Abstract
Extracellular matrix molecule chondroitin sulfate proteoglycans (CSPGs) are highly upregulated in scar tissues and form a potent chemical barrier for CNS axon regeneration. Recent studies support that the receptor protein tyrosine phosphatase σ (PTPσ) and its subfamily member leukocyte common antigen related phosphatase (LAR) act as transmembrane receptors to mediate CSPG inhibition. PTPσ deficiency increased regrowth of ascending axons into scar tissues and descending corticospinal tract (CST) axons into the caudal spinal cord after spinal cord injury (SCI). Pharmacological LAR inhibition enhanced serotonergic axon growth in SCI mice. However, transgenic LAR deletion on axon growth in vivo and role of LAR in regulating regrowth of other fiber tracts have not been studied. Here, we studied role of LAR in restricting regrowth of injured descending CNS axons in deficient mice. LAR deletion increased regrowth of serotonergic axons into scar tissues and caudal spinal cord after dorsal overhemitransection. LAR deletion also stimulated regrowth of CST fibers into the caudal spinal cord. LAR protein was upregulated days to weeks after injury and co-localized to serotonergic and CST axons. Moreover, LAR deletion improved functional recovery by increasing BMS locomotor scores and stride length and reducing grid walk errors. This is the first transgenic study that demonstrates crucial role of LAR in restricting regrowth of injured CNS axons.
Keywords: CSPG receptor, LAR phosphatase, spinal cord injury, scar inhibition, axon regeneration, functional recovery
Introduction
Failure of CNS axons to regenerate after injury has been ascribed to a non-permissive environment and a developmental reduction in intrinsic growth capacity (Busch and Silver, 2007; Goldberg et al., 2002; McGee and Strittmatter, 2003). Among the extrinsic factors, chondroitin sulfate proteoglycans (CSPGs) are highly upregulated by reactive scar tissues and strongly suppress axon extension into and beyond the lesion by forming a non-permissive perineuronal nets with other extracellular matrix molecules (Bradbury et al., 2002; Busch and Silver, 2007; Jones et al., 2003). Although the potent inhibitory property of CSPGs on neuronal regeneration has been identified for over two decades (McKeon et al., 1991; Snow et al., 1990), the underlying molecular mechanisms are not well understood. The inhibitory actions of CSPGs depend on sulfation pattern of their glycosaminoglycan (GAG) chains because preventing GAG sulfation eliminates most of the inhibitory activity on axon growth in vitro (Gilbert et al., 2005; Sherman and Back, 2008; Wang et al., 2008). CSPGs may mediate suppression of axon growth by several mechanisms, including binding and activating functional transmembrane receptors, sterically hindering growth-promoting adhesion molecules, and facilitating inhibitory effects of chemo-repulsive molecules (Condic et al., 1999; Kantor et al., 2004; Tan et al., 2011). Although CSPGs may hinder function of matrix molecules non-specifically, several receptors appear to be important in conveying CSPG inhibition, including PTPσ, LAR, Nogo receptor (NgR) 1 and 3 (Dickendesher et al., 2012; Fisher et al., 2011; Shen et al., 2009). In particular, PTPσ and LAR, two members in the LAR subfamily, could bind CSPGs with high affinity and mediate their suppression of axon elongation.
PTPσ deficiency in adult mice increased regrowth of sensory axons into scar tissues and CST axons into the caudal spinal cord after injury (Dickendesher et al., 2012; Shen et al., 2009). Pharmacological blockade of LAR with small peptides stimulated regrowth of 5-HT axons (Fisher et al., 2011). However, many critical issues regarding CSPG receptor function remain unclear, including validation of LAR function on axon growth in vivo by a transgenic approach. In this study, we studied the effect of LAR deletion on CNS axonal regeneration and functional recovery. LAR deletion increases regrowth of serotonergic and CST axon tracts in the caudal spinal cord after spinal cord injury (SCI) and improves locomotor functional recovery several weeks after injury. Digestion of CSPG GAG chains with locally applied bacterial chondroitinase ABC (ChABC) has been the main in vivo approach to surmount CSPG inhibition, but ChABC has many important disadvantages for therapeutic use in patients (Lee et al., 2010; Lemons et al., 2003; Sharma et al., 2012). Therefore, a better understanding of CSPG receptor function may facilitate surmounting scar-mediated growth inhibition and developing more effective therapies for CNS injury.
Materials and Methods
LAR protein expression in the spinal cord
To determine expression changes of LAR after a CNS injury, we examine levels of this protein in lesioned spinal cord in female C57BL/6 mice (9 weeks old) after dorsal over-transection injury at T7 (see below for injury induction). To check LAR expression at different time points, we perfused SCI mice transcardially with cold PBS for 5 min 1, 3, 7, 14 and 21 days after injury (4 mice per time point). Immediately after perfusion, 3 blocks of fresh spinal cord (4 mm/block) in each mouse were collected onto dry ice and stored in −80°C freezer: 2–6 mm rostral to lesion center, 0–2 mm rostral to and caudal to the lesion (containing the lesion area), and 2–6 mm caudal to the lesion. After preparation of the spinal cord blocks in lysis buffer with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 mM orthovanadate, 10 µg/ml leupeptin and 10 µg/ml aprotinin, Sigma), the supernatants of these samples were analyzed for LAR protein levels with Western blots. In brief, following total protein quantification with Bio-Rad DC protein assay reagents, the samples containing same amount of total proteins (20 µg/well) were prepared for Western blots using antibodies against LAR (Clone 7, BDB610351, BD Biosciences). Proteins were transferred to nitrocellulose membrane and bands were visualized with enhanced chemiluminescence reagents (Amersham, Piscataway, NJ). The same blots were reprobed for actin clone C4 (MP Biomedicals). For blot densitometry, the images of protein bands were captured with a Bio-Rad Gel Doc XR documentation system and band density was determined using Quantity One software (Fu et al., 2007). The band density of LAR protein calculated by software at different time points was calibrated with that actin reprobed with the same blots. To confirm LAR upregulation 7 days after a dorsal over-transection, we immunostained parasagittal sections of the spinal cord 4 mm rostral and caudal to the lesion epicenter for LAR protein in adult mice. No-injury controls received sham surgery (2 mice/group).
To determine expression of LAR in serotonergic and CST axons, 4 adult wild-type mice received biotin dextran amine (BDA) tracer injections into the left sensorimotor cortex. Tissue sections (30 µm) of fixed spinal cord were doubly stained for LAR and 5-HT (raphespinal axons) or for LAR and BDA (CST axons) 14 days after BDA tracer injections. Coronal sections of the brainstem (40 um) were also collected and used to localize LAR to 5-HT-labeled serotonergic neuronal cell bodies in the raphe nuclei.
LAR knockout mice
All the experimental procedures with animals were approved by the Institutional Animal Care and Use Committee at Temple University. LAR knockout mice on a DBA background were provided by Dr. Frank Longo (Xie et al., 2001) and originally generated by Dr. William Skarnes (Skarnes et al., 1995). LAR+/+, +/− and −/− mice were generated by crossing LAR+/− mice and genotypes of mice were determined by regular PCR and reverse transcript PCR as reported previously(Yeo et al., 1997).
Dorsal over-hemisection of the spinal cord, axon tracing, histology and behavioral tests
To study axonal growth and functional recovery in LAR deficient mice after SCI, we performed 3 sets of in vivo experiments in LAR mutant mice as summarized in Table 1. We examined regrowth of serotonergic axons in experiment (Exp.) 1 and 2 and CST axons in Exp. 2 and 3. Because it is extremely time-consuming to quantify axon numbers from all the parasagittal sections in each animal (~25 sections/mouse), we evaluated regrowth of serotonergic and CST axons from one experiment for each fiber tract, Exp. 1 and 3, respectively. We monitored the recovery of the Basso mouse scale (BMS) scores in all the 3 experiments (Basso et al., 2006). A complete laminectomy was performed and the dorsal part of the spinal cord was fully exposed at T6 and T7 levels (Li and Strittmatter, 2003). A dorsal overhemisection at 1.0 mm was performed at T7 (~1.5 mm in dorsoventral diameter) with a 30-gauge needle and microscissors to completely sever dorsal part of the spinal cord (Fig. 2A) as reported previously (Fisher et al., 2011; Li and Strittmatter, 2003; Ohtake et al., 2014). The lesion depth of 1 mm was ensured by passing a marked 30-gauge of needle 4 times across the dorsal spinal cord. Three weeks after SCI, mice received BDA tracer injections into the sensorimotor cortex with 5 injection sites and the coordinates summarized in Table 2 (0.9 mm depth, 1 µl/spot) (Li and Strittmatter, 2003).
Table 1.
Summary of LAR mutant mouse experiments to study axon growth and recovery after SCI
| Experiments | Groups & mouse No. | Axonal growth analysis | Behavioral tests |
|---|---|---|---|
| Exp. 1 | LAR+/+, n=7 LAR+/−, n=7 LAR−/−, n=12 |
5-HT axons: transverse & parasagittal sections | BMS, grid walk |
| Exp. 2 | LAR+/+, n=7 LAR+/−, n=5 LAR−/−, n=7 |
5-HT and CST axons: Transverse sections | BMS, footprint |
| Exp. 3 | LAR+/+, n=9 LAR−/−, n=8 |
CST axons: transverse & parasagittal sections | BMS |
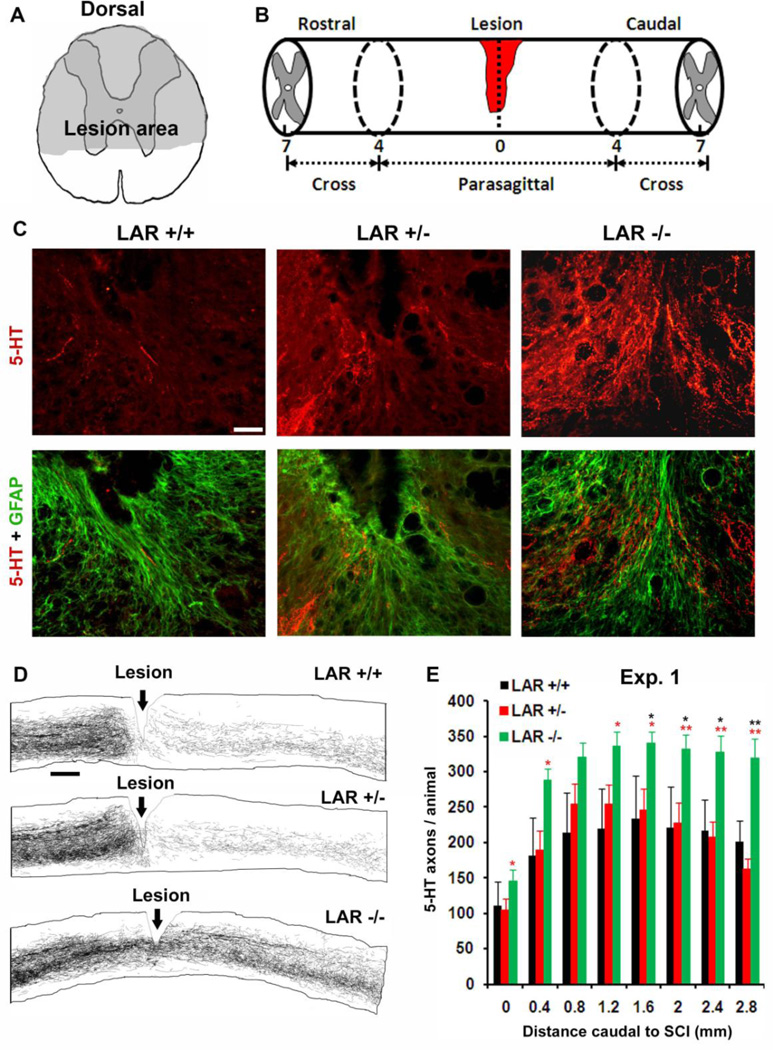
Figure 2. LAR deletion in adult mice enhances growth of serotonergic fibers in the scar tissue and caudal spinal cord after T7 SCI.
A, B, Schematic of the transected area at T7 and histological processing of the spinal cord around the lesion. C, Parasagittal sections of the spinal cord around the lesion from representative mouse in each group indicate growth of 5-HT-labeled serotonergic axons (red) into the GFAP-stained astrocytic scar tissue (green) in LAR −/− mouse, but not in LAR +/+ SCI controls. Scale: 50 µm. D, Camera lucida drawings of 5-HT fibers from all parasagittal sections of the representative mouse in each group indicate higher density of serotonergic axons in the caudal spinal cord of LAR −/− animal than that in SCI controls. Scale: 400 µm. E, Serotonergic fiber number was counted from all parasagittal sections of the spinal cord 0–2.8 mm caudal to the lesion epicenter in each group. The indicated differences were compared to LAR +/+ (black *) or LAR +/− (red *) group (*p<0.05, **p<0.01).
Table 2.
Coordinates for BDA injections for CST tracing.
| Injection sites | From Bregma | From midline |
|---|---|---|
| Spot 1 | 0 | 1 |
| Spot 2 | −0.5 | 0.5 |
| Spot 3 | −0.5 | 1 |
| Spot 4 | −0.5 | 1.5 |
| Spot 5 | −1 | 1 |
Mice were perfused 5 weeks after SCI and the spinal cord around the lesion was processed for 5-HT and/or CST labeling as we reported previously (Fisher et al., 2011; Li and Strittmatter, 2003). The spinal cord extending from 0–4 mm rostral to and caudal to the lesion (8 mm long, containing the injury site) was cut parasagittally (30 ∝m, Fig. 2B). The spinal cord 5–7 mm rostral to and caudal to the injury was transversely sectioned (30 ∝m). All the parasagittal sections and 20 transverse sections (10 rostral to injury, 10 caudal to injury) were processed for BDA tracer with avidin/biotin complex and 3,3′-diaminobenzidine (DAB) based reaction (GrandPre et al., 2002; Li and Strittmatter, 2003). For raphe axon labeling, all the parasagittal sections and 20 transverse sections rostral to and caudal to the lesion (10 sections per location) were immunostained with a rabbit anti-5-HT serotonergic antibody (1:4000, ImmunoStar) and an Alexa594-conjugated secondary antibody. To visualize the lesion area, all the parasagittal sections were also stained for glial fibrillary acidic protein (GFAP, 1:400, Sigma) with an anti-mouse Alexa488-conjugated secondary antibody.
To compare axon numbers in the caudal spinal cord among different groups in Exp. 1, we counted 5-HT axons in all the parasagittal sections of the spinal cord from 0–2.8 mm caudal to the lesion epicenter in each animal, using Nikon 80i microscope, digital DXM1200 color camera and ACT-1 software. The injury center was determined as the midpoint of histological abnormalities produced by the lesion cavitations, reactive astrocytes, and morphological changes of injured axons. The 5-HT-positive axons that crossed lines perpendicular to the parasagittal plane at the lesion center and at 0.8, 1.6, 2.4 and 2.8 mm caudal to the lesion were counted from live images (10× lens) on a computer screen. The numbers of 5-HT axons at a given distance were summed from all the sections of each mouse and compared among different groups. To determine serotonergic fiber density in the spinal cord 5–7 mm caudal to the lesion (at the upper lumbar levels) in Exp. 1 and 2, individual 5-HT-labeled fibers in the ventral and dorsal half of transverse spinal cord sections were traced manually from images captured through a 10× objective. The mean density of traced 5-HT fibers was measured from 4 randomly-selected sections in each mouse using Photoshop and NIH software.
To detect BDA-labeled axons in the spinal cord, free floating transverse or parasagittal sections were preincubated with 0.5% bovine serum albumin in 1% Triton TBS for 1 hr and then incubated with avidinbiotin- peroxidase complex (ABC, PK-6100, Vector Lab) in TBS with 0.1% bovine serum albumin, 0.5% Triton for 3–4 days at room temperature. BDA tracer was visualized by daily processing with nickel-enhanced horseradish peroxidase-based DAB (D12384, Sigma) reaction (GrandPre et al., 2002). To quantify CST axons in the caudal spinal cord in Exp. 3, we photographed all the parasagittal sections caudal to the lesion in each animal and traced BDA-labeled individual axons 0–3.6 mm caudal to the lesion. Total length of CST axons was measured from every 0.4 mm of the spinal cord with Photoshop and Image J software. In Exp. 2 and 3, CST fibers were counted from transverse sections 5–7 mm caudal to the lesion and the average number of BDA-labeled axons was obtained from 3 randomly-selected sections in each animal. Transection depth at the lesion site was confirmed from parasagittal sections immunostained for GFAP in each animal. To determine possible role of LAR deletion on scar tissue formation after CNS injury, we measured the areas of GFAP-positive reactive astrocyte tissues around the lesion from all the parasagittal sections in each animals with Photoshop software (Zukor et al., 2013).
