Abstract
Background
Echinococcosis in humans is a disease caused by the larvae of Echinococcus granulosus (E. granulosus) and Echinococcus multilocularis (E. multilocularis). Serological tests are valuable, especially in the clarification of unexplained clinical findings and imaging methods. For this reason, indirect hemagglutination (IHA), latex agglutination, immunoelectrophoresis, immunoblotting, immuno-enzymatic tests, indirect fluorescence antibody test (IFAT), and enzyme-linked immunosorbent assay (ELISA) are used. The purpose of this study was to investigate the value of an immunochromatographic test (ICT) specific for E. granulosus antibodies in the diagnosis of echinococcosis.
Material/Methods
ICT evaluated 102 cases of cystic echinococcosis, 38 cases of other parasitic diseases, and 50 healthy individuals. ELISA (DRG, Germany) that detects IgG antibodies specific for E. granulosus was used as the reference method.
Results
The sensitivity, specificity, and positive and negative predictive values of ICT were 96.8%, 87.5%, 98.9%, and 70%, respectively. Diagnostic value was 96.1%. No significant differences and high degrees of agreement were found between ELISA and immunochromatographic test for cystic echinococcosis. Serum samples included 4 taeniasis, 2 leishmaniasis, and 2 healthy individuals were diagnosed to be positive with immunochromatographic test.
Conclusions
The ability of test to give fast results without need for equipment, devices, and specific storage conditions is an advantage. This test may be used due to its advantages in endemic regions for screening and diagnostic purposes.
Keywords: Echinococcosis, Hepatic; Echinococcus granulosus; Serologic Tests
Background
Echinococcosis in humans is a disease caused by the larvae of Echinococcus granulosus (E. granulosus) and Echinococcus multilocularis (E. multilocularis). Echinococcosis emerges as an important public health problem in Turkey as well as throughout the world. The course of early-term infections is generally asymptomatic. Early diagnosis decreases morbidity and mortality. Imaging methods such as ultrasonography, computerized tomography, and magnetic resonance are valuable for the diagnosis of the disease. However, there are some difficulties in distinction of the disease from abscess and malignancy in some cases. Serological tests are valuable, especially in the clarification of unexplained clinical findings and imaging methods and in early diagnosis. The relationship between disease-specific IgG antibodies and the clinical presentation of the disease was shown by several studies [1–3]. Performance of the serological diagnosis varies depending on the antigen used and the test performed [2]. In the diagnosis of cystic echinococcosis, hydatid cyst fluid and crude antigens have shown higher sensitivity compared to purified cyst fluid components [4]. Indirect hemagglutination (IHA), latex agglutination, immunoelectrophoresis, immunoblotting, immuno-enzymatic tests, indirect fluorescence antibody test (IFAT), and enzyme-linked immunosorbent assay (ELISA) are used in serological diagnosis [3]. However, these diagnostic methods require special tools and equipment. The use of rapid diagnostic methods in the detection, isolation, identification, and count of pathogen factors in clinical, food, and environmental samples and their metabolites was first started in the 1960s [5–7]. These tests are cheap, rapid, and have high specificity and sensitivity. Moreover, the test requires no special equipment for use and can be read by eye, and can be used by people who are not expert. This qualitative and semi-quantitative test detects antibody, antigen, and nucleic acid products [8,9]. Its shelf-life can be increased up to 2 years by packaging inside a plastic cassette. The immunochromatographic VIRapid® HYDATIDOSIS (Vircell, Spain) test is a tape test that contains 5/B antigen of E. granulosus, works for serum and plasma samples, and gives legible and qualitative results within 30 min. There are few studies performed on this subject [10,11]. The aim of this study was to investigate the diagnostic value of an immunochromatographic test specific for the detection of E. granulosus antibodies in cystic echinococcosis.
Material and Methods
This study was performed after ethics approval from the local ethics committee (call no. 15/14, project no. 2014/208). Written consent was obtained from all subjects in the patient and control groups. We included 102 patients (57 men, 45 women; age range: 13–78 years) who were clinically, radiologically, and surgically diagnosed with cystic echinococcosis in Kocaeli University Faculty of Medicine. Serum samples were collected before treatement for cystic echinococcosis. A total of 38 sero-positive serum samples of other parasitic diseases (including 10 leishmaniasis, 13 toxoplasmosis, and 15 taeniasis) were included in the study for cross-reactions (Table 1). Serum samples of 50 healthy individuals were included in the study as a control group. ELISA detecting E. granulosus-specific IgG antibodies (DRG, Germany) was used as the reference method. The test was used in all serum samples according to the manufacturer’s recommendations. Ten IU was taken as the threshold value and values exceeding it were considered as positive. The immunochromatographic VIRapid® HYDATIDOSIS test (Vircell, Spain) using 5/B antigen of E. granulosus was performed on all serum samples according to the manufacturer’s recommendations.
