Abstract
Eyebright, Euphrasia rostkoviana Hayne (Scrophulariaceae), is a medicinal plant traditionally used in Europe for the treatment of various health disorders, especially as eyewash to treat eye ailments such as conjunctivitis and blepharitis that can be associated with bacterial infections. Some Euphrasia species have been previously reported to contain essential oil. However, the composition and bioactivity of E. rostkoviana oil are unknown. Therefore, in this study, we investigated the chemical composition and antimicrobial activity of the eyebright essential oil against some organisms associated with eye infections: Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, S. epidermidis, Pseudomonas aeruginosa, and Candida albicans. GC-MS analysis revealed more than 70 constituents, with n-hexadecanoic acid (18.47%) as the main constituent followed by thymol (7.97%), myristic acid (4.71%), linalool (4.65%), and anethole (4.09%). The essential oil showed antimicrobial effect against all organisms tested with the exception of P. aeruginosa. The best activity was observed against all Gram-positive bacteria tested with the minimum inhibitory concentrations of 512 µg/mL. This is the first report on the chemical composition of E. rostkoviana essential oil and its antimicrobial activity.
1. Introduction
Eyebright, Euphrasia rostkoviana Hayne (Scrophulariaceae), has been used in Europe for centuries as a traditional medicine for treatment of various diseases. Decoctions and infusions of flowering aerial parts are used against dry cough, hoarseness, symptomatic treatment of cold, earache, and headache, hay fever, purulent skin lesion, or catarrhal diseases of the intestinal tract, but especially as eyewash to treat and prevent eye disorders such as conjunctivitis, blepharitis, eye fatigue, purulent ocular inflammation, and sties [1–3]. The use of eyebright tea has also been reported in ethnoveterinary medicine for cow eye infection treatment [4]. Despite centuries of the traditional use for the treatment of eye ailments, there has been only one prospective cohort trial carried out confirming the efficacy of Euphrasia eye drops in the treatment of conjunctivitis [5] and a single clinical study investigating the effect of local application of the eye drops on antibiotic consumption in neonates [6]. Moreover, until the recent reports on anticandidal [7] and antibacterial activity [8] of some Euphrasia extracts, the spectrum of antimicrobial action has been completely unknown.
The therapeutic effect of E. rostkoviana can be attributed mainly to its antioxidant, anti-inflammatory, and antimicrobial activity [2, 4, 8, 9]. Among the compounds previously identified in E. rostkoviana extracts [8–10], apigenin, luteolin, kaempferol, quercetin, caffeic acid, coumaric acid, and rosmarinic acid may be responsible for the antimicrobial action. Although the presence of essential oil (EO) in E. officinalis L. [11] and E. stricta Kunt [12] has previously been reported, the composition and bioactivity of the E. rostkoviana EO are unknown. Therefore, in this study, we investigated the chemical composition and antimicrobial activity of the eyebright EO against the panel of three Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus, and S. epidermidis) and three Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa), and one yeast (Candida albicans), organisms commonly associated with eye infections.
2. Material and Methods
2.1. Chemicals and Plant Material
The authentic standards borneol, camphor, carvacrol, carvone, caryophyllene, p-cymene, estragole, eucalyptol, limonene, linalool, menthol, menthone, β-myrcene, γ-terpinene, and thymol for EO components identification as well as the control antibiotics ciprofloxacin and tioconazole were purchased from Sigma-Aldrich (Prague, Czech Republic). Hexane (Merck, Prague, Czech Republic), dimethyl sulfoxide (DMSO) (Lach-Ner, Neratovice, Czech Republic), and Tween 80 (Sigma-Aldrich, Prague, Czech Republic) were used as solvents. The plant material used for the EO distillation was purchased from commercial sources (F-DENTAL, Hodonín, Czech Republic). The EO was extracted by hydrodistillation using Clevenger type apparatus.