To determine functional recovery, we evaluated locomotion recovery during 5 weeks of survival by measuring the BMS scores two days and once per week after SCI (Exp. 1–3), performance on a grid walk (Exp. 1) and footprint analysis (Exp. 2) 5 weeks after SCI. The BMS scores were evaluated while animal was walking in an open field and re-evaluated from digital video documents. The grid walk errors were counted from videotapes played at a slow speed (4 separate trials per test) and averaged from different trials. For footprint analysis, walking patterns of mouse hindpaws were recorded with ink during continuous locomotion across a 50-cm runway, and the stride length on each side and stride width between two sides of the prints were measured and calculated from multiple steps (Li and Strittmatter, 2003). All of the behavioral tests were performed by two persons. For axon quantification and behavioral analysis, the number of animals used in LAR+/+, LAR+/− and LAR−/− groups was summarized in Table 1.
Statistical analysis
SigmaPlot software was used for statistical analysis. Data in graphs are shown as means ± SEM. The comparisons between multiple groups including the BMS data were analyzed with a repeated measures ANOVA followed by Bonferroni post-hoc tests. The experiments involving a single determination of means between two independent groups were analyzed with Student’s t-test. Differences between groups with p<0.05 were considered significant. During the experimental procedures, including surgery, histology, axon quantification and behavioral evaluations, researchers were blind to the animal genotypes.
Results
Traumatic SCI altered LAR expression in the spinal cord
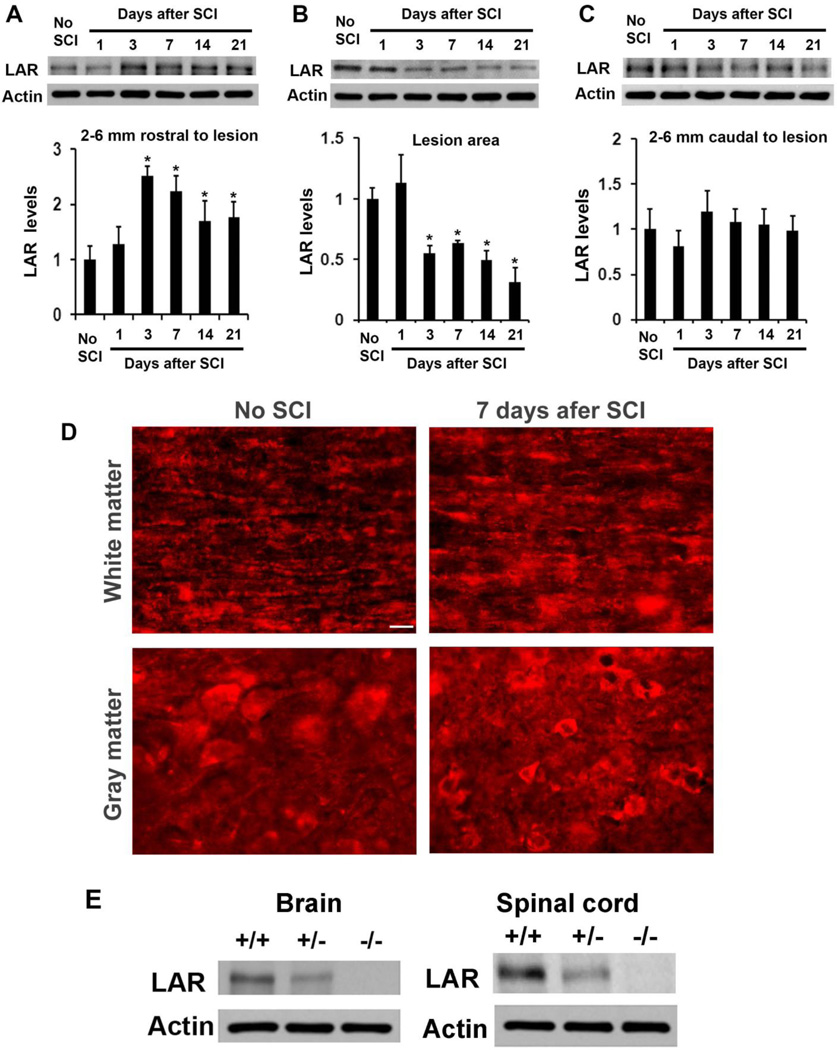
Given dramatic upregulation of CSPGs after CNS injury and expression of LAR in uninjured CNS (Fisher et al., 2011; Jones et al., 2003; Xie et al., 2001), traumatic axonal injury may alter LAR expression. We examined levels of LAR protein in the lesioned spinal cord of C57BL/6 mice 1–21 days after a dorsal over-transection injury at T7. Compared to uninjured controls, LAR levels in the rostral spinal cord were significantly enhanced 3–21 days after SCI although LAR in the lesion center area was moderately reduced (Fig. 1A–C). LAR reduction in the lesion area is probably due to tissue damage and reactive scar formation days to weeks after the lesion. In contrast, LAR levels in the caudal spinal cord were not changed. Consistently, immunostaining for LAR protein in parasagittal sections of the spinal cord displayed stronger LAR signals in the spinal cord several mm rostral to the lesion epicenter (7 days after injury) than sham controls at the same spinal cord level (Fig. 1D). The LAR signals presented in the areas of axon cylinders, neuronal bodies and glial cells (Fisher et al., 2011). Thus, LAR is upregulated in the rostral spinal cord days-weeks after a traumatic SCI.
Figure 1. Traumatic injury alters LAR expression in the lesioned spinal cord.
A–C, The levels of LAR were analyzed with Western blots from tissue lysates of fresh spinal cord blocks 2–6 mm rostral to the lesion (A), at lesion site (2 mm rostral to and caudal to injury center, containing the lesion (B), 2–6 mm caudal to the lesion (C), or from the same level of the spinal cord in no-SCI controls. Actin was also detected in the same blots (bottom bands). Bar graphs below the bands indicate densitometric analysis of LAR from multiple animals. Trauma significantly increased LAR levels rostral to the lesion although there was a reduction at the lesion site. n=3 mice in each group, *p<0.05 compared to no-SCI controls. D, Immunostaining for LAR protein in parasagittal sections of the spinal cord indicated stronger signals in the spinal cord 3 mm rostral to a dorsal over-transection 7 days after injury than sham controls at the same spinal cord level. Scale: 25 µm. E, Western blots confirm the reduction and deletion of LAR protein in the brain and spinal cord of adult LAR +/− and −/− mice.
LAR deletion enhances growth of raphespinal axonal tracts in adult mice with SCI
Several PTPs, including LAR and PTPσ, regulate neuronal growth and guidance during development in vertebrates (Gonzalez-Brito and Bixby, 2009; Stepanek et al., 2005). LAR blockade with peptides stimulated growth of descending serotonergic axons after SCI (Fisher et al., 2011). To determine the role of LAR deletion in limiting descending axon growth in vivo, we examined growth of descending axonal tracts in adult LAR mutant mice 5 weeks after SCI. We confirmed the lack and reduction of LAR protein in LAR −/− and +/− mice with Western blots (Fig. 1E). The number of progeny in LAR −/− mice (17%) is lower than that of LAR +/+ mice (25%) (Yeo et al., 1997), but LAR −/− and +/− mice are viable and grossly normal in appearance. Behavioral evaluation with multiple assays, including BMS score, grid walk, thermal withdrawal and grip force, indicate the overall normal motor and sensory function in LAR −/− mice compared to LAR +/+ and +/− controls (not shown). Examination of 5-HT-stained raphespinal and BDA-traced CST axons indicates overall normal distribution of these descending pathways in the spinal cord (not shown).