Table 1.
Positive serum samples of cystic echinococcosis, other parasitic diseases and healthy individuals tested by ELISA and immunochromatographic tests.
| Serum sample | Number of serums | Positive samples | |
|---|---|---|---|
| ELISA | ICT | ||
| Cystic echinococcosis | 102 | 95 | 92 |
| Leishmaniasis | 10 | 1 | 2 |
| Toxoplasmosis | 13 | 0 | 0 |
| Taeniasis | 15 | 5 | 4 |
| Healthy individuals | 50 | 1 | 2 |
Statistical analysis
The IBM SPSS for Windows 20.0 (SPSS, Chicago, IL, USA) software package was used for data analysis. Diagnostic tests were used for the assessment of data performance. Sensitivity, specificity, and diagnostic value were calculated based on true-positive, true-negative, false-positive, and false-negative values in 2×2 tables. The performance of the immunochromatographic test was calculated based on ELISA and IHA values. For the rejection of the null hypothesis, p<0.05 was considered as significant. Compliance of the immunochromatographic test with the ELISA test was tested by kappa value, which was assessed as very good within the range of 0.75–1.0.
Results
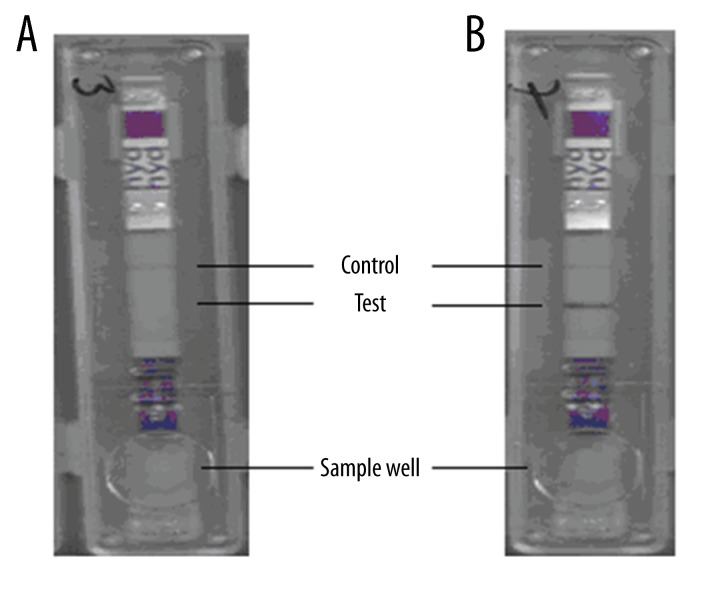
Serum samples tested with the immunochromatographic test are shown in Figure 1. While negative serum samples gave a reaction only with the control line (Figure 1A), samples seropositive for cystic echinococcosis gave a reaction on both test and control lines (Figure 1B). In the study, 92 out of 102 serum samples were detected to be positive with the immunochromatographic test, while 95 samples were detected to be positive with ELISA (Table 1). The immunochromatographic test was evaluated for other parasitic diseases in terms of cross-reactivity and the highest reactivity was observed with taeniasis (T. saginata); however, this value was found to be lower than ELISA. When sensitivity and specificity of ICT was tested with serum samples from patients diagnosed with leishmaniasis, toxoplasmosis, and taeniasis, 4 of 15 patients with taeniasis and 2 of 10 patients with leishmaniasis were found to be positive, indicating the presence of some cross-reactivity. Among serum samples of 50 healthy individuals, seropositivity was found in 2 people by the immunochromatographic test and in 1 patient by ELISA. The p value indicated that the difference between ELISA and immunochromatographic VIRapid® HYDATIDOSIS test for cystic echinococcosis was not statistically significant (McNemar chi-Square p=0.62; Kappa=0.757). In this study, sensitivity, specificity, positive predictive value, and negative predictive values for the immunochromatographic test were 96.8%, 87.5%, 98.9%, and 70%, respectively. The diagnostic value was determined to be 96.1%.
Figure 1.
Some of the immunochromatographic test results of patient serum samples. (A) Negative serum sample; only control line is pink, no color change in test line. (B) Positive serum sample; control line and test line are pink.
Discussion
In the last 20 years there have been significant improvements on the serological diagnosis of echinococcosis. Sensitivity and specificity of the diagnostic tests were increased by use of new antigens and methods. Many serological tests were developed for the diagnosis of this disease [2,3,12,13]. In this study, cystic echinococcosis-specific IgG type antibodies were studied with the immunochromatographic test. We found that it agrees with ELISA for specificity and sensitivity. Sensitivity of the immunochromatographic test was 96.8%, its specificity was 87.5%, positive predictive value was 98.9%, and negative predictive value was 70%. Similar results were obtained in other studies [2,14]. The highest performance was in active cysts in the serological diagnosis of echinococcosis. The immunochromatographic VIRapid® HYDATIDOSIS (Vircell, Spain) test used for the diagnosis of cystic echinococcosis is a tape test that contains 5/B antigen of E. granulosus, works for serum and plasma samples, and gives legible and qualitative results within 30 min. While 92 out of 102 samples from individuals with a diagnosis of cystic echinococcosis were positive by VIRapid® HYDATIDOSIS immunochromatographic test, reactivation was observed on test line in 2 of 50 seronegative samples.
The successful use of rapid diagnostic tests for the diagnosis of infections of Echinococcus species was shown in several studies. Researchers have evaluated 50 alveolar echinococcosis patients with an immunochromatographic test developed by using recombinant Em 18 antigen, with a reported sensitivity of 94.2% and specificity of 95.4%. They found these results agree with the results of ELISA and immunoblot [10]. In another study, camel hydatid cyst fluid was used as the antigen and 26 cystic echinococcosis patients and 35 patients with other parasitic infections were evaluated by this rapid test. Sensitivity of the test was detected as 100% and specificity as 91.4%. It was reported that this test was easy to use and gives results in 15 min [11]. A similar result was obtained by rapid dot immunogold filtration assay (DIGFA) that gives the result in as fast as 3 min in the diagnosis of cystic and alveolar echinococcosis. In this study, E. granulosus purified hydatid cyst fluid extract (Eg CF and AgB), crude antigens, E. granulosus protoscolex extract (EgP), and E. multilocularis metacestode antigen (Em2) were used. This is a rapid diagnostic method that is as easily read as the immunochromatographic test; its sensitivity is 80.7% in cystic echinococcosis and 92.9% in alveolar echinococcosis [13]. In a performed study, 72 serum samples taken from 12 patients with a diagnosis of alveolar echinococcosis at different stages were evaluated by the immunochromatographic test and ELISA. It was reported that the immunochromatographic test showed a high correlation with ELISA absorbance values in the follow-up of different stages of the disease. Moreover, it was emphasized that it did not require expertise or special equipment and it gave results within 20 min. It was reported that it was highly successful in the evaluation of active inactive lesions and during followup after radical surgery [15]. In another study on 144 cystic echinococcosis patients who were assessed by immunochromatographic test that was developed by using hydatid cyst fluid, sensitivity of the test was 91% and specificity was 96.9%. No significant difference was found between ELISA and the immunochromatographic test for cystic echinococcosis. Positivity was detected in 1 of 60 healthy individuals and 5 of 25 cysticercosis patients who were evaluated by this test [16]. We found false-positivity in 2 of 50 healthy individuals and 4 of 15 taeniasis patients by immunochromatographic test. These results were higher than the results by Carmena et al. [2] and Zhang et al. [14]. Seropositivity in echinococcosis varies depending on active cysts and organ involvement [17–20]. Again, it was reported that natural and recombinant antigens were not better than crude hydatid cyst fluid antigens in serological diagnosis of E. granulosus [14]. Cystic echinococcosis patients were assessed by a serological test using recombinant antigen B (rAgB) and positivity was detected in 77.6% [21]. In a performed study, 5 different antigens – hydatid cyst fluid, AgB, AgB/1, AgB/2, and AgB/3 – were compared in the serological diagnosis of echinococcosis and reported that a higher positivity and specificity were detected in the tests using hydatid cyst fluid [22]. In this study, a lower seropositivity was found depending on the antigen used. Lower specificity is observed in immunochromatographic tests due to cross reactions, especially an antigenic similarity often seen between Echinococcus and Taenia species [23]. We observed the most frequent cross-reactions in taeniasis among parasitic diseases in our study.
Conclusions
In our study, the immunochromatographic VIRapid® HYDATIDOSIS test was shown to work well in detecting antibodies against E. granulosus in human serum samples. It is important to have high specificity and sensitivity and high positive and negative predictive values for rapid diagnostic tests. However, in specificity studies among different parasitic diseases, antigenic similarities may be observed between Echinococcus and Taenia species. Therefore, the ICT test can also detect the antibody in taeniasis patient serum, which decreases its specificity. Moreover, these tests have some advantages, such as their performance within a short time, obtaining rapid results, their lower costs, and no need for any special equipment or storage conditions. However, more extensive studies are needed on this subject. We suggest that rapid diagnostic tests may be used for supporting clinical diagnosis in combination with ultrasonography and for screening purposes in regions where echinococcosis is endemic.
Footnotes
Source of support: Departmental sources
References
- 1.Siracