protein (DBP), that is, the 416 variant Glu (rs7041) and the 420 variant Lys (rs4588), were analysed. A significantly reduced frequency of the 420 variant Lys was found in IBD patients compared to controls [42].
In conclusion, the influence of VDR variants on IBD risk is still poorly defined. Interesting approaches are represented by investigations on the association between polymorphisms and vitamin D levels and those examining proteins involved in vitamin D-related pathways, but all need further studies and confirmation.
4. Vitamin D Status and Related Risk Factors in IBD
Starting in the late seventies, investigations on the vitamin D status of IBD patients have been carried out with different methodological approaches and results. By comparing IBD patients (CD alone or mixed populations) versus healthy controls (HC), no differences were found for circulating 25(OH)D3 concentrations in 6 studies on adult IBD populations [43–48] and in 1 study on a pediatric cohort [49], whereas lower plasma levels were reported in undernourished CD patients [50], in CD patients after intestinal resections [51], in 2 studies on adult, and in 1 study on pediatric CD patients [52–54] and in 3 mixed IBD populations [55–57].
Comparing 25(OH)D3 levels between CD and UC patients, no differences were found in 8 studies on adult or pediatric patients in basal conditions [47, 54, 55, 57–61] and in 1 pediatric study on partially vitamin D supplemented patients [62]. Lower levels in CD compared with UC were found in 5 studies [46, 63–66].
Finally, investigations concerning the active form of vitamin D, 1,25(OH)2D3, reported normal levels after bowel resections in CD [67] but no differences between well- and undernourished CD patients compared to HC or in well-nourished UC patients [50]. Similar findings were reported in a pediatric study including CD, UC and HC [61]. Lower 1,25(OH)2D3 concentrations compared to controls were found in 2 studies including CD and UC patients [45, 68]. Conversely, elevated levels of 1,25(OH)2D3 were reported after ileal resections in CD [51]. In this latter study, a positive correlation with 25(OH)D3 levels and PTH was reported.
Changing methodology and introducing vitamin D reference values as parameter, the importance of vitamin D in IBD has become more convincing. Defining vitamin sufficiency as serum values above 30 ng/mL, vitamin D insufficiency as values between 10/20 and 30 ng/mL, and vitamin deficiency as concentrations below 10 to 15 ng/mL, data from 27 studies from all over the world were available [44, 46, 52, 53, 56–58, 60, 61, 63, 64, 69–77], 6 of them on cohorts over 100 participants [59, 62, 66, 78–80], and one with more than 1,000 patients [81]. In synthesis, vitamin deficiency was found in 8-100% of patients with CD and in 15-60% of patients with UC, vitamin insufficiency in 12-72.3% in CD or in mixed IBD populations and in 7-64% of UC patients. Five papers [53, 61, 74, 76, 82] differentiated vitamin D levels according to seasonal variations in CD patients reporting vitamin deficiency in 50–76% in winter and in 10–19% in summer months; vitamin insufficiency, where reported [76, 82], was indicated in 73–100% in winter and 55–59% in summer months.
Studies evaluating vitamin D levels in IBD patients were all conducted after disease onset and established diagnosis, but it is not clear if vitamin D deficiency is the cause or a consequence. Pathogenesis of vitamin D hypovitaminosis in patients with IBD may depend on various mechanisms such as decreased exposure to sunlight or oral vitamin D intake, ileal resections leading to malabsorption or a disturbed enterohepatic circulation, and/or increased losses through the gastrointestinal system by protein-losing enteropathy [59].
To identify the reasons for the differences of the vitamin D status, the ability to absorb vitamin D2 was evaluated in a study by Farraye et al. [77] comparing CD patients and HC. In this study, 42% of CD patients were vitamin D deficient 25(OH)D3 (≤20 ng/mL), while 29% were insufficient (25(OH)D3: 21–29 ng/mL); 12 h after ingesting 50,000 IU of vitamin D2, circulating levels of this metabolite were significantly lower in CD compared with HC indicating a significant 30% reduction of the ability to absorb vitamin D2. In another study, on 31 CD patients and 15 HC, the capacity of absorbing orally administered vitamin D (5 μg of 25(OH)D3/kg body weight) was evaluated; 10% of CD patients showed decreased absorption of 25(OH)D3 after 4 and 8 hours [71]. Finally, a wide variability of absorption of vitamin D2 was reported in vitamin deficient and insufficient CD patients, but vitamin D2 absorption was significantly reduced compared with HC [77].
Several studies evaluated factors influencing vitamin D status hypothesizing reduced sun exposure as cause for hypovitaminosis, since a geographical north-south gradient was noted also for other autoimmune T helper- (Th-) 1-mediated diseases, like multiple sclerosis. The link between this gradient and the pathophysiological mechanisms that involve vitamin D status depends not only on dietary intake but also from UV exposure [83]. Indeed, a negative association between sun exposure and lower levels of 25(OH)D in CD was reported in Indian patients [52] and, most recently, also in Dutch CD [84] where reduced exposure to sunlight (defined as no sunny holidays, no solarium use, and more sun protection) was associated with low 25(OH)D serum levels.
The relationship between sun exposure and the risk of developing CD or UC has been investigated by Nerich et al. [85]. High residential sunlight exposure was associated with a significant decreased risk of CD, but not UC. Four years later, the same group published similar results, that is, an increased incidence of CD with reduced sunlight exposure, in a cohort of women living in France, whereas vitamin D intake was not associated with a risk reduction in CD or UC [86].
Reduced UV exposure seems therefore not only to increase risk for CD, but it also seems associated with a worse outcome of disease. In a recent nationwide North-American study, the influence of UV exposure on hospitalization rates, length of hospital stay, and surgeries was investigated in an impressive number of IBD patients (649,932 CD, 384,267 UC, and 288,894,297 non-IBD controls). Reduced UV exposure led to significantly longer hospitalizations in all groups and to more frequent intestinal surgeries and deaths in CD [87]. Data on 25(OH)D3 were not available in this study. The finding that more UV exposure is associated with a minor number of surgical procedures in CD was confirmed in a subsequent study on 481,712 CD-related hospitalizations reporting 67,751 major surgical procedures [88].
Finally, a prospective cohort study of 72,179 women enrolled in the Nurses' Health Study addressed the question if vitamin D hypovitaminosis may, per se, represent a risk factor for the development of IBD. Incident cases of CD and UC were recorded over a follow-up period of 22 years. A 25(OH)D3 prediction score based on diet and lifestyle was developed and validated against effectively measured levels of 25(OH)D3. The authors showed that higher predicted plasma levels of 25(OH)D3 were associated with a significant risk reduction for CD but not for UC, suggesting that vitamin D status may contribute to the pathogenesis of CD [89].
After a series of contradictory and mostly negative studies on vitamin D levels in IBD patients compared with HC, more conclusive data have been produced introducing reference values. However, most of these studies have been aimed to investigate bone and calcium metabolism. Recent large cohort studies investigating UV exposure or vitamin D status estimating the risk to develop IBD have pushed forward our understanding on the potential role of vitamin D in the context of IBD.
5. Vitamin D Status and Clinical Outcome in IBD Patients
Several studies concerning the relationship between vitamin D status and clinical outcome in IBD patients have been published (Table 2). Almost 30 years ago, 25(OH)D3 levels in active CD were found to be lower than in quiescent CD [50]. Twenty years later, another study showed that low serum 25(OH)D3 levels were predicted by disease duration and activity scores in both, CD and UC [46]. This inverse association between disease activity and serum 25(OH)D3 levels was confirmed in a small prospective study in CD [52] and in a retrospective study on a much larger, mixed IBD population [59]. In this latter study, low serum 25(OH)D3 levels were associated with higher clinical activity scores in CD and in UC, but not with the risk for medical or surgical hospitalizations. Moreover, regression analysis found that low vitamin D levels were independently associated with quality of life (QoL) in CD patients but not in UC patients. A reduced QoL was reproduced by another study where vitamin insufficient patients had significantly lower QoL scores than those who were sufficient [82]. Finally, in a mixed IBD population, an inverse correlation between serum 25(OH)D3 concentrations and fecal calprotectin, a marker for gut inflammation, was found whereas serum CRP as a marker of systemic inflammation did not correlate with 25(OH)D3 levels [90].
Table 2.
Vitamin D versus disease activity and outcome in IBD (chronological order).
| Author | Year | Population | Methodology | Main findings |
|---|---|---|---|---|
| Harries et al. [50] | 1985 | U.S.A 40 CD 20 UC 9 HC |
Single-center cohort; CD divided into 2 groups (undernourished and well nourished); 2 control groups: 20 well-nourished UC and 9 HC | 25(OH)D3 significantly lower in CD with active disease versus inactive disease (P < 0.05) |
|
| ||||
| Tajika et al. [46] | 2004 | Japan 33 CD, 11 UC, 15 HC |
Single-center cohort; 25(OH)D3 and disease activity assessed by CDAI and IOIBD score | Serum 25(OH)D3 significantly related to disease duration (r = 0.46, P = 0.003), CDAI (r = 0.44, P = 0.005), IOIBD score (r = 0.30, P < 0.05), serum ferritin (r = 0.34, P = 0.03), CRP (r = 0.34, P = 0.03) |
|
| ||||
| Joseph et al. [52] | 2009 | India 34 CD, 34 HC |
Single-center cohort; disease activity evaluated by HBI in CD | Serum 25(OH)D3 in CD significantly lower versus controls (P < 0.05). Disease activity correlated negatively with 25(OH)D3 level (P < 0.004). 25(OH)D3 levels were comparable to controls in mild CD but were significantly lower in moderate and severe CD |
|
| ||||
| Nakajima et al. [68] | 2011 | Japan 47 CD, 40 UC, 41 HC |
Single-center cohort; disease activity measured using CAI/CDAI scores | No decrease 1,25(OH)2D3 in CD with high CDAI No significant correlation between serum 1,25(OH)2D3 levels and CAI or CDAI in UC or CD |
|
| ||||
| Ulitsky et al. [59] | 2011 | U.S.A. 504 IBD (403 CD, 101 UC) |
Single-center cohort; retrospective observational study HRQOL measured with SIBDQ, disease activity measured using HBI/UCDI scores |
25(OH)D3 deficiency significantly associated with lower SIBDQ (P = 0.002) and higher mean HBI/UCDI (P = 0.002) in IBD versus vit D sufficient patients. Analyzed separately, vit D deficiency associated with lower HRQOL scores only in CD (P = 0.04), not in UC |
|
| ||||
| El-Matary et al. [54] | 2011 | Canada 60 IBD (39 CD, 21 UC) |
Cross-sectional pediatric study. Disease activity measured by PCDAI e PUCAI | No correlation between PCDAI and serum 25(OH)D3. Marginal evidence against the null hypothesis (P = 0.05) between serum 25(OH)D3 and PUCAI, but without statistical significance |
|
| ||||
| Hassan et al. [60] | 2013 | Iran 60 IBD (34 UC, 26 CD) |
Cross-sectional study. Disease activity measured by CDAI and Truelove index | Serum vit D lower in active versus inactive disease (non significantly). VitD deficiency was not associated with IBD activity (also considering CD and UC separately), however was associated with a history of IBD related intestinal surgery |
|
| ||||
| Ananthakrishnan et al. [81] | 2013 | U.S.A. 3,217 IBD (55% CD, 45% UC) |
Multicenter cohort; 25(OH)D3: Normal (>30 ng/mL), Insufficient (20–29.9 ng/mL) or Deficient (<20 ng/mL) |
IBD-related surgery: CD: 10% patients never vitamin D deficient versus 13% vitamin D insufficient versus 17% vitamin D deficient. UC: vitamin D deficiency associated with elevated risk of surgery and hospitalization with effect similar to CD; no statistical significance in patients vitamin D insufficient. Normalization of 25(OH)D3 associated with reduction in the risk of related surgery but not in UC |
|
| ||||
| Zator et al. [92] | 2014 | U.S.A. 101 IBD (74 CD, 27 UC) |
Retrospective single-center cohort; patients on anti-TNF therapy evaluated for loss of response; 25(OH)D3 insufficiency: <30 ng/mL | Patients with insufficient vitamin D demonstrated earlier cessation of anti-TNF-α therapy (P = 0.04). This effect was significant in patients who stopped treatment for loss of response, stronger for CD than UC (P = NS) |
|
| ||||
| Ananthakrishnan et al. [91] | 2014 | U.S.A. 3188 IBD patients (45% UC, 55% CD) |
Retrospective multi-center analysis of 25(OH)D3 in 35 patients who developed CDI | 25(OH)D3 level was significantly lower in IBD who developed CDI compared to non-CDI-IBD (P = 0.002). Levels below 20 ng/mL were associated with a two-fold increase in risk of CDI. 25(OH)D3 level was an independent predictor of CDI |
|
| ||||
| Ham et al. [93] | 2014 | U.S.A. 37 CD |
Prospectively collected samples for 25(OH)D3 analysis; assessment of HBI and CRP PBMC tested for VDR, Cyp |
25(OH)D3 levels lower in patients with active disease versus inactive disease, 25(OH)D3 correlated with HBI (not with CRP) PBMC: mean gene expression of VDR and CypB1 higher in active disease |
|
| ||||
| Garg et al. [90] | 2013 | Australia 40 CD 31 UC 23 HC |
Assessment of 25(OH)D3, fecal calprotectin and CRP | Inverse correlation between serum 25(OH)D3 and fecal calprotectin in CD and UC patients, but not with CRP |
|
| ||||
| Hlavaty et al. [82] | 2014 | Slovakia 141 CD 49 UC |
SIBDQ assessment in vitamin D sufficient or -deficient patients and in vitamin supplement (800 IU/day for 3 months) patients | SIBDQ was significantly better in vitamin D-sufficient patients; vitamin D supplements did not influence vitamin D status or sIBDQ |
|
| ||||
| Govani et al. [88] | 2015 | U.S.A. 67,751 CD |
Retrospective, national, analysis of UV exposure and inpatient surgery risk | UV exposure protective for inpatients surgery |
Abbreviations: CD: Crohn's disease; UC: ulcerative colitis; HC: healthy controls; IBS: irritable bowel syndrome; IBD: inflammatory bowel disease; CDAI: Crohn's Disease Activity Index; IOIBD: international organization for the study of inflammatory bowel disease score; CAI: Lichtiger's clinical activity index; 25(OH)D3: 25-Hydroxycholecalciferol; 1,25(OH)2D3: 1,25dihydroxycholecalciferol; SIBDQ: Short IBD Questionnaire; HBI: Harvey-Bradshaw index; UCDI: Ulcerative colitis disease activity index; HRQOL: health-related quality of life; PCDAI: pediatric Crohn's disease activity index; PUCAI: pediatric ulcerative colitis activity index; CDI: Clostridium difficile infection; CRP: C-reactive protein; UV: ultraviolet; TNF: tumor necrosis factor; PBMC: peripheral blood mononuclear cells; Cyp: Cyp27b1 gene; VDR: vitamin D receptor.
Conversely, other studies on CD and UC patients failed to show a correlation between serum 25(OH)D3 levels and disease activity [60]. The same findings, that is, no association between 25(OH)D3 concentrations and disease activity, were published on a pediatric IBD population [54].
Going beyond disease activity, in a prospective study on the largest multicenter cohort involving 3,217 patients, low plasma 25(OH)D3 levels (<20 ng/mL) were associated with an increased risk of hospitalizations and surgery for CD as well as for UC patients [81]. In a subset of CD patients, but not UC patients, who normalized vitamin D status, a reduction of CRP levels and the need for hospitalizations was observed.
The likelihood for developing Clostridium difficile (Cl) colitis related to vitamin D status was investigated retrospectively. There was an increased risk for developing Cl colitis in patients with low plasma 25(OH)D3 levels (<20 ng/mL), and an increase by 1 ng/mL of 25(OH)D3 was accompanied by a 4% risk reduction of developing Cl colitis. Lastly, death from Cl colitis occurred in those with lower 25(OH)D3 levels compared with survivors [91]. A recent study investigated the relationship between 25(OH)D3 concentrations and duration of anti-TNF therapy in IBD patients. Interestingly, low vitamin D levels were associated with loss of response during maintenance therapy in CD patients [92], whereas serum 25(OH)D3 levels increased with anti-TNF therapy [93].
The only study that investigated plasma 1,25(OH)2D3 levels found no association between 1,25(OH)2D3 levels and CDAI or CAI in Japanese patients [68].
From the above, it appears that low vitamin D is inversely correlated to disease activity documented by clinical scores and surrogate markers of inflammation such as CRP and fecal calprotectin; moreover, low levels were also associated with clinical outcomes, that is, surgery, response to anti-TNF therapy, Cl superinfection, and, finally, death. Inflammation per se has been shown to upregulate conversion from 25(OH)D3 to 1,25(OH)2D3 which may lead to a reduction of available 25(OH)D3. In this discussion, an observation of two recent papers may be relevant, coming from orthopaedic surgery, showing an acute reduction of 25(OH)D3 levels following a systemic inflammatory response induced by surgery, considering serum 25(OH)D3 as a negative acute phase reactant [94, 95].
6. Therapeutic Studies In Vitro and in Experimental Animals
As a result of this evidence, vitamin D should be proposed as a therapy for IBD. Several experimental studies, both on animals and IBD patients, have been carried out (Table 3). Starting with the former, in a model of spontaneous colitis, interleukin- (IL-) 10 knock-out (KO) mice on a vitamin D deficient diet showed growth retardation and weight loss, together with a high mortality rate (58% at week 9) compared to mice on a vitamin D sufficient diet; 1,25(OH)2D3 (0.005 μg/day) supplementation starting from week 2 reduced weight loss and ameliorated histology scores, but vitamin D supplementation after symptom onset at week 7 (1,25(OH)2D3, 0.2 μg/day) did not induce significant differences compared with untreated animals, except for bowel weight indicating a reduction of inflammation in supplemented animals [96]. In another study, the efficacy of a low calcemic vitamin D analogue (22-ene-25-oxa-vitamin D (ZK156979)) was investigated in 2,4,6-trinitrobenzene sulfonic acid (TNBS) colitis [97]. Treatment was performed with 1,25(OH)2D3 (0.2 μg/kg) versus ZK156979 (0.1–2.0 μg/kg), both administered intraperitoneally (i.p.) before or after colitis induction. Assessment of inflammation and colitis severity was established by scoring colitis, macroscopic and histological analysis, and measurement of myeloperoxidase activity (MPO) and cytokine levels. The authors found that ZK156978 reduced the severity of TNBS-induced colitis with a potency comparable with that of 1,25(OH)2D3, downregulating MPO activity, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) tissue levels, and T-box transcription factor (T-bet) expression, together with an increase of interleukin IL-10 and IL-4 tissue concentrations, without calcemic effects.
Table 3.
Therapeutic studies in experimental and human IBD (chronological order).
| Author | Year | Species/cells | Investigational agent | Methodology | Main findings |
|---|---|---|---|---|---|
| Animal and in vitro studies | |||||
|
| |||||
| Cantorna et al. [96] | 2000 | IL-10 KO mice | 1,25(OH)2D3 p.o. | Exp. 1. Vit. D-deficient IL-10 KO mice versus vit. D-sufficient mice (treated with cholecalciferol); Exp. 2. Vit. D-deficient IL10 KO mice versus 1,25(OH)2D3- treated; Exp. 3. Vit. D treatment after onset of GI symptoms |
Vitamin D sufficiency prevents enterocolitis in IL-10 KO mice up to 13 weeks; 1,25(OH)2D3 treatment ameliorates inflammation |
|
| |||||
| Daniel et al. [97] | 2006 | BALB/c mice | TNBS colitis; 22-ene-25-oxa-vitamin D (ZK156979) i.p. (vitamin D analogue) |
Treatment with ZK156979 versus 1,25(OH)2D3 before or after induction of colitis with TNBS; investigation of tissue MPO, TNF-α, IFN-γ, T-bet, IL-10, and IL-4 | ZK156979 versus 1,25(OH)2D3 prevents or ameliorates TNBS colitis decreasing pro-inflammatory and increasing anti-inflammatory cytokines |
|
| |||||
| Laverny et al. [98] | 2010 | C57BL/6 mice | DSS-colitis, 1α,25(OH)2-16-ene-20-cyclopropyl-vitamin D3 (BXL-62) (=VDR agonist) intrarectally | Daily administration of BXL-62 versus 1,25(OH)2D3; Macro- and microscopic scoring; mucosal concentrations of TNF-α, IL-12/23p40, IL-6, and IFN-γ and assessment of mRNA |
Higher potency of BXL-62 versus 1,25(OH)2D3 in reducing tissue inflammation |
|
| |||||
| Verlinden et al. [99] | 2013 | C57BL/6 mice | DSS- colitis 1α,25(OH)2-19-nor-14,20-bisepi-23-yne-vitamin D3 (TX527) | Histological examination; measurement of transcript levels of cytokines (IL-1, IL-6, IFN-γ, and TNF-α) | TX527 reduced “clinical” disease scores and attenuated histological scores, downregulation of transcript levels of inflammatory cytokines |
|
| |||||
| Ooi et al. [100] | 2013 | C57BL/6 mice Cyp KO VDR KO |
1,25(OH)2D3 p.o. | DSS colitis; characterization of gut microbiota, and gut macrophages; E-cadherin expression |
Lower expression of E cadherin and tolerogenic macrophages Less beneficial microbiota in KO mice Vitamin D treatment ameliorates colitis and reduces Helicobacteraceae |
|
| |||||
| Wu et al. [21] | 2014 | Conditional VDR KO and IL-10 KO mice DSS-colitis cells: MEF, SKCO15, HCT116 human tissue |
DSS colitis BUT feeding in IL-10 KO |
VDR KO: colitis evaluation, pyrosequencing for microbiota, Paneth cells, lysozyme production, autophagy MEF (VDR−/− VDR+/− VDR+/+) and VDR knockdown in SKCO15 with evaluation of ATG16L1 and LC3B proteins IL-10 KO: VDR and ATG16L1 expression with or w/o BUT feeding Human tissue (UC, inflamed versus normal) VDR, ATG16L1, Bacteroides concentration (FISH) HCT116 and HIEC: VDR expression with and w/o incubation with BUT |
Conditional VDR KO mice: worse colitis, increased E. coli and Bacteroides (B. fragilis), and decreased BUT-producing bacteria; less and abnormal Paneth cells and reduced lysozyme and ATG16L1 protein; in SKCO15 and MEF reduced expression of ATG16L1 and LC3B proteins In UC: reduced expression of VDR and ATG16L1, increase of Bacteroides; BUT increases VDR expression in HIEC and HCT116 |
|
| |||||
| Tao et al. [102] | 2014 | C57BL/6 mice | TNBS-colitis Vitamin D sufficient or deficient diet |
At week 14, assessment of ECM and total collagen production, together with determination in isolated colonic SEMF, of expression of VDR, α-SMA, and Collagen I in normal SEMF | Histological scoring, ECM, and collagen production in the colon reduced in vitamin D supplemented mice; in SEMF decreased levels of TGF-β1, Smad-3, p-Smad3, and Collagen I and induced VDR expression and decreased TGF-β1-induced α-SMA and Collagen I expression |
|
| |||||
| Assa et al. [101] | 2015 | Caco cells C57BL/6 mice |
DSS- colitis Vitamin D sufficient or deficient diet 1,25(OH)2D3 for Caco |
Caco cells incubated with or w/o 1,25(OH)2D3 challenged with AIEC C57BL/6 mice on normal or low 1,25(OH)2D3 diet infected with AIEC |
1,25(OH)2D3 protects Caco cells against AIEC induced loss of TER and TJ protein redistribution 1,25(OH)2D3 reduces DSS colitis and AIEC invasion low vitamin D diet and DSS colitis increased Bacteroides |
|
| |||||
| In vivo and ex vivo studies in IBD patients | |||||
|
| |||||
| Stio et al. [105] | 2007 | 4 CD and 4 HC | TX 527 [19-nor-14,20-bisepi-23-yne-1,25(OH)2D3], Vitamin D analogue | Single-center, ex vivo study; experimental study on PBMC of CD patients |
TX 527 inhibits TNF-α mediated effects on PBMC and the activation of NF-κB; its action is mediated by VDR |
|
| |||||
| Miheller et al. [107] | 2009 | 37 CD | Group A treated with aVD versus group B treated with pVD | Single-center study; evaluation of bone parameters and CDAI, CRP, and SIBDQ after 6, 12, 52 weeks | In aVD, after 6 weeks (but not at 52 weeks) a significant reduction of CDAI, IBDQ, and CRP together with a significant change of bone parameters |
|
| |||||
| Ardizzone et al. [103] | 2009 | 9 UC, 8 CD | 1,25(OH)2D3 | Single-center ex vivo study; PBMC with or without calcitriol; determination of TNF-α, IFN-γ, IL-2, and IL-10 | In UC PBMC 1,25(OH)2D3 reduced IFN-γ and enhanced IL-10 production In CD PBMC 1,25(OH)2D3 reduced TNF-α production |
|
| |||||
| Jørgensen et al. [108] | 2010 | 94 CD | Vitamin D3 versus placebo | Multi-center randomized double-blind placebo-controlled study; 1200 IU vit D3/day or placebo; estimation of clinical relapse rate |
Vit. D3 significantly increased serum vit. D levels, but the decrease of relapse was not significant (13% versus 29%, P = 0.06) |
|
| |||||
| Bendix-Struve et al. [104] | 2010 | 108 CD | Vitamin D3 versus placebo | Randomized, placebo-controlled, clinical trial After 0, 36, and 52 weeks, PBMC tested in 10 patients treated with Vitamin D3 (1200 IU/day) and in 10 patients treated with placebo for cytokine production and proliferation |
Vit. D3 treatment of CD patients increased the IL-6 levels and enhance the CD4+ T-cell proliferation |
|
| |||||
| Laverny et al. [98] | 2010 | 22 CD, 21 UC | 1α,25(OH)2-16-ene-20-cyclopropyl-vitamin D3 (BXL-62) |
Ex vivo preparations of PBMC (+LPS) and (CD2/CD28 activated)-LPMCs incubated with or without BXL-62. Determination of mRNA and protein concentrations of TNF-α, IL-12/23p40, IL-6, and IFN-γ |
Higher anti-inflammatory potency compared to 1,25(OH)2D3 demonstrated by the significantly more potent inhibition in PBMC and in LPMCs of the proinflammatory cytokines TNF-α, IL-12/23p40, IL-6, and IFN-γ |
|
| |||||
| Yang et al. [109] | 2013 | 18 CD | Vitamin D3 | Open-label prospective clinical trial over 24 weeks, multi-center study; vitamin D3 at 1000 IU/day; dose increase every two week of 1000 IU/day up to 5000 IU/day to achieve serum 25(OH)D3 >40 ng/mL | Vit. D3 supplementation significantly raised serum 25(OH)D3, reduced CDAI scores, and improved IBDQ scores |
|
| |||||
| Bartels et al. [106] | 2014 | 10 CD | Vitamin D3 | Single-center study, oral vitamin D supplementation (or placebo) and assessment of maturation marker expression and cytokine production of monocyte-derived dendritic cells | Dendritic cells from vitamin supplemented CD patients exhibited reduced expression of CD80 and reduced production of the cytokines IL-10, IL-1β, and IL-6 |
|
| |||||
| Ham et al. [93] | 2014 | PBMC | Incubation of CD4+ with vit D 50 nM | Determination of CD25+ and CD39+ cells | 3-fold increase of CD25+ cells, CD39+ unchanged |
CD: Crohn's disease; UC: ulcerative colitis; HC: healthy controls; vit: vitamin; p.o.: per os; GI: gastrointestinal; KO: knock-out; TNBS: 2,4,6-trinitrobenzene sulfonic acid; i.p.: intraperitoneal; DSS: dextran sodium sulfate; 25(OH)D: 25-hydroxycholecalciferol; 1,25(OH)2D3: 1,25-dihydroxycholecalciferol; vitamin D3 (vit D3): cholecalciferol; VDR: vitamin D receptor; MEF: mouse embryonic fibroblasts; AIEC: adherent-invasive Escherichia coli; TER: transepithelial electrical resistance; TJ: tight-junction; aVD: active vitamin D (1,25(OH)2D3); pVD: plain vitamin D (25(OH)vitamin D); CDAI: Crohn's disease activity index; CRP: C-reactive protein; SIBDQ: Short IBD questionnaire; PBMC: peripheral blood mononuclear cells; LPS, lipopolysaccharide; LPMCs: lamina propria mononuclear cells; IBDQ: IBD questionnaire; IL: interleukin; Cyp: Cyp27b1 gene; IFN: interferon; TNF: tumor necrosis factor; BUT butyrate; SEMF: subepithelial myofibroblasts; ECM: extracellular matrix; α-SMA: alpha smooth muscle actin; FISH: fluorescent in situ hybridization; HIEC: human intestinal epithelial cells; ATG16L1: autophagy related 16-like 1 (S. cerevisiae); LC3B: autophagy-related protein LC3B; SKCO15: human colorectal adenocarcinoma cells; HCT116: human colon cancer cell.
Laverny et al. [98] studied the effect of an intrarectally administered vitamin D receptor agonist (1α,25(OH)2-16-ene-20-cyclopropyl-vitamin D3; BXL-62) in C57Bl/6 mice with dextran-sodium sulfate- (DSS-) induced (3%) colitis. BXL-62 treatment (1 μg/kg) compared to 1,25(OH)2D3 (0.3 μg/kg) was superior in preventing weight loss and visible fecal blood, together with better stool consistency and histology scores without inducing hypercalcemia. Another synthetic vitamin D agonist, 1α,25(OH)2-19-nor-14,20-bisepi-23-yne-vitamin D3 (TX527), has been shown to attenuate inflammation in the DSS model of colitis by downregulating IL-1, IL-6, IFN-γ, and TNF-α as well as the gastrointestinal glutathione peroxidase 2 [99].
There are three very interesting studies which associate vitamin D or its receptor with intestinal microbiota. First, in Cyp27b1-KO mice, that is, mice unable to produce 1,25(OH)2D3, an increased susceptibility to DSS colitis was observed [100]. Oral vitamin supplementation reduced weight loss, whereas treatment with antibiotics greatly attenuated colitis. In these mice, a reduced expression of E-cadherin on epithelial and immune cells was observed pointing towards a more “leaky” gut. Moreover, a reduced number of tolerogenic dendritic cells were observed in the gut of Cyp27b1-KO mice. In these mice, as well as in VDR-KO mice, dysbiosis of the microbiota was observed with an increase of the Helicobacteraceae family and a reduction of the Firmicutes and Deferribacteres phyla. The authors concluded that vitamin D (production or its receptor) is involved in the regulation of the gut microbiota. Second, DSS-induced colitis was reduced together with a lower penetration of adherent-invasive E. coli (AIEC) in mice on a vitamin-sufficient diet compared to those fed a vitamin D deficient diet. Moreover, vitamin D hypovitaminosis and DSS colitis led to an increase of Bacteroidetes. In the same paper in Caco cells incubated with or without vitamin D and then challenged with AIEC, vitamin D maintained transepithelial resistance and prevented tight junctional protein redistribution [101]. The third paper, that reported changes of the microbiota related to interference in the vitamin D system, assessed susceptibility to DSS colitis in conditional VDR KO mice (deletion restricted to the intestinal epithelial cells), along with Paneth cell quantity and quality by means of quantification of lysozyme and ATG16L1 protein expression. The latter is a protein involved in autophagy, and its genetic variants are well known as risk factors for CD. In this model, an increase of E. coli and Bacteroides, together with a decrease of butyrate producing bacteria was reported. Supplementing butyrate to IL-10 KO mice reverses reduced VDR and ATG16L1 expression. Similar results, that is, an increased expression of VDR and ATG16L1, were obtained incubating several cell lines with butyrate [21].
Finally, a reduction of intestinal fibrosis, assessed by production of extracellular matrix and total collagen, was seen in mice with TNBS colitis on a vitamin supplemented diet compared to mice fed a vitamin D deficient diet [102]. Moreover, in isolated subepithelial myofibroblasts from the colon, a vitamin D sufficient diet reduced concentrations of TGF-β1, Smad 3, p-Smad 3, and collagen I. It was concluded that preventive vitamin D administration reduces fibrosis inhibiting the VDR-mediated TGF-β1/Smad 3 pathway.
In the above studies, in various types of spontaneous or chemically induced colitis and in several cell lines, vitamin D and synthetic agonists have been shown to reduce colitis severity and intestinal fibrosis. Vitamin D hypovitaminosis or knocking down Cyp27b1 or VDR had the opposite results. Interestingly, these latter conditions were all associated with changes of the intestinal microbiota.
7. Therapeutic Studies in Human Ex Vivo Preparations
In an ex vivo study on PBMC obtained from IBD patients and incubated in the presence of 1,25(OH)2D3, a reduction of interferon- (IFN-) γ and an increase of IL-10 production were observed in PBMC from UC patients whereas in CD the production of TNF-α were reduced [103]. The effect of orally administered vitamin D3 on monocyte-depleted PBMC from vitamin D3-treated (1200 IU vitamin D daily over 1 year) versus placebo-treated patients was investigated [104]. CD4+ T-cell proliferation and T-cell cytokine production were assessed. IL-6 production in vitamin D3-treated patients increased, whereas TNF-α, IFN-γ, and IL-4 did not. No change was observed for IL-10 and the percentage of the CD4+, CD25+, and Foxp3+ regulatory T cells compared to placebo. The amount of proliferating CD4+ T cells was significantly increased (from 41% to 56%) in the vitamin-D-treated group.
Another ex vivo study employed the vitamin D analogue (19-nor-14,20-bisepi-23-yne-1,25(OH)2D3; TX 527). This analogue significantly inhibited PBMC proliferation and TNF-α release in CD and HC [105]. The increase of VDR protein levels after incubation with TX 527 was higher in CD compared with HC. Moreover, in PBMC of both, HC and CD, stimulated with TNF-α, a decrease in nuclear NF-κB protein levels together with an increase in cytoplasmic IKB-α levels were observed pointing to an inhibition of TNF-α induced effects on PBMC exerted by the vitamin D analogue.
The effect of the vitamin receptor agonist BXL-62 on PBMC from CD and UC patients and lamina propria mononuclear cells (LPMC) obtained from biopsies of two CD (ileum) and two UC (colon) patients was investigated [98]. After incubation, in LPS-stimulated PBMC and in activated LPMC from IBD patients, BXL-62 significantly inhibited, with a significantly higher potency compared with 1,25(OH)2D3, TNF-α, IL-6, and IL-12/23p40 transcription and cytokine concentrations measured in culture supernatants without differences between CD and UC.
In PBMC of CD patients, expression of the CYP27B1 gene, that is, the gene that encodes the enzyme that converts 25(OH)D3 to 1,25(OH)2D3, and that of the VDR gene was investigated, showing a higher expression in active compared to inactive disease [93]. Moreover, CD4+ T cells incubated in the presence of vitamin D showed a threefold increase of CD25+ cells.
Finally, the effect of oral vitamin D supplementation on the maturation and cytokine production of monocyte-derived dendritic cells of CD patients was studied [106]. Compared to placebo-treated CD patients, vitamin D supplementation led to reduced CD80 expression in LPS-stimulated dendritic cells together with reduced production of IL-10, IL-1β, and IL-6.
8. Therapeutic Studies in Human IBD
There are only few studies with vitamin D addressing the clinical course of IBD (Table 3). In one of these studies, the effect of supplementation of the active form of vitamin D 1,25(OH)2D3 (aVD, 1000 IU 1.25(OH)2D3 daily) versus the plain vitamin D 25(OH)D (pVD; 2 × 0.25 μg alphacalcidiol daily) was investigated in CD patients in clinical remission (CDAI < 150) [107]. Both groups received oral calcium supplementation (1000 mg/day). At 6 weeks, the mean CDAI and IBDQ scores, as well as the CRP concentrations, decreased in the aVD-treated group, but not in the pVD-treated group. These differences between the groups however disappeared by week 52. Serum calcium concentrations did not change at any time point. Jørgensen et al. [108] performed a randomized double-blind placebo-controlled multicenter study to assess the benefit of vitamin D3 treatment in CD. They included 94 CD patients in clinical (CDAI < 150) and biochemical remission, randomized to receive 1200 IU of vitamin D3 + 1200 mg of calcium or 1200 mg of calcium alone. During 1-year follow-up, serum 25(OH)D3 levels increased significantly in vitamin D-supplemented patients, on average from 27 to 38 ng/mL, but free serum calcium did not change. The relapse rate (defined as increase of CDAI >70 over baseline and CDAI ≥150) was not significantly lowered. Adjustment for the use of azathioprine and smoking resulted in minor changes of the risk estimate. However, the authors concluded that vitamin D might be effective in CD but claimed the need for larger studies.
In an uncontrolled study, 18 active CD patients were initially treated with 1000 IU vitamin D daily over 2 weeks. Thereafter, the dose was escalated (to a maximum of 5000 IU) until a serum concentration of 40 ng/mL of 25(OH)D3 was reached [109]. After 24 weeks, a significant reduction of the CDAI and an improvement of the IBDQ score were observed. No differences were observed for CRP, erythrocyte sedimentation rate (ESR), TNF-α, IL-17, IL-10, and vascular endothelial growth factor (VEGF). Data on serum calcium levels were not reported.
In this last paragraph, the therapeutic effects of vitamin D supplementation on disease activity mainly given to patients in remission yielded modest results; the daily administered dose ranged in these studies between 1000 and 5000 IU, with an increase of serum vitamin D levels but apparently without hypercalcemia.
9. Conclusions
Literature data highlighting the importance of vitamin D in different aspects of immune regulation, for example, in chronic immune-mediated diseases and cancer, suggest considering this metabolite not simply as a vitamin involved in bone and calcium homeostasis but as an autocrine mediator with an active role in numerous physiological processes, particularly in the innate immune system. Since most studies concerning the calcium status in IBD yielded contradictory data, in the most recent literature, the discussion has focused on the possible role of vitamin D as a risk factor for the onset and evolution of gut inflammation. The potential role of 25(OH)2D as negative acute phase reactant has yet to be proven in IBD but may explain its frequently reduced levels in active disease. Besides lower vitamin D levels due to reduced UV exposure, genetic induced loss of function of VDR may contribute to defects involving vitamin D pathways. It has been shown in VDR KO animals that this deletion profoundly alters innate immune response and the gut microbiota. Further studies in this field are needed to provide more insight in the link between vitamin D/VDR and bowel inflammation.
Simple vitamin D supplementation does not seem to lead to significant improvement of the clinical course of IBD but may be indicated for a subset of patients. Vitamin D synthetic analogues of vitamin D seem to be more promising, at least in animal studies and in ex vivo experiments.
Acknowledgment
The authors wish to thank Trays Ricciardi for language editing.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Jones G., Strugnell S. A., DeLuca H. F. Current understanding of the molecular actions of vitamin D. Physiological Reviews. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 2.Narula N., Marshall J. K. Management of inflammatory bowel disease with vitamin D: beyond bone health. Journal of Crohn's & Colitis. 2012;6(4):397–404. doi: 10.1016/j.crohns.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Holick M. F., Chen T. C., Lu Z., Sauter E. Vitamin D and skin physiology: a D-lightful story. Journal of Bone and Mineral Research. 2007;22(2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 4.Baeke F., Etten E. V., Gysemans C., Overbergh L., Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Molecular Aspects of Medicine. 2008;29(6):376–387. doi: 10.1016/j.mam.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Pike J. W., Meyer M. B. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheumatic Disease Clinics of North America. 2012;38(1):13–27. doi: 10.1016/j.rdc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman M., Milestone A. N., Walters J. R. F., Har A. L., Ghosh S. Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therapeutic Advances in Gastroenterology. 2011;4(1):49–62. doi: 10.1177/1756283x10377820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun R. F., Liu P. T., Modlin R. L., Adams J. S., Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Frontiers in Physiology. 2014;5, article 151:15. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J. Vitamin D and mucosal immune function. Current Opinion in Gastroenterology. 2010;26(6):591–595. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Bruce D., Cantorna M. T. Vitamin D receptor expression controls proliferation of naïve CD8+ T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunology. 2014;15(6):1–11. doi: 10.1186/1471-2172-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kühne H., Schutkowski A., Weinholz S., et al. Vitamin D receptor regulates intestinal proteins involved in cell proliferation, migration and stress response. Lipids in Health and Disease. 2014;13, article 51 doi: 10.1186/1476-511x-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Etten E., Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. The Journal of Steroid Biochemistry and Molecular Biology. 2005;97(1-2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Tang J., Zhou R., Luger D., et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. Journal of Immunology. 2009;182(8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffery L. E., Burke F., Mura M., et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of Immunology. 2009;183(9):5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haussler M. R., Haussler C. A., Whitfield G. K., et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the ‘Fountain of Youth’ to mediate healthful aging. The Journal of Steroid Biochemistry and Molecular Biology. 2010;121(1-2):88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artaza J. N., Sirad F., Ferrini M. G., Norris K. C. 1,25(OH)2 vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. The Journal of Steroid Biochemistry and Molecular Biology. 2010;119(1-2):73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof M. G., Redlich K., Schiller C., et al. Growth inhibitory effects on human colon adenocarcinoma-derived Caco-2 cells and calcemic potential of 1α,25- dihydroxyvitamin D3 analogs: structure-function relationships. Journal of Pharmacology and Experimental Therapeutics. 1995;275(3):1254–1260. [PubMed] [Google Scholar]
- 17.Cross H. S., Pavelka M., Slavik J., Peterlik M. Growth control of human colon cancer cells by vitamin D and calcium in vitro. Journal of the National Cancer Institute. 1992;84(17):1355–1362. doi: 10.1093/jnci/84.17.1355. [DOI] [PubMed] [Google Scholar]
- 18.Hugot J. P., Chamaillard M., Zouali H., et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 19.Inohara N., Ogura Y., Fontalba A., et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. The Journal of Biological Chemistry. 2003;278(8):5509–5512. doi: 10.1074/jbc.c200673200. [DOI] [PubMed] [Google Scholar]
- 20.Wang T. T., Dabbas B., Laperriere D., et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. The Journal of Biological Chemistry. 2010;285(4):2227–2231. doi: 10.1074/jbc.c109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S., Zhang Y. G., Lu R., et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2014 doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verway M., Behr M. A., White J. H. Vitamin D, NOD2, autophagy and Crohn's disease. Expert Review of Clinical Immunology. 2010;6(4):505–508. doi: 10.1586/eci.10.31. [DOI] [PubMed] [Google Scholar]
- 23.Simmons J. D., Mullighan C., Welsh K. I., Jewell D. P. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47(2):211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin K., Radlmayr M., Borchers R., Heinzlmann M., Folwaczny C. Candidate genes colocalized to linkage regions in inflammatory bowel disease. Digestion. 2002;66(2):121–126. doi: 10.1159/000065592. [DOI] [PubMed] [Google Scholar]
- 25.Köstner K., Denzer N., Müller C. S. L., Klein R., Tilgen W., Reichrath J. The relevance of Vitamin D Receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Research. 2009;29(9):3511–3536. [PubMed] [Google Scholar]
- 26.Xu Y., He B., Pan Y., et al. Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumour Biology. 2014;35(5):4153–4169. doi: 10.1007/s13277-013-1544-y. [DOI] [PubMed] [Google Scholar]
- 27.Tizaoui K., Berraies A., Hamdi B., Kaabachi W., Hamzaoui K., Hamzaoui A. Association of vitamin d receptor gene polymorphisms with asthma risk: systematic review and updated meta-analysis of case-control studies. Lung. 2014;192(6):955–965. doi: 10.1007/s00408-014-9648-8. [DOI] [PubMed] [Google Scholar]
- 28.Santoro D., Caccamo D., Gagliostro G., et al. Vitamin D metabolism and activity as well as genetic variants of the vitamin D receptor (VDR) in chronic kidney disease patients. Journal of Nephrology. 2013;26(4):636–644. doi: 10.5301/jn.5000203. [DOI] [PubMed] [Google Scholar]
- 29.Hughes D. J., McManus R., Neary P., O'Morain C., O'Sullivan M. Common variation in the vitamin D receptor gene and risk of inflammatory bowel disease in an Irish case-control study. European Journal of Gastroenterology & Hepatology. 2011;23(9):807–812. doi: 10.1097/meg.0b013e328349283e. [DOI] [PubMed] [Google Scholar]
- 30.Pluskiewicz W., Zdrzałek J., Karasek D. Spine bone mineral density and VDR polymorphism in subjects with ulcerative colitis. Journal of Bone and Mineral Metabolism. 2009;27(5):567–573. doi: 10.1007/s00774-009-0072-8. [DOI] [PubMed] [Google Scholar]
- 31.Pei F. H., Wang Y. J., Gao S. L., et al. Vitamin D receptor gene polymorphism and ulcerative colitis susceptibility in Han Chinese. Journal of Digestive Diseases. 2011;12(2):90–98. doi: 10.1111/j.1751-2980.2011.00483.x. [DOI] [PubMed] [Google Scholar]
- 32.Xia S. L., Yu L. Q., Chen H., et al. Association of vitamin D receptor gene polymorphisms with the susceptibility to ulcerative colitis in patients from Southeast China. Journal of Receptors and Signal Transduction. 2014 doi: 10.3109/10799893.2014.975248. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y. Y., Shu X. L., Zhao H., Yu J. D., Ma M., Chen J. Association between vitamin D receptor gene polymorphisms and pediatric Crohn's disease in China: A study based on gene sequencing. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15(11):1006–1008. doi: 10.7499/j.issn.1008-8830.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Noble C. L., McCullough J., Ho W., et al. Low body mass not vitamin D receptor polymorphisms predict osteoporosis in patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2008;27(7):588–596. doi: 10.1111/j.1365-2036.2008.03599.x. [DOI] [PubMed] [Google Scholar]
- 35.Bentley R. W., Keown D., Merriman T. R., et al. Vitamin D receptor gene polymorphism associated with inflammatory bowel disease in New Zealand males. Alimentary Pharmacology and Therapeutics. 2011;33(7):855–856. doi: 10.1111/j.1365-2036.2011.04588.x. [DOI] [PubMed] [Google Scholar]
- 36.Dresner-Pollak R., Ackerman Z., Eliakim R., Karban A., Chowers Y., Fidder H. H. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genetic Testing. 2004;8(4):417–420. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- 37.Naderi N., Farnood A., Habibi M., et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. Journal of Gastroenterology and Hepatology. 2008;23(12):1816–1822. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 38.Xue L.-N., Xu K.-Q., Zhang W., Wang Q., Wu J., Wang X.-Y. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. Inflammatory Bowel Diseases. 2013;19(1):54–60. doi: 10.1002/ibd.22966. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Wang Z. T., Hu J. J., Fan R., Zhou J., Zhong J. Polymorphisms of the vitamin D receptor gene and the risk of inflammatory bowel disease: a meta-analysis. Genetics and Molecular Research. 2014;13(2):2598–2610. doi: 10.4238/2014.april.8.2. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho A. Y. O. M., Bishop K. S., Han D. Y., et al. The role of vitamin D level and related single nucleotide polymorphisms in Crohn's disease. Nutrients. 2013;5(10):3898–3909. doi: 10.3390/nu5103898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones G., Stewart G. Association of PHF11 polymorphisms with asthma and allergy. Thorax. 2010;65(7):659–660. doi: 10.1136/thx.2009.124511. [DOI] [PubMed] [Google Scholar]
- 42.Eloranta J. J., Wenger C., Mwinyi J., et al. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenetics and Genomics. 2011;21(9):559–564. doi: 10.1097/FPC.0b013e328348f70c. [DOI] [PubMed] [Google Scholar]
- 43.Sonnenberg A., Ehms H., Sonnenberg G. E., Strohmeyer G. 25-Hydroxycholecalciferol serum levels in patients with Crohn's disease. Acta Hepato-Gastroenterologica. 1977;24(4):293–295. [PubMed] [Google Scholar]
- 44.Nic Suibhne T., Cox G., Healy M., O'Morain C., O'Sullivan M. Vitamin D deficiency in Crohn's disease: prevalence, risk factors and supplement use in an outpatient setting. Journal of Crohn's & Colitis. 2012;6(2):182–188. doi: 10.1016/j.crohns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Ardizzone S., Bollani S., Bettica P., Bevilacqua M., Molteni P., Porro G. B. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn's disease and ulcerative colitis. Journal of Internal Medicine. 2000;247(1):63–70. doi: 10.1046/j.1365-2796.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 46.Tajika M., Matsuura A., Nakamura T., et al. Risk factors for vitamin D deficiency in patients with Crohn's disease. Journal of Gastroenterology. 2004;39(6):527–533. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 47.Abreu M. T., Kantorovich Y., Vasiliauskas E. A., et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53(8):1129–1136. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Bruyn J. R., van Heeckeren R., Ponsioen C. Y., et al. Vitamin D deficiency in Crohn's disease and healthy controls: a prospective case-control study in the Netherlands. Journal of Crohn's and Colitis. 2014;8(10):1267–1273. doi: 10.1016/j.crohns.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Veit L. E., Maranda L., Fong J., Nwosu B. U. The vitamin D status in inflammatory bowel disease. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101583.e101583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harries A. D., Brown R., Heatley R. V., Williams L. A., Woodhead S., Rhodes J. Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut. 1985;26(11):1197–1203. doi: 10.1136/gut.26.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudnicki M., Frølich A., Transbøl I. Inappropriate hypercalcitriolemia in ileum-resected patients with Crohn's disease. Mineral and Electrolyte Metabolism. 1992;18(1):52–55. [PubMed] [Google Scholar]
- 52.Joseph A. J., George B., Pulimood A. B., Seshadri M. S., Chacko A. 25 (OH) vitamin D level in Crohn's disease: association with sun exposure & disease activity. Indian Journal of Medical Research. 2009;130(2):133–137. [PubMed] [Google Scholar]
- 53.McCarthy D., Duggan P., O'Brien M., et al. Seasonality of vitamin D status and bone turnover in patients with Crohn's disease. Alimentary Pharmacology & Therapeutics. 2005;21(9):1073–1083. doi: 10.1111/j.1365-2036.2005.02446.x. [DOI] [PubMed] [Google Scholar]
- 54.El-Matary W., Sikora S., Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Digestive Diseases and Sciences. 2011;56(3):825–829. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 55.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. Journal of Internal Medicine. 1996;239(2):131–137. doi: 10.1046/j.1365-2796.1996.420765000.x. [DOI] [PubMed] [Google Scholar]
- 56.Gilman J., Shanahan F., Cashman K. D. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn's disease and ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2006;23(7):1007–1016. doi: 10.1111/j.1365-2036.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 57.de Souza H. N., Lora F. L., Kulak C. A. M., Mañas N. C. P., Amarante H. M. B., Borba V. Z. C. Low levels of 25-hydroxyvitamin D (25OHD) in patients with inflammatory bowel disease and its correlation with bone mineral density. Arquivos Brasileiros de Endocrinologia e Metabologia. 2008;52(4):684–691. doi: 10.1590/s0004-27302008000400015. [DOI] [PubMed] [Google Scholar]
- 58.Leslie W. D., Miller N., Rogala L., Bernstein C. N. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. American Journal of Gastroenterology. 2008;103(6):1451–1459. doi: 10.1111/j.1572-0241.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 59.Ulitsky A., Ananthakrishnan A. N., Naik A., et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. Journal of Parenteral and Enteral Nutrition. 2011;35(3):308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 60.Hassan V., Hassan S., Seyed-Javad P., et al. Association between serum 25 (OH) vitamin D concentrations and inflammatory bowel diseases (IBDS) activity. Medical Journal of Malaysia. 2013;68(1):34–38. [PubMed] [Google Scholar]
- 61.Alkhouri R. H., Hashmi H., Baker R. D., Gelfond D., Baker S. S. Vitamin and mineral status in patients with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition. 2013;56(1):89–92. doi: 10.1097/MPG.0b013e31826a105d. [DOI] [PubMed] [Google Scholar]
- 62.Pappa H. M., Gordon C. M., Saslowsky T. M., et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118(5):1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahnsen J., Falch J. A., Mowinckel P., Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scandinavian Journal of Gastroenterology. 2002;37(2):192–199. doi: 10.1080/003655202753416876. [DOI] [PubMed] [Google Scholar]
- 64.Kuwabara A., Tanaka K., Tsugawa N., et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporosis International. 2009;20(6):935–942. doi: 10.1007/s00198-008-0764-2. [DOI] [PubMed] [Google Scholar]
- 65.Ezzat Y., Hamdy K. The frequency of low bone mineral density and its associated risk factors in patients with inflammatory bowel diseases. International Journal of Rheumatic Diseases. 2010;13(3):259–265. doi: 10.1111/j.1756-185x.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 66.Atia A., Murthy R., Bailey B. A., et al. Vitamin D Status in Veterans with inflammatory bowel disease: relationship to Health care costs and services. Military Medicine. 2011;176(6):711–714. doi: 10.7205/milmed-d-10-00371. [DOI] [PubMed] [Google Scholar]
- 67.Hessov I., Mosekilde L., Melsen F., et al. Osteopenia with normal vitamin D metabolites after small-bowel resection for Crohn's disease. Scandinavian Journal of Gastroenterology. 1984;19(5):691–696. [PubMed] [Google Scholar]
- 68.Nakajima S., Iijima H., Egawa S., et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition. 2011;27(10):1023–1028. doi: 10.1016/j.nut.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Laakso S., Valta H., Verkasalo M., Toiviainen-Salo S., Viljakainen H., Mäkitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcified Tissue International. 2012;91(2):121–130. doi: 10.1007/s00223-012-9617-2. [DOI] [PubMed] [Google Scholar]
- 70.Driscoll R. H., Jr., Meredith S. C., Sitrin M., Rosenberg I. H. Vitamin D deficiency and bone disease in patients with Crohn's disease. Gastroenterology. 1982;83(6):1252–1258. [PubMed] [Google Scholar]
- 71.Vogelsang H., Schöfl R., Tillinger W., Ferenci P., Gangl A. 25-Hydroxyvitamin D absorption in patients with Crohn's disease and with pancreatic insufficiency. Wiener Klinische Wochenschrift. 1997;109(17):678–682. [PubMed] [Google Scholar]
- 72.Schoon E. J., Müller M. C. A., Vermeer C., Schurgers L. J., Brummer R.-J. M., Stockbrügger R. W. Low serum and bone vitamin K status in patients with longstanding Crohn's disease: another pathogenetic factor of osteoporosis in Crohn's disease? Gut. 2001;48(4):473–477. doi: 10.1136/gut.48.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haderslev K. V., Jeppesen P. B., Sorensen H. A., Mortensen P. B., Staun M. Vitamin D status and measurements of markers of bone metabolism in patients with small intestinal resection. Gut. 2003;52(5):653–658. doi: 10.1136/gut.52.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilman J., Shanahan F., Cashman K. D. Determinants of vitamin D status in adult Crohn's disease patients, with particular emphasis on supplemental vitamin D use. European Journal of Clinical Nutrition. 2006;60(7):889–896. doi: 10.1038/sj.ejcn.1602395. [DOI] [PubMed] [Google Scholar]
- 75.Levin A. D., Wadhera V., Leach S. T., et al. Vitamin D deficiency in children with inflammatory bowel disease. Digestive Diseases and Sciences. 2011;56(3):830–836. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 76.Kini G. P., Young B., Herbison P., Schultz M. Does seasonal level of serum 25-OH vitamin D correlate with the activity of Crohn’s disease? New Zealand Medical Journal. 2014;127(1394):51–59. [PubMed] [Google Scholar]
- 77.Farraye F. A., Nimitphong H., Stucchi A., et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn's disease. Inflammatory Bowel Diseases. 2011;17(10):2116–2121. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 78.Sentongo T. A., Semaeo E. J., Stettler N., Piccoli D. A., Stallings V. A., Zemel B. S. Vitamin D status in children, adolescents, and young adults with Crohn disease. The American Journal of Clinical Nutrition. 2002;76(5):1077–1081. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 79.Siffledeen J. S., Siminoski K., Steinhart H., Greenberg G., Fedorak R. N. The frequency of vitamin D deficiency in adults with Crohn's disease. Canadian Journal of Gastroenterology. 2003;17(8):473–478. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- 80.Pappa H. M., Langereis E. J., Grand R. J., Gordon C. M. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition. 2011;53(4):361–364. doi: 10.1097/MPG.0b013e3182250b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ananthakrishnan A. N., Cagan A., Gainer V. S., et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflammatory Bowel Diseases. 2013;19(9):1921–1927. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hlavaty T., Krajcovicova A., Koller T., et al. Higher vitamin D serum concentration increases health related quality of life in patients with inflammatory bowel diseases. World Journal of Gastroenterology. 2014;20(42):15787–15796. doi: 10.3748/wjg.v20.i42.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peyrin-Biroulet L., Oussalah A., Bigard M.-A. Crohn's disease: the hot hypothesis. Medical Hypotheses. 2009;73(1):94–96. doi: 10.1016/j.mehy.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 84.de Bruyn J. R., van Heeckeren R., Ponsioen C. Y., et al. Vitamin D deficiency in Crohn's disease and healthy controls: a prospective case-control study in the Netherlands. Journal of Crohn's & Colitis. 2014;8(10):1267–1273. doi: 10.1016/j.crohns.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Nerich V., Jantchou P., Boutron-Ruault M.-C., et al. Low exposure to sunlight is a risk factor for Crohn's disease. Alimentary Pharmacology and Therapeutics. 2011;33(8):940–945. doi: 10.1111/j.1365-2036.2011.04601.x. [DOI] [PubMed] [Google Scholar]
- 86.Jantchou P., Clavel-Chapelon F., Racine A., Kvaskoff M., Carbonnel F., Boutron-Ruault M.-C. High residential sun exposure is associated with a low risk of incident Crohn's disease in the prospective E3N cohort. Inflammatory Bowel Diseases. 2014;20(1):75–81. doi: 10.1097/01.MIB.0000436275.12131.4f. [DOI] [PubMed] [Google Scholar]
- 87.Limketkai B. N., Bayless T. M., Brant S. R., Hutfless S. M. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Alimentary Pharmacology & Therapeutics. 2014;40(5):508–517. doi: 10.1111/apt.12845. [DOI] [PubMed] [Google Scholar]
- 88.Govani S. M., Higgins P. D. R., Stidham R. W., Montain S. J., Waljee A. K. Increased ultraviolet light exposure is associated with reduced risk of inpatient surgery among patients with Crohn's disease. Journal of Crohn's and Colitis. 2015;9(1):77–81. doi: 10.1093/ecco-jcc/jju002. [DOI] [PubMed] [Google Scholar]
- 89.Ananthakrishnan A. N., Khalili H., Higuchi L. M., et al. Higher predicted vitamin D status is associated with reduced risk of crohn's disease. Gastroenterology. 2012;142(3):482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garg M., Rosella O., Lubel J. S., Gibson P. R. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflammatory Bowel Diseases. 2013;19(12):2634–2643. doi: 10.1097/01.MIB.0000436957.77533.b2. [DOI] [PubMed] [Google Scholar]
- 91.Ananthakrishnan A. N., Cagan A., Gainer V. S., et al. Higher plasma vitamin D is associated with reduced risk of Clostridium difficile infection in patients with inflammatory bowel diseases. Alimentary Pharmacology and Therapeutics. 2014;39(10):1136–1142. doi: 10.1111/apt.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zator Z. A., Cantu S. M., Konijeti G. G., et al. Pretreatment 25-hydroxyvitamin D levels and durability of anti–tumor necrosis factor-α therapy in inflammatory bowel diseases. Journal of Parenteral and Enteral Nutrition. 2014;38(3):385–391. doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 93.Ham M., Longhi M. S., Lahiff C., Cheifetz A., Robson S., Moss A. C. Vitamin D levels in adults with Crohn's disease are responsive to disease activity and treatment. Inflammatory Bowel Diseases. 2014;20(5):856–860. doi: 10.1097/mib.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reid D., Toole B. J., Knox S., et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. The American Journal of Clinical Nutrition. 2011;93(5):1006–1011. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]
- 95.Waldron J. L., Ashby H. L., Cornes M. P., et al. Vitamin D: a negative acute phase reactant. Journal of Clinical Pathology. 2013;66(7):620–622. doi: 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- 96.Cantorna M. T., Munsick C., Bemiss C., Mahon B. D. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. Journal of Nutrition. 2000;130(11):2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 97.Daniel C., Radeke H. H., Sartory N. A., et al. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. Journal of Pharmacology and Experimental Therapeutics. 2006;319(2):622–631. doi: 10.1124/jpet.106.107599. [DOI] [PubMed] [Google Scholar]
- 98.Laverny G., Penna G., Vetrano S., et al. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunology Letters. 2010;131(1):49–58. doi: 10.1016/j.imlet.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Verlinden L., Leyssens C., Beullens I., et al. The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. The Journal of Steroid Biochemistry and Molecular Biology. 2013;136(1):107–111. doi: 10.1016/j.jsbmb.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 100.Ooi J. H., Li Y., Rogers C. J., Cantorna M. T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced coliti. Journal of Nutrition. 2013;143(10):1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Assa A., Vong L., Pinnell L. J., et al. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflammatory Bowel Diseases. 2015;21(2):297–306. doi: 10.1097/MIB.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 102.Tao Q., Wang B., Zheng Y., Jiang X., Pan Z., Ren J. Vitamin D prevents the intestinal fibrosis via induction of vitamin D receptor and inhibition of transforming growth factor-beta1/smad3 pathway. Digestive Diseases and Sciences. 2014 doi: 10.1007/s10620-014-3398-6. [DOI] [PubMed] [Google Scholar]
- 103.Ardizzone S., Cassinotti A., Trabattoni D., et al. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on Th1/Th2 cytokines in inflammatory bowel disease: an in vitro study. International Journal of Immunopathology and Pharmacology. 2009;22(1):63–71. doi: 10.1177/039463200902200108. [DOI] [PubMed] [Google Scholar]
- 104.Bendix-Struve M., Bartels L. E., Agnholt J., Dige A., Jørgensen S. P., Dahlerup J. F. Vitamin D3 treatment of Crohns disease patients increases stimulated T cell IL-6 production and proliferation. Alimentary Pharmacology & Therapeutics. 2010;32(11-12):1364–1372. doi: 10.1111/j.1365-2036.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- 105.Stio M., Martinesi M., Bruni S., et al. The Vitamin D analogue TX 527 blocks NF-kappaB activation in peripheral blood mononuclear cells of patients with Crohn's disease. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(1):51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Bartels L. E., Bendix M., Hvas C. L., et al. Oral vitamin D3 supplementation reduces monocyte-derived dendritic cell maturation and cytokine production in Crohn's disease patients. Inflammopharmacology. 2014;22(2):95–103. doi: 10.1007/s10787-013-0197-1. [DOI] [PubMed] [Google Scholar]
- 107.Miheller P., Muzes G., Hritz I., et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflammatory Bowel Diseases. 2009;15(11):1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 108.Jørgensen S. P., Agnholt J., Glerup H., et al. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Alimentary Pharmacology and Therapeutics. 2010;32(3):377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 109.Yang L., Weaver V., Smith J. P., Bingaman S., Hartman T. J., Cantorna M. T. Therapeutic effect of vitamin D supplementation in a pilot study of Crohn's patients. Clinical and Translational Gastroenterology. 2013;4, article e33 doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]