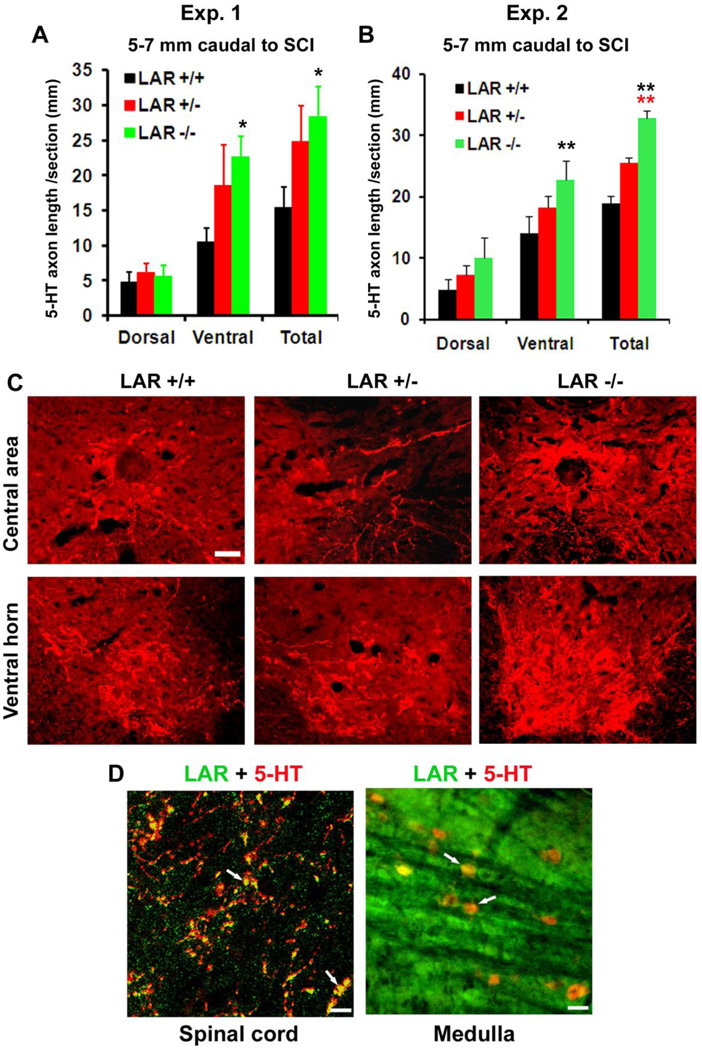
We examined regrowth of 5-HT-stained serotonergic axons in LAR mutant mice 5 weeks after a dorsal transection injury at T7 (Fig. 2A, B). Serotonergic tracts projecting to the spinal cord contribute to fictive rhythmic activity of flexor and extensor motoneurons and to recovery after SCI (Jordan et al., 2008; Li et al., 2004; Ribotta et al., 2000; Viala and Buser, 1969). Dorsal over-transection injury disconnected the majority of 5-HT-labeled serotonergic fibers (~70%) at the lesion site and markedly reduced the number of 5-HT axons in the caudal spinal cord (Li and Strittmatter, 2003; Ohtake et al., 2014). However, LAR−/− mice displayed projection of a greater number of 5-HT-labeled axons into reactive scar tissues around the lesion and the caudal spinal cord (Fig. 2C–E). Quantification at different distance from the lesion demonstrates increased number of 5-HT fibers in parasagittal sections 0–2.8 mm caudal to the lesion center in LAR−/− mice. Transverse sections of the spinal cord 5–7 mm caudal to the lesion at the upper lumbar levels also display augmented length of 5-HT axons in both the dorsal and ventral spinal cord in LAR−/− mice (Fig. 3A–C). Double staining for LAR and 5-HT indicate LAR expression in raphespinal axons and raphe neuronal cell bodies in the brainstem of wild-type mice (Fig. 3D).
Figure 3. LAR deletion in adult mice enhances growth of serotonergic fibers in the caudal spinal cord 5–7 mm caudal to the lesion at the upper lumbar levels.
A, B, Serotonergic fiber length was measured from the gray and white matter in dorso-central areas and from the gray matter in ventral horn of the spinal cord 5–7 mm caudal to the lesion. LAR +/+ or +/− mice show a number of 5-HT axons in the caudal spinal cord, indicating the spared serotonergic axons following dorsal over-transection. In contrast, LAR−/− group exhibits a greater number of 5-HT axons in the caudal spinal cord, particularly in the ventral areas. The indicated differences were compared to LAR +/+ (black asterisk) or LAR +/− (red asterisk) group (*p<0.05, **p<0.01). C, Transverse sections of the spinal cord 5–7 mm caudal to the lesion displayed reduced 5-HT fibers 5 weeks after dorsal transection at T7 in SCI controls, but sections from LAR −/− mice displayed increased density of serotonergic fibers in central and ventral part of the spinal cord compared to control mice. The dorsal is up in all these sections. Scale bar: 75 µm. D, LAR protein is partially co-localized to 5-HT-labeled axons (white arrows) in the spinal cord and to 5-HT-labeled neuronal cell bodies (white arrows) in the medulla. Scales: 20 (left) and 50 (right) µm.
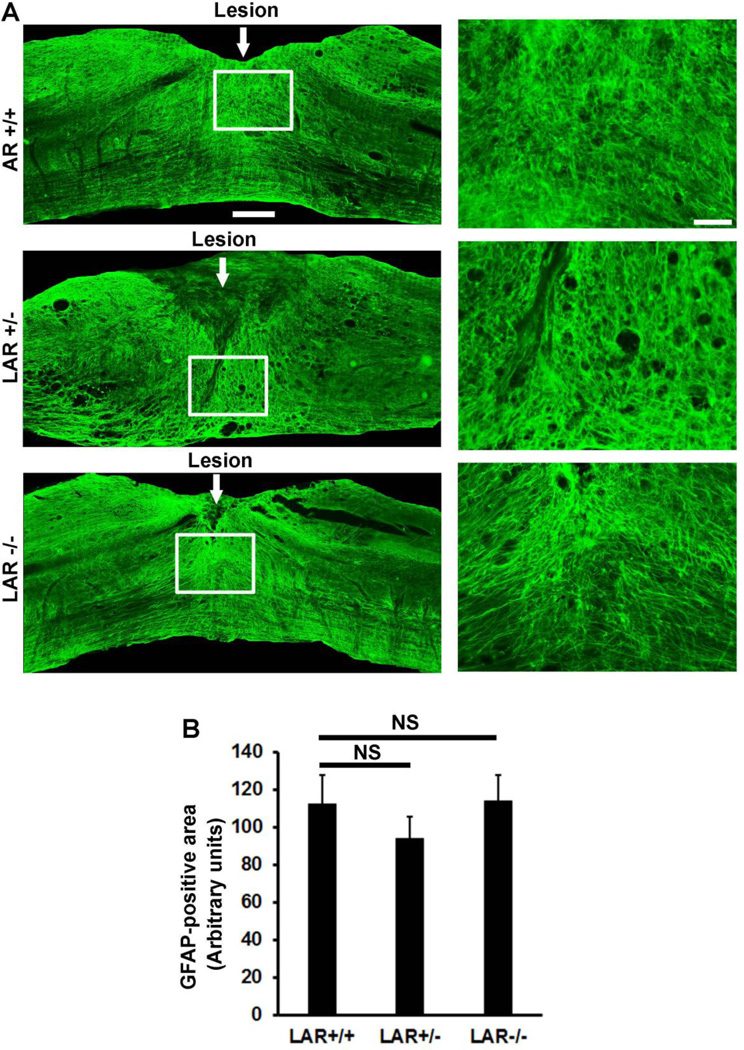
Several weeks after a dorsal over-transection, the lesion epicenter areas close to the dorsal spinal cord surface usually formed connective fibrous tissue matrix and multiple cystic cavities (Fig. 2C, Fig. 7). Hypertrophic and overlapped GFAP+ reactive astrocytes radially encircled these fibrous tissue and cavity areas. Although some GFAP+ astrocytic processes were heavily interwoven and densely packed in certain areas (especially close to the superficial epicenter), most reactive astrocytes run long and parallel processes that directed towards to the dorsal lesion epicenter, including in the deeply transected areas. Interestingly, most regrown 5-HT positive axons in LAR −/− mice typically parallel the GFAP+ reactive astrocytic processes surrounding the dorsal lesion epicenter and also present in the deeply transected areas close to the central canal and ventral spinal cord (Fig. 2C). Of note, regrown serotonergic axons inside of the scar tissues were not co-localized with GFAP+ processes and rarely detected in the areas of GFAP-negative connective tissues and cystic cavities. These findings suggest that LAR deletion surmounts suppression of CSPGs generated principally by reactive astrocytes and that reactive astrocytic processes might guide elongation of regenerative axons inside and around the lesion areas. Consistently, GFAP+ processes have been suggested to guide CST axon regeneration in injured spinal cord following PTEN knockdown with siRNA (Zukor et al., 2013).
Figure 7. LAR deletion did not alter the size of reactive astrocytic scar tissues around the lesion.
A, Images for GFAP staining from parasagittal sections of the spinal cord around the lesion displayed similar patterns of reactive astrocytes in 3 groups of mice. White line squares indicate the areas of images shown on the right side at a higher magnification. Scale bar: 250 µm (left images) and 50 µm (right images). B, Graph indicates quantification of GFAP-positive reactive astrocyte areas from all parasagittal sections in LAR +/+, +/− and −/− groups.
LAR deficiency increases regrowth of CST axons in adult mice with SCI
PTPσ deletion in adult mutant mice resulted in a degree of CST axon regrowth after either a dorsal hemisection or a contusion injury (Fry et al., 2010). Given wide expression of LAR in adult CNS and its role in mediating CSPG inhibition (Fisher et al., 2011), it may also contribute to growth failure of other descending tracts including CSTs. To evaluate potential role of LAR in limiting CST growth in the lesioned spinal cord, we evaluated the integrity of BDA-traced CSTs in adult LAR mutant mice with dorsal overtransection injury. CST axons are essential for controlling fine motor function (Weidner et al., 2001) and it appears more difficult to induce CST axons to regenerate than other fiber tracts in adult mammals (Pearse et al., 2004). Rostral to the lesion, 3 groups of mice exhibited similar tracing pattern of CST axons (Fig. 4–6). CST axons in SCI controls usually retracted 0.5~1 mm from the lesion 5 weeks after SCI, but LAR−/− mice exhibited regrowth of CST axons into the scar tissues around the lesion and the caudal spinal cord. Most CST axons in the caudal spinal cord presented in the gray matter and followed a branching trajectory (Fig. 5E–H, Fig. 6J, K, L). In contrast, very few of CST axons were observed in the scar tissues and caudal spinal cord in SCI controls. Immunostaining for GFAP around the lesion indicates similar extent of injury areas and reactive scar tissues in these animals (not shown). Moreover, a number of CST axons extended into the spinal cord 5–7 mm caudal to the lesion at the upper lumbar spinal cord levels in most (16 out of 18 mice) of LAR−/− mice (Fig. 6M, N). Consistently, LAR protein was partially co-localized to CST axons in the spinal cord of adult mice (Fig. 6O).
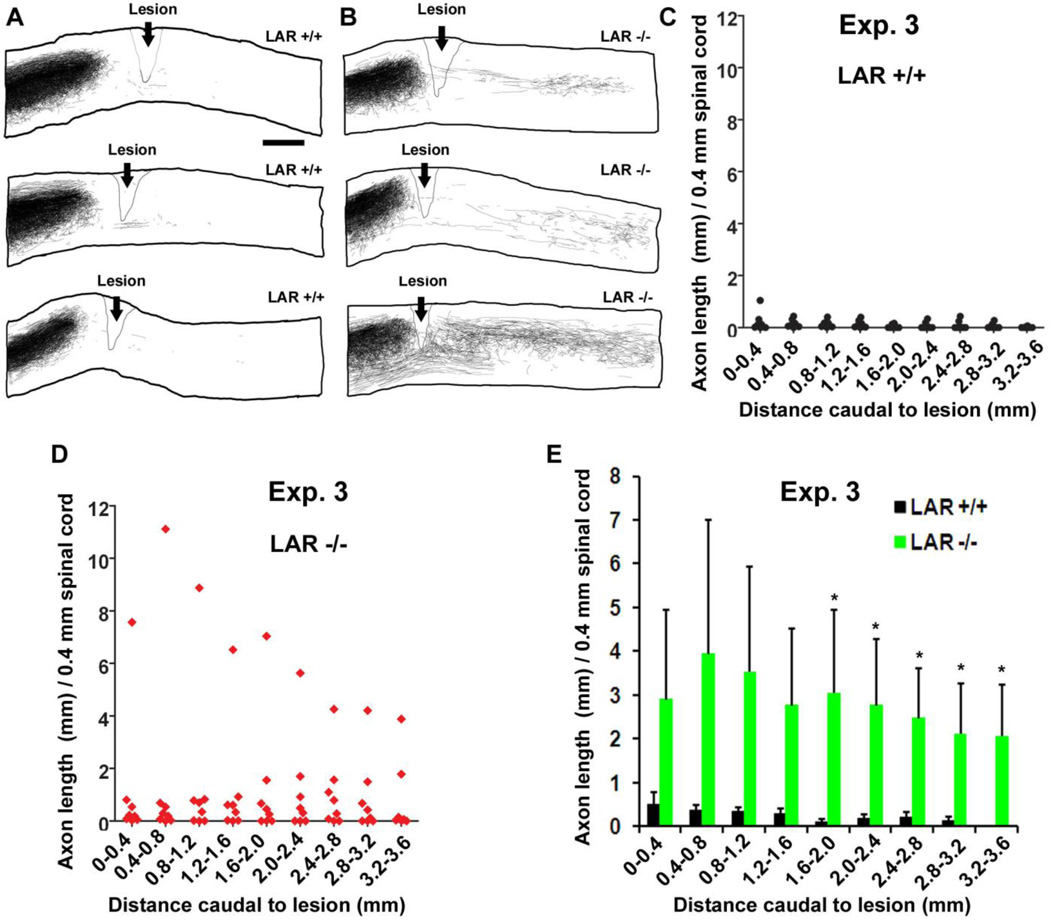
Figure 4. LAR deletion induces regrowth of CST axons in the caudal spinal cord after SCI.
A, B, Camera lucida drawings indicate BDA-labeled CST fibers from all parasagittal sections of 3 representative mice in each group. CST axons terminated at the lesion area in LAR +/+ mice, but LAR −/− animals display regrowth of CST axons around lesion and in the caudal spinal cord. Scale: 500 µm. C, D, E, BDA-labeled CST fibers were traced from all parasagittal sections of the spinal cord 0–3.6 mm caudal to the lesion and the length of CST axons was quantified from every 0.4 mm length of the caudal spinal cord. The indicated differences in E were compared between LAR +/+ and −/− groups (*p<0.05).
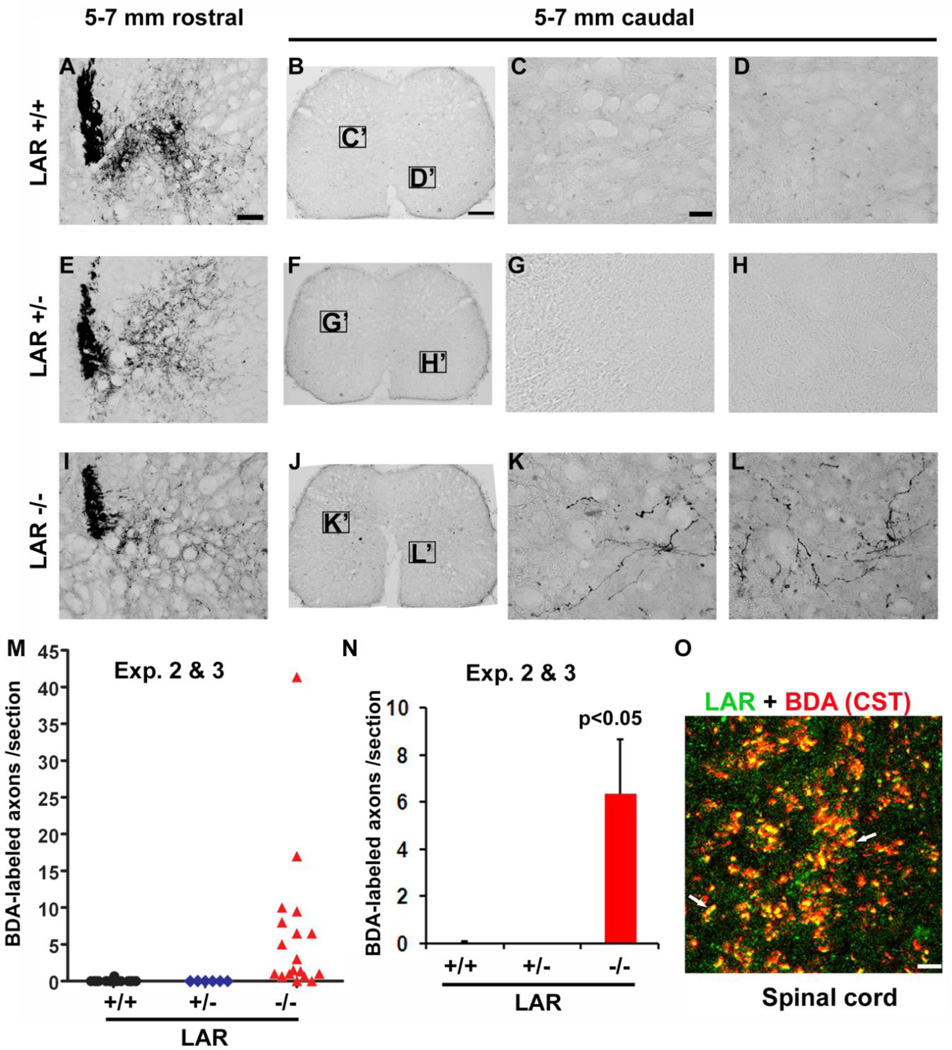
Figure 6. LAR deletion induces regrowth of CST axons in the caudal spinal cord at the upper lumbar level.
A–L, Transverse sections 5–7 mm rostral to the lesion display the similar pattern of CST labeling in LAR +/+, +/− and −/− mice (A, E, I). In contrast to no BDA-traced CST axons in the spinal cord 5–7 mm caudal to the lesion in LAR +/+ and +/− mice (B–D, F–H), a number of CST axons were observed at the same levels of the spinal cord in LAR−/− mice (J–L). The midline dorsal is up in all the sections. Scale: 50 µm in A, E and I; 200 µm in B, F and J; 25 µm in C, D, G, H, K and L. M, N, Quantification of the BDA-labeled axons indicates increased CST fibers in the spinal cord 5–7 mm caudal to the lesion in LAR −/− group (*p<0.05). O, Double staining indicates the expression of LAR protein in BDA-labeled CST axons in LAR +/+ mice. White arrows indicate co-localization of LAR protein to CST axons. C', D', G', H', K' and L' indicate the sampling areas for the high magnification images in the caudal spinal cord. Scale: 20 µm.
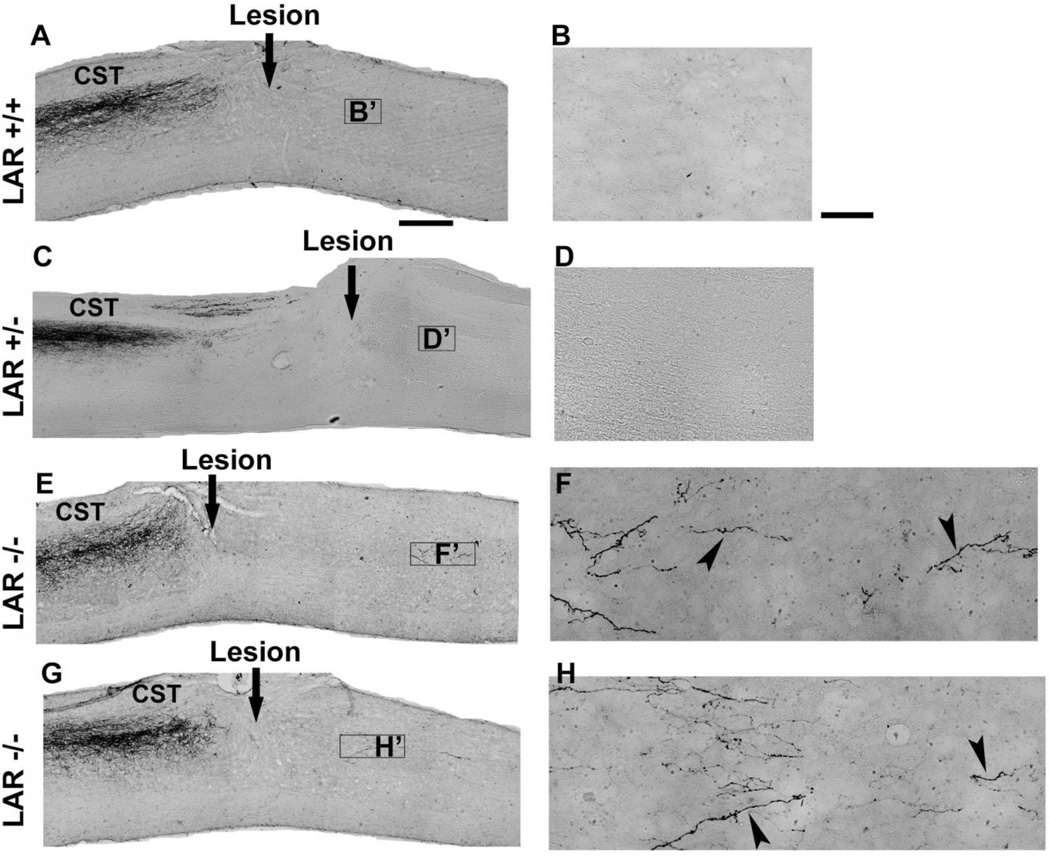
Figure 5. Images of parasagittal sections indicate regrowth of CST axons in the caudal spinal cord of LAR −/− mice.
A, B, Sagittal sections in SCI controls show no regenerative CST growth in the caudal spinal cord 5 weeks after a dorsal over-transection at T7. C, D, Sections from a LAR+/− mouse indicate termination of CST fibers at the lesion site and no CST axons in the caudal spinal cord. E–H, Similar sections from two LAR −/− mice demonstrate a number of CST fibers in the spinal cord caudal to the transection, particularly in the gray matter areas. Higher-magnification images from F and H demonstrate the meandering course of BDA-traced CST fibers (arrowheads) around the lesion in the caudal spinal cord, but not in LAR +/+ and +/− controls (B, D). The dorsal is up in all these sections. Scale bars: 500 µm in A, C, E, G; 50 µm in B, D, F, H.
LAR deletion did not alter the size of reactive astrocytic scars in SCI mice
LAR deletion stimulated regrowth of both 5-HT and CST axons in SCI mice. We next examined whether LAR deficiency would have any effects on scar generation by measuring the areas of GFAP+ reactive astrocytic scar tissues from all parasagittal sections in each animal. Compared to normal astrocytes, reactive astrocytes around the lesion displayed stronger GFAP staining signals and unique process structures, including the elongated and radial distribution. Evaluations of GFAP+ reactive astrocytes around the lesions indicated similar size and structures of reactive scar tissues in the LAR +/+, +/− and −/− groups (Fig. 7). This result suggests that LAR deletion promotes axon regrowth principally by surmounting CSPG inhibition, but not by reducing physical barrier of the scar tissues.
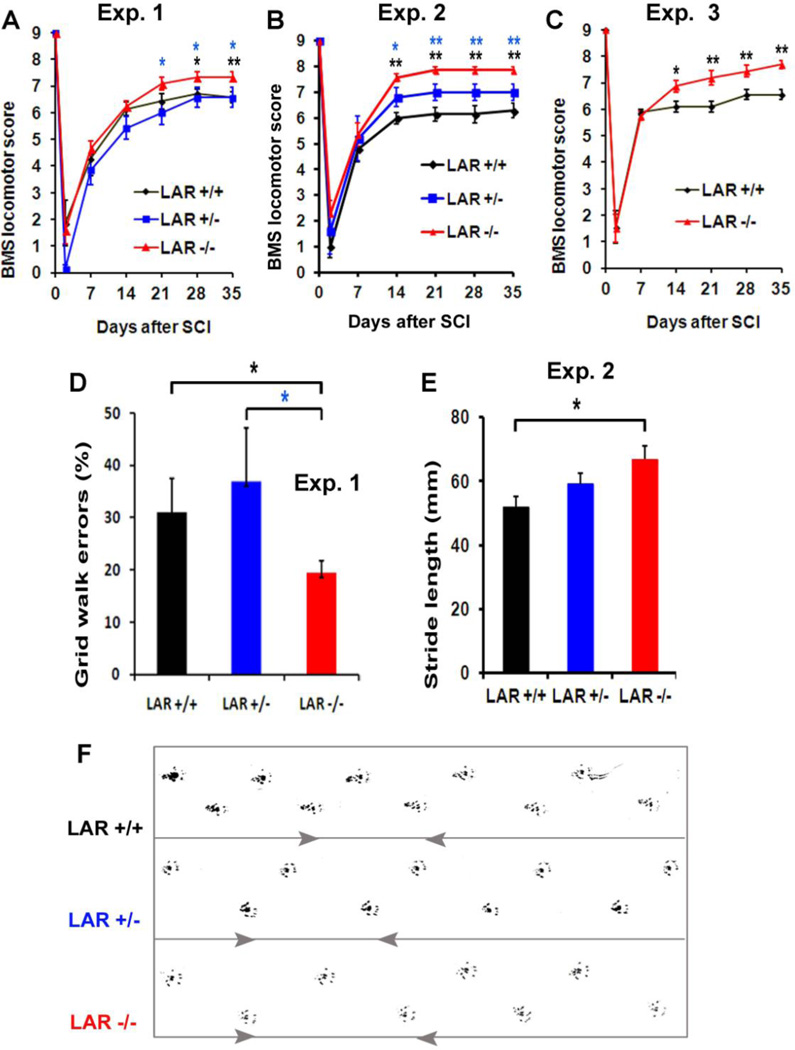
LAR deletion improves locomotor recovery in mice with SCI
We monitored functional recovery in LAR mutant mice a few weeks after SCI by performing several behavioral tests. All mice had the BMS scores of 0–4 at day two post-injury. The control mice gradually recovered partial function over a 21-day observation period. However, the BMS scores of LAR−/− mice were significantly higher than the controls 21–35 day after SCI in 3 separate experiments (Fig. 8A–C). By 5 weeks after SCI, control mice basically exhibited frequent or consistent plantar stepping, but they had no or some coordination and rotated paw position at initial contact and lift-off with the testing surface (BMS score: 5 or 6). In contrast, Most LAR−/− mice displayed consistent plantar stepping, consistent coordination and parallel paw position at initial contact and lift-off although they might have rotation at lift off and mild-severe trunk instability (BMS score: 7 or 8). The control SCI mice made numerous errors by misplacing their hindpaws and falling into grid holes, but LAR−/− mice made significantly fewer errors by correctly placing their hindpaws on the grid (Fig. 8D). Moreover, LAR−/− mice had enhanced stride length of the hindpaws measured from footprints 5 weeks after injury (Fig. 8E, F). Therefore, LAR deficiency in adult mice enhanced functional recovery after SCI, in addition to increasing regrowth of descending axonal tracts.
Figure 8. LAR deletion improves locomotor recovery in adult mice with SCI.
A–C, Graphs indicate the locomotor BMS scores in different groups of LAR mutant mice with SCI from 3 separate experiments. D, Graph indicates grid walk errors in LAR +/+, +/− and −/− mice 5 weeks after SCI in experiment 1. E, Graph indicates stride length of the hindlimbs 5 weeks after injury in 3 groups in experiment 2. F, The representative footprints of the hindlimbs are shown in 3 mice from experiment 2. The indicated differences were compared to LAR +/+ or +/− SCI controls (*p<0.05, **p<0.01).
Discussion
CSPGs have at least two PTP receptors and also bind to two NgRs at the sites remote from the binding domains for myelin-associated inhibitors NgR3 (Dickendesher et al., 2012; Fisher et al., 2011; Shen et al., 2009). Identification of CSPG receptors is an important advance for better understanding the scarmediated suppression, but many critical questions remain unanswered regarding CSPG receptor-mediated suppression of neuronal growth. Does deleting each of the receptors stimulate significant regeneration and functional recovery after CNS axon injury? In this study, we demonstrate that transgenic deletion of LAR increased growth of descending serotonergic axons and CST fibers in reactive scar tissues and caudal spinal cord after SCI. Furthermore, LAR deletion improved functional recovery by increasing BMS locomotor scores, reducing grid walk errors and enhancing stride length of the hindlimbs weeks after SCI. We have recently demonstrated that pharmacological LAR blockade with sequence-targeting peptides enhanced regrowth of descending 5-HT axons in SCI (Fisher et al., 2011), but confirmation of in vivo LAR function with a transgenic method is critical, especially for exclusion of potential non-selective effects of the peptide study. This is the first transgenic study on the role of LAR for regulating axonal growth and functional recovery in vivo. This report, also for the first time, provided clear evidence that LAR suppression stimulates regrowth of injured CSTs, which are important descending tracts for controlling voluntary and fine movements in mammals (Metz and Whishaw, 2002; Weidner et al., 2001). Thus, our findings support the crucial role of LAR in restricting axon regrowth after CNS injury.
LAR deletion stimulates axonal growth after CNS injury
Our recent studies support that LAR, another member in the LAR subfamily, regulates CSPG inhibition on neuronal growth as a functional receptor. CSPGs bind LAR with high affinity and CSPG stimulation enhances LAR activity in vitro. Blockade of LAR with sequence-targeting peptides reverses neurite growth inhibition induced by CSPGs and enhances serotonergic axon growth in the caudal spinal cord and locomotor functional recovery in SCI mice (Fisher et al., 2011). However, the role of LAR in limiting CNS axonal regeneration has not been examined with genetic methods previously. Here, we have analyzed mice lacking LAR protein and demonstrated the function of LAR on restricting regrowth of axotomized CNS axons in adult rodents. Although LAR mutant mice have a different genetic background (DBA) from C57BL/6 animals used in peptide study and it is hard to compare the number of serotonergic axon growth between two studies, analysis of axonal regrowth and functional recovery in LAR KO mice has provided firm conclusion on LAR function and insights into the molecular mechanisms of scar-mediated suppression on neuronal growth.
Like most other studies on axon growth, we employed a partial injury model because of the experimental difficulties posed by a complete spinal cord transection. In these circumstances, the existence of spared axons make it very difficult to be certain that the increase in descending axons in the caudal spinal cord represents regeneration of severed axons, sprouts of spared fibers, or both. In particular, the increased 5-HT fibers detected in the caudal spinal cord in LAR −/− mice could be derived from regenerating fibers of lesioned tracts and/or sprouting of spared fibers because dorsal overhemisection spared a small portion of 5-HT axons in the ventral spinal cord (Fisher et al., 2011; Li and Strittmatter, 2003; Ohtake et al., 2014). Application of a complete injury model should be able to clarify this issue.
Many studies demonstrated a limited degree of sprouting or regenerative growth of injured CST axons by inhibiting myelin-associated inhibitors and scar-rich proteoglycans (Bradbury et al., 2002; Li and Strittmatter, 2003), applying neurotrophic factors (Schnell et al., 1994), suppressing intracellular RhoA and GSK-3β signals (Dill et al., 2008; McKerracher and Higuchi, 2006), transplanting grafts, or combining different strategies (Diener and Bregman, 1998; Li et al., 1997; Ruitenberg et al., 2005). We thus also evaluated the integrity of CST axons in mice with a dorsal transection in LAR mutant mice. Although most transected CST axons terminated before reaching the lesion center, LAR deletion induced regrowth of CST axons into the scar tissues and the caudal spinal cord in most SCI mice. Importantly, some CST axons were observed in the spinal cord 5–7 mm caudal to the lesion at the upper lumbar spinal cord. Similarly, adult PTPσ deficient mice also exhibited long distant axon regrowth after a thoracic dorsal hemisection or a contusion (Fry et al., 2010). Because of the differences in mouse strains, injury models and axon counting methods between the present and PTPσ studies, it is difficult to compare the degrees of CST axon regrowth between LAR −/− and PTPσ −/− mice directly. It will be very interesting to study whether inhibition of both LAR and PTPσ would induce greater axon regeneration than targeting one receptor alone.
Although a partial injury model was employed in this study, most of the BDA-traced CST fibers detected in the caudal spinal cord are probably regenerating axons from transected axons for the following reasons. Dorsal over-transection at 1 mm depth transected all the CSTs in LAR mutant mice, including the dorsal, dorsolateral and lateral CSTs, because no BDA-traced ventral CST fibers were detected in these mice. Consistently, we did not detect any obvious regrowth of CSTs in the caudal spinal cord in either LAR +/+ or +/− mice although LAR reduction in +/− mice (Fig. 1E) induced a moderate increase in 5-HT axons in the caudal spinal cord compared to LAR+/+ group (Fig. 3A, B). Moreover, all the BDA-labeled axons in the caudal spinal cord of LAR −/− mice followed branching and tortuous courses (Figs. 5F, H and 6K, L) and did not have the linear trajectory of uninjured fibers as reported previously (Steward et al., 2007). The reasons for CST axon regrowth failure in a small portion of LAR−/− mice (Fig. 6M) are not clear.
LAR deletion on functional recovery after CNS axon injury
Although PTPσ deletion promotes regrowth of lesioned spinal cord axons (Fry et al., 2010; Shen et al., 2009), the functional significance of increased axon growth in PTPσ mutant mice is not known. LAR deficiency improves recovery of locomotor function in adult rodents, in addition to enhancing regrowth of descending projecting axons. Surmounting inhibition of scar tissues around the lesion is probably the molecular basis for increased axon growth and improved behavioral recovery in LAR −/− mice. Purified CSPGs dose-dependently bind LAR with high affinity and CSPG stimulation activates LAR phosphatase in vitro (Fisher et al., 2011). LAR transgenic deletion or blockade with blocking peptides partially overcomes growth inhibition by CSPGs, but not by CNS myelin inhibitors, in adult neuronal cultures (Fisher et al., 2011). Consistently, transgenic or pharmacological inhibition of LAR stimulates regrowth of descending axons into CSPG-rich scar tissues following CNS injury. Together, LAR plays a significant role in mediating scar-sourced inhibition as a functional receptor.
The enhanced supraspinal sprouting and/or regeneration to the caudal spinal cord probably contribute to the improved functional recovery in LAR −/− mice although segmental mechanisms and short-range sprouts appeared to play a major role in the locomotor recovery of control SCI mice. LAR deficiency stimulated growth of the spinal cord axons and enhanced the number of raphespinal and CST fibers in the caudal spinal cord. Because anterograde tracing with BDA usually labels a small portion of total CSTs (Brosamle and Schwab, 1997), the actual regenerating CSTs should be much greater than those observed. Given widespread expression of LAR protein in adult CNS (Fisher et al., 2011; Zhang et al., 1998), LAR deletion might also stimulate sprouting, regeneration and reorganization of ascending tracts as well as the other descending fibers (such as rubrospinal axons), which probably contributed to the enhanced functional recovery. Of note, we cannot exclude the possibility that LAR inhibition promotes functional recovery by other mechanisms, such as protecting injured tissues and enhancing differentiation of endogenous stem cells and myelination. Several PTPs, including LAR, activate caspases and contribute to cell apoptosis (Halle et al., 2007a; Halle et al., 2007b; Weng et al., 1998; Zhang et al., 2013). LAR is expressed in neural progenitors in adult rodents and its downregulation promotes neurogenesis and generation of granule cell layer neurons (Bernabeu et al., 2006).
Conclusion
Current major approach to surmount CSPG-mediated inhibition is to digest sulfated proteoglycans with local application of the bacterial ChABC (Busch and Silver, 2007; Kwok et al., 2011). Because important disadvantages of ChABC, such as partial removal of inhibitory components of CSPGs, a short period of enzymatic activity at the body temperature and failure to cross the blood–brain barrier, may prevent use of this enzyme to treat patients (Sharma et al., 2012). It is extremely important to discover more feasible approaches to surmount scar-mediated inhibition on neuronal growth. Recent elucidation of the mechanisms by which CSPG acts may not only lead to a more profound understanding of CNS axon regeneration, but facilitate development of therapies that restore function to persons disabled by CNS injuries (Fisher et al., 2011). Because inhibiting each of the recently-identified CSPG receptors, including PTPσ, LAR or NgRs, could partly overcome suppression of the scar-sourced inhibitors (Dickendesher et al., 2012; Fisher et al., 2011; Fry et al., 2010; Shen et al., 2009), simultaneous blockade of these receptors may achieve greater axonal regeneration and functional recovery. Moreover, targeting these extracellular inhibitors together with the neuron-intrinsic factors, such as PTEN, mTor and Krüppel-like factors (Liu et al., 2010; Moore et al., 2009; Ohtake et al., 2014; Park et al., 2008; Sun et al., 2011), is likely to promote more robust axon regeneration and neuronal plasticity in adult CNS.
Highlights.
Deletion of CSPG receptor LAR increased regrowth of serotonergic axons after spinal cord injury.
LAR deficiency stimulated regrowth of CST fibers into caudal spinal cord after injury.
LAR deletion improved functional recovery spinal cord injury.
Receptor LAR plays a crucial role of in restricting axon regeneration after CNS injury.
Acknowledgements
We thank Sherry Rovinsky, Cynthia R. Pennise and Tao Yang for technical support. This work was supported by research grants to S.L. from NIH (1R21NS066114, 1R01NS079432 and 1R01EY024575), Christopher & Dana Reeve Foundation (LA1-1002-2) and Shriners Research Foundation (86300).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Basso DM, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, et al. Downregulation of the LAR protein tyrosine phosphatase receptor is associated with increased dentate gyrus neurogenesis and an increased number of granule cell layer neurons. Mol Cell Neurosci. 2006;31:723–738. doi: 10.1016/j.mcn.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Condic ML, et al. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J Neurosci. 1999;19:10036–10043. doi: 10.1523/JNEUROSCI.19-22-10036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener PS, Bregman BS. Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat. J Neurosci. 1998;18:779–793. doi: 10.1523/JNEUROSCI.18-02-00779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill J, et al. Inactivation of glycogen synthase kinase-3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, et al. Leukocyte Common Antigen-Related Phosphatase Is a Functional Receptor for Chondroitin Sulfate Proteoglycan Axon Growth Inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry EJ, et al. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- Fu Q, et al. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, et al. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, et al. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Brito MR, Bixby JL. Protein tyrosine phosphatase receptor type O regulates development and function of the sensory nervous system. Mol Cell Neurosci. 2009;42:458–465. doi: 10.1016/j.mcn.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, et al. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Halle M, et al. Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol Cell Biol. 2007a;27:1172–1190. doi: 10.1128/MCB.02462-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle M, et al. Protein tyrosine phosphatases: emerging regulators of apoptosis. Cell Cycle. 2007b;6:2773–2781. doi: 10.4161/cc.6.22.4926. [DOI] [PubMed] [Google Scholar]
- Jones LL, et al. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jordan LM, et al. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kantor DB, et al. Semaphorin 5A Is a Bifunctional Axon Guidance Cue Regulated by Heparan and Chondroitin Sulfate Proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kwok JC, et al. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Lee H, et al. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, et al. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp Neurol. 2003;184:981–990. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- Li S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, et al. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, Higuchi H. Targeting Rho to stimulate repair after spinal cord injury. J Neurotrauma. 2006;23:309–317. doi: 10.1089/neu.2006.23.309. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y, et al. Systemic PTEN antagonist peptides promote axon growth and recovery after spinal cord injury. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.02.037. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Ribotta MG, et al. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg MJ, et al. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain. 2005;128:839–853. doi: 10.1093/brain/awh424. [DOI] [PubMed] [Google Scholar]
- Schnell L, et al. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Sharma K, et al. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Back SA. A 'GAG' reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Skarnes WC, et al. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc Natl Acad Sci U S A. 1995;92:6592–6596. doi: 10.1073/pnas.92.14.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, et al. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Stepanek L, et al. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, et al. Response to: Kim et al., "axon regeneration in young adult mice lacking Nogo-A/B". Neuron. 2007;38:187–199. doi: 10.1016/j.neuron.2007.04.004. Neuron. 54, 191–5. [DOI] [PubMed] [Google Scholar]
- Sun F, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, et al. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng LP, et al. Overexpression of the transmembrane tyrosine phosphatase LAR activates the caspase pathway and induces apoptosis. Curr Biol. 1998;8:247–256. doi: 10.1016/s0960-9822(98)70106-x. [DOI] [PubMed] [Google Scholar]
- Xie Y, et al. The leukocyte common antigen-related protein tyrosine phosphatase receptor regulates regenerative neurite outgrowth in vivo. J Neurosci. 2001;21:5130–5138. doi: 10.1523/JNEUROSCI.21-14-05130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo TT, et al. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J Neurosci Res. 1997;47:348–360. doi: 10.1002/(sici)1097-4547(19970201)47:3<348::aid-jnr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Zhang G, et al. Expression of CSPG receptors PTPsigma and LAR selectively in poorly regenerating reticulospinal neurons of lamprey. J Comp Neurol. 2013 doi: 10.1002/cne.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, et al. LAR Tyrosine Phosphatase Receptor: A Developmental Isoform Is Present in Neurites and Growth Cones and Its Expression Is Regional- and Cell-Specific. Mol Cell Neurosci. 1998;10:271–286. doi: 10.1006/mcne.1998.0663. [DOI] [PubMed] [Google Scholar]
- Zukor K, et al. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]