2.2. Chemical Analysis of the EO by Gas Chromatography-Mass Spectrometry (GC-MS)
The E. rostkoviana EO was analyzed by GC-MS using Agilent 7890A GC coupled to Agilent 5975C single-quadrupole mass detector equipped with a HP-5MS column (30 m × 0.25 mm, 0.25 μm film) from Agilent (Santa Clara, CA, USA). Hexane was used as a solvent and the sample volume of 1 μL was injected in split mode (ratio 20 : 1) into the injector heated to 250°C. The starting oven temperature was set at 60°C for 3 min, programmed to 230°C at a rate of 3°C/min, and then kept constant for 10 min. Helium was used as carrier gas with the flow rate of 1 mL/min. The MS analysis was carried out in full-scan mode and the electron ionization energy was set at 70 eV. The identification of individual components was based on the comparison of their mass spectra and relative retention indices with the National Institute of Standards and Technology Library (NIST, USA) and literature [13], as well as coinjection of authentic standard.
2.3. Bacterial Strains and Cultivation Media
The standard strains of three Gram-positive bacteria Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, and S. epidermidis ATCC 12228, three Gram-negative bacteria Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa ATCC 27853, and one yeast Candida albicans ATCC 10231 were obtained from Oxoid (Basingstoke, United Kingdom). Cation adjusted Mueller-Hinton broth (MHB) and Sabouraud dextrose broth (SDB) were used as cultivation media for antibacterial and antifungal microdilution assay, respectively, and were equilibrated with Tris-buffered saline (Sigma-Aldrich, Prague, Czech Republic). Mueller-Hinton agar (MHA) and Sabouraud dextrose agar (SDA) were used for subsequent determination of bactericidal and fungicidal concentrations, respectively. All media were purchased from Oxoid (Basingstoke, United Kingdom).
2.4. Minimum Inhibitory Concentration (MIC) Determination
The MICs were determined using the in vitro broth microdilution method following the guidelines of Clinical and Laboratory Standards Institute (CLSI) [14, 15] modified according to the recommendations proposed for effective assessment of the anti-infective potential of natural products [16] using 96-well microtiter plates. Briefly, the EO was dissolved in DMSO with addition of Tween 80 and two-fold serial dilutions were prepared in MHB for bacteria and in SDB for the yeast whereas the concentrations tested ranged from 4 to 2048 μg/mL. The inoculum was prepared from overnight cultures so that the initial CFU concentrations in the microplates were 5 × 105 and 2 × 103 CFU/mL for bacteria and yeast, respectively. The inoculated plates were examined after 24 h of incubation at 35°C and once more after 48 h in case of C. albicans. The microbial growth was measured spectrophotometrically by Multiscan Ascent Microplate Photometer (Thermo Fisher Scientific, Waltham, USA) at 405 nm. MICs were expressed as the lowest concentrations able to inhibit ≥ 80% of bacterial growth compared to the positive growth control. The experiments were performed in triplicate in three independent tests and median values were used for MICs calculation. Due to the recently reported possibility of EO volatile components' influence on the microbial growth in adjoining wells [17], positive growth control rows were inserted in between the EO dilution rows to detect eventual growth influence. The solvents used did not inhibit the bacterial growth at concentrations tested. Ciprofloxacin and tioconazole were used as reference antibiotics for bacteria and yeast, respectively.
2.5. Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration(MFC) Determination
The aliquots of 20 μL were transferred from each well without microbial growth to the MHA plates (SDA plates for C. albicans) after 24 h and 48 h of incubation of bacteria and yeast tested, respectively. The plates were then incubated for 24 h at 35°C. The MBC and MFC were evaluated as ≥99.9% decrease in CFU comparing to inoculum, all performed in triplicate in three independent tests.
3. Results and Discussion
3.1. Chemical Characterization of Oils and Bioactive Fractions Constituents
The EO hydrodistillation by Clevenger-type apparatus yielded 0.02% (w/v) of yellowish-brown oil that tends to solidify at room temperature which is probably caused by high proportion of fatty acids (32.23% in total). GC-MS analysis of the EO revealed the presence of more than 70 constituents, with palmitic acid (18.47%) being the most abundant component followed by thymol (7.97%), myristic acid (4.71%), linalool (4.65%), anethole (4.09%), linolenic acid (3.81%), hexahydrofarnesyl acetone (3.16%), lauric acid (2.79%), α-terpineol (2.39%), and borneol (2.39%). The main compounds are shown also in the chromatogram (Figure 1) and the complete list of EO constituents is presented in Table 1.
Figure 1.
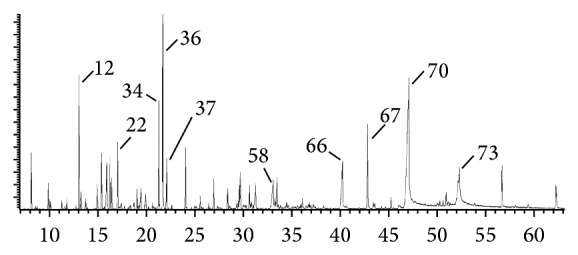
Typical chromatogram of Euphrasia rostkoviana essential oil. The main components are labeled according to the order of their retention times. (12) Linalool; (22) α-terpineol; (34) anethole; (36) thymol; (37) carvacrol; (58) lauric acid; (66) myristic acid; (67) hexahydrofarnesyl acetone; (70) palmitic acid; (73) linolenic acid.
Table 1.
Chemical composition of Euphrasia rostkoviana Hayne essential oil.
| Peak number | Component | RI | Area (%)∗ | ID | Peak number | Component | RI | Area (%) | ID |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1-Hexanold | — | 0.10 | a | 41 | Damascenone | 1385 | 0.56 | a, b |
| 2 | 1-Octen-3-ol | 981 | 1.82 | a, b | 42 | Methyl eugenol | 1406 | 0.23 | a, b |
| 3 | β-Myrcene | 992 | 0.14 | a, b, c | 43 | Caryophyllene | 1419 | 1.28 | a, b, c |
| 4 | 3-Octanol | 996 | 0.13 | a, b | 44 | Geranyl acetone | 1455 | 0.89 | a, b |
| 5 | p-Cymene | 1027 | 0.81 | a, b, c | 45 | Trans-β-farnesene | 1460 | 0.13 | a, b |
| 6 | Limonene | 1032 | 0.34 | a, b, c | 46 | Alloaromadendrene | 1462 | 0.12 | a, b |
| 7 | Eucalyptol | 1034 | 0.25 | a, b, c | 47 | γ-Muurolene | 1478 | 0.25 | a, b |
| 8 | γ-Terpinene | 1062 | 0.34 | a, b, c | 48 | Germacrene D | 1482 | 0.31 | a, b |
| 9 | Sabinene hydrate | 1070 | 0.14 | a, b | 49 | Curcumene | 1484 | 1.21 | a, b |
| 10 | 1-Octanol | 1074 | 0.40 | a, b | 50 | Trans-β-ionone | 1487 | 1.53 | a, b |
| 11 | 3,5-Octadienoned | 1094 | 0.16 | a, b | 51 | Valencene | 1493 | 0.13 | a, b |
| 12 | Linalool | 1101 | 4.65 | a, b, c | 52 | α-Selinened | 1495 | 0.17 | a, b |
| 13 | α-Thujone | 1106 | 0.88 | a, b | 53 | Epizonarened | 1498 | 0.26 | a, b |
| 14 | β-Thujone | 1118 | 0.48 | a, b | 54 | α-Muurolene | 1500 | 0.18 | a, b |
| 15 | Camphor | 1146 | 1.00 | a, b, c | 55 | β-Bisabolene | 1510 | 0.94 | a, b |
| 16 | Menthone | 1156 | 1.98 | a, b, c | 56 | γ-Cadinene | 1515 | 0.40 | a, b |
| 17 | 2-Nonenal, (E)- | 1163 | 0.10 | a, b | 57 | Nerolidold | 1566 | 0.10 | a, b |
| 18 | Borneol | 1168 | 2.39 | a, b, c | 58 | Lauric acid | 1574 | 2.79 | a, b |
| 19 | (+/−)Lavandulol | 1170 | 0.16 | a, b | 59 | Spathulenol | 1578 | 0.61 | a, b |
| 20 | Menthol | 1175 | 2.02 | a, b, c | 60 | Caryophyllene oxide | 1583 | 1.47 | a, b |
| 21 | 4-Terpineol | 1179 | 1.13 | a, b | 61 | Pseudoiononed | 1587 | 0.18 | a, b |
| 22 | α-Terpineol | 1191 | 2.39 | a, b | 62 | Humulene epoxide II | 1609 | 0.25 | a, b |
| 23 | Estragole | 1199 | 0.34 | a, b, c | 63 | Longifolenaldehyded | 1613 | 0.20 | a, b |
| 24 | Decanal | 1207 | 0.15 | a, b | 64 | τ-Cadinol | 1643 | 0.18 | a, b |
| 25 | β-Cyclocitrald | 1222 | 0.21 | a, b | 65 | β-Eudesmol | 1651 | 0.13 | a, b |
| 26 | Thymol methyl ether | 1238 | 0.75 | a, b | 66 | Myristic acid | 1771 | 4.71 | a, b |
| 27 | Cumin aldehyde | 1242 | 0.23 | a, b | 67 | Hexahydrofarnesyl acetone | 1847 | 3.16 | a, b |
| 28 | Neral | 1244 | 0.14 | a, b | 68 | Pentadecanoic acid | 1865 | 0.28 | a, b |
| 29 | Carvone | 1246 | 1.28 | a, b, c | 69 | Farnesyl acetone | 1919 | 0.50 | a, b |
| 30 | Piperitone | 1256 | 0.18 | a, b | 70 | Palmitic acid | 1977 | 18.47 | a, b |
| 31 | Geraniol | 1258 | 0.79 | a, b | 71 | Phytold | 2114 | 0.12 | a, b |
| 32 | Trans-2-decenald | 1264 | 0.11 | a, b | 72 | Linoleic acid | 2143 | 1.90 | a, b |
| 33 | Geranial | 1273 | 0.15 | a, b | 73 | Linolenic acid | 2148 | 3.81 | a, b |
| 34 | Anethole | 1287 | 4.09 | a, b | 74 | Tricosane | 2300 | 1.79 | a, b |
| 35 | Safrole | 1289 | 0.21 | a, b | 75 | Tetracosane | 2400 | 0.16 | a, b |
| 36 | Thymol | 1295 | 7.97 | a, b, c | 76 | Pentacosane | 2500 | 1.36 | a, b |
| 37 | Carvacrol | 1304 | 1.96 | a, b, c | |||||
| 38 | (E,E)-2,4-Decadienal | 1318 | 0.21 | a, b | Total identified | 98.91 | |||
| 39 | Capric acid | 1374 | 0.26 | a, b | |||||
| 40 | α-Copaene | 1377 | 0.15 | a, b |
R: retention indices relative to n-alkanes on HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm); ∗peak area relative to total peak area in %; ID: identification method; a: identification based on mass spectra matching; b: identification based on retention index; c: identification based on coinjection of authentic sample; d: tentative identification.
The high content of fatty acids has previously been found in the E. stricta EO (25.7% in total) also with the highest proportion of palmitic acid (20.3%) and myristic acid (3.9%) [12]. However, there is no other compound present in significant amount that would indicate the relatedness of these two Euphrasia species.
3.2. Antimicrobial Activity
The E. rostkoviana EO showed activity against six out of seven organisms tested with MICs ranging from 512 to 2048 μg/mL. The Gram-positive bacteria were more sensitive than the Gram-negative ones and the yeast whereas P. aeruginosa was the only organism that was not inhibited by the oil at the highest concentrations tested. The MICs, MBCs, and MFCs of the EO against all microorganisms tested are summarized in Table 2. The active concentrations are comparable to those previously reported for, for example, EOs of Artemisia annua, Eucalyptus globulus, Mentha suaveolens, Myrtus communis, Ocimum basilicum, or Thymus vulgaris, especially in the case of anticandidal activity [18–20]. The oil was also more effective than E. rostkoviana extracts tested by Teixeira and Silva [8] against E. coli, E. faecalis, S. aureus, and S. epidermidis. The MICs of the reference antibiotics against the bacteria and yeast susceptible to the E. rostkoviana EO were in accordance with the CLSI acceptable limits and previous reports, respectively [21–23].
Table 2.
The inhibitory and cidal concentrations of E. rostkoviana essential oil.
| Microorganism | Euphrasia rostkoviana EO | CIP | TIO | ||||
|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | IC50 (µg/mL) | MBC/MFC (µg/mL) | MIC (µg/mL) | MIC (µg/mL) | |||
| 24 h | 48 h∗ | 24 h | 48 h | 24 h | 24 h | 48 h | |
| Enterococcus faecalis | 512 | — | 128 | — | 1024 | 0.5 | — |
| Staphylococcus aureus | 512 | — | 128 | — | >2048 | 0.5 | — |
| Staphylococcus epidermidis | 512 | — | 256 | — | >2048 | 0.25 | — |
| Klebsiella pneumoniae | 2048 | — | 1024 | — | >2048 | 0.125 | — |
| Escherichia coli | 2048 | — | 1024 | — | >2048 | 0.015 | — |
| Pseudomonas aeruginosa | >2048 | — | >2048 | — | >2048 | 0.125 | — |
| Candida albicans | 128 | 1024 | 128 | 1024 | 2048 | — | 0.063 |
∗The growth inhibition was measured after 24 h and 48 h of incubation in case of C. albicans; EO: essential oil; CIP: ciprofloxacin; TIO: tioconazole; MIC: minimum inhibitory concentration; IC50: inhibitory concentration causing ≥50% of bacterial growth; MBC: minimum bactericidal concentration; MFC: minimum fungicidal concentration.
Since the content of the main EO constituent palmitic acid does not exceed 20% and there are more than 10 other antimicrobially active compounds ranging from 1 to 8% it is difficult to suggest the main agents responsible for the E. rostkoviana EO antimicrobial effect. Palmitic acid has been previously identified as the major compound of fractions active against Gram-negative, but not Gram-positive, bacteria [24]. On the other hand, medium-chain saturated fatty acids and long-chain unsaturated fatty acids are known to inhibit especially Gram-positive bacteria [25]. Moreover, lauric acid exerts also activity against a number of fungi [26]. Thus the antimicrobial activity of the EO is probably due to a complex action of the antimicrobial fatty acids with the other well-known antimicrobial compounds identified in the EO such as anethole, borneol, camphor, carvacrol, linalool, menthol, α-terpineol, or thymol.
4. Conclusions
In conclusion, the chemical analysis revealed a number of antimicrobially active substances present in the E. rostkoviana EO and its antifungal and antibacterial activity against Gram-positive as well as Gram-negative bacteria was confirmed. To the best of our knowledge, this is the first report on the composition and antimicrobial activity of E. rostkoviana EO.
Acknowledgments
This work was financially supported by the European Science Foundation and the Ministry of Education, Youth and Sports of the Czech Republic Project CZ.1.07/2.3.00/30.0040; by the S-grant of the Ministry of Education, Youth and Sports of the Czech Republic; and by the Czech University of Life Sciences Prague Project IGA 20155012. The authors are grateful to Slavka Barlakova for English proofreading.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Darshan S., Doreswamy R. Patented antiinflammatory plant drug development from traditional medicine. Phytotherapy Research. 2004;18(5):343–357. doi: 10.1002/ptr.1475. [DOI] [PubMed] [Google Scholar]
- 2.Barnes J., Anderson L. A., Phillipson J. D. Herbal Medicines. 3rd. London, UK: Pharmaceutical Press; 2007. [Google Scholar]
- 3.European Medicines Agency. Assessment Report on Euphrasia officinalis L. and Euphrasia rostkoviana Hayne, Herba. London, UK: European Medicines Agency; 2010. (A Report of Committee on Herbal Medicinal Products). http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2011/01/WC500100385.pdf. [Google Scholar]
- 4.Lans C., Turner N., Khan T., Brauer G., Boepple W. Ethnoveterinary medicines used for ruminants in British Columbia, Canada. Journal of Ethnobiology and Ethnomedicine. 2007;3, article 11 doi: 10.1186/1746-4269-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoss M., Michels C., Peter E., Beutke R., Gorter R. W. Prospective cohort trial of Euphrasia single-dose eye drops in conjunctivitis. Journal of Alternative and Complementary Medicine. 2000;6(6):499–508. doi: 10.1089/acm.2000.6.499. [DOI] [PubMed] [Google Scholar]
- 6.Stoffel L., Zimmermann D., Hunkeler R., et al. Euphrasia eye drops in neonates: a pilot project. Schweizerische Zeitschrift fur GanzheitsMedizin. 2007;19(5):254–259. doi: 10.1159/000283798. [DOI] [Google Scholar]
- 7.Trovato A., Monforte M. T., Forestieri A. M., Pizzimenti F. In vitro anti-mycotic activity of some medicinal plants containing flavonoids. Bollettino Chimico Farmaceutico. 2000;139(5):225–227. [PubMed] [Google Scholar]
- 8.Teixeira R., Silva L. R. Bioactive compounds and in vitro biological activity of Euphrasia rostkoviana Hayne extracts. Industrial Crops and Products. 2013;50:680–689. doi: 10.1016/j.indcrop.2013.08.035. [DOI] [Google Scholar]
- 9.Blazics B., Alberti Á., Béni S., Kursinszki L., Tölgyesi L., Kéry Á. Identification and LC-MS-MS determination of acteoside, the main antioxidant compound of Euphrasia rostkoviana, using the isolated target analyte as external standard. Journal of Chromatographic Science. 2011;49(3):203–208. doi: 10.1093/chrsci/49.3.203. [DOI] [Google Scholar]
- 10.Blazics B., Ludanyi K., Szarka S., Kery A. Investigation of Euphrasia rostkoviana Hayne using GC-MS and LC-MS. Chromatographia. 2008;68(1):S119–S124. doi: 10.1365/s10337-008-0630-6. [DOI] [Google Scholar]
- 11.Harkiss K. J., Timmins P. Studies in the Scrophulariaceae part VIII1 phytochemical investigation of Euphrasia officinalis . Planta Medica. 1973;23(4):342–347. doi: 10.1055/s-0028-1099453. [DOI] [PubMed] [Google Scholar]
- 12.Miladinovic D. L., Ilic B. S., Nikolic D. M., et al. Volatile constituents of Euphrasia stricta . Chemistry of Natural Compounds. 2014;49(6):1146–1147. doi: 10.1007/s10600-014-0845-8. [DOI] [Google Scholar]
- 13.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th. Carol Stream, Ill, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. 8th. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2009. (CLSI document M7-A8). [Google Scholar]
- 15.Espinel-Ingroff A. V., Pfaller M. A. Susceptibility test methods: yeasts and filamentous fungi. In: Murray P. R., Baron E. J., Jorgensen J. H., Landry M. L., Pfaller M. A., editors. Manual of Clinical Microbiology. 9th. Washington, DC, USA: ASM Press; 2007. [Google Scholar]
- 16.Cos P., Vlietinck A. J., Berghe D. V., Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. Journal of Ethnopharmacology. 2006;106(3):290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Novy P., Kloucek P., Rondevaldova J., Havlik J., Kourimska L., Kokoska L. Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia. 2014;94:102–107. doi: 10.1016/j.fitote.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 18.De Rapper S., Kamatou G., Viljoen A., van Vuuren S. The in vitro antimicrobial activity of Lavandula angustifolia essential oil in combination with other aroma-therapeutic oils. Evidence-based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/852049.852049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilia A. R., Santomauro F., Sacco C., Bergonzi M. C., Donato R. Essential oil of Artemisia annua L.: an extraordinary component with numerous antimicrobial properties. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/159819.159819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringaro A., Vavala E., Colone M., et al. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans . Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/125904.125904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2009. (CLSI document M100-S19). [Google Scholar]
- 22.El-Sherbeny M. A., Maarouf A. R., Hassan A. H. E., Abdel-Aziz N. I. Design and synthesis of new benzimidazole derivatives as potential antimicrobial agents. Journal of American Science. 2012;8(12):785–798. [Google Scholar]
- 23.Lulekal E., Rondevaldova J., Bernaskova E., et al. Antimicrobial activity of traditional medicinal plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharmaceutical Biology. 2014;52(5):614–620. doi: 10.3109/13880209.2013.858362. [DOI] [PubMed] [Google Scholar]
- 24.Moradali M.-F., Mostafavi H., Ghods S., Hejaroude G. A. Investigation of antimicrobial fatty acids from medicinal artist conk mushroom Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) by TLC and spectroscopic detection. International Journal of Medicinal Mushrooms. 2008;10(2):149–154. doi: 10.1615/intjmedmushr.v10.i2.50. [DOI] [Google Scholar]
- 25.Zhang H., Zhang L., Peng L.-J., et al. Quantitative structure-activity relationships of antimicrobial fatty acids and derivatives against Staphylococcus aureus . Journal of Zhejiang University: Science B. 2012;13(2):83–93. doi: 10.1631/jzus.b1100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayrit F. M. The properties of Lauric acid and their significance in coconut oil. Journal of the American Oil Chemists' Society. 2015;92(1):1–15. doi: 10.1007/s11746-014-2562-7. [DOI] [Google Scholar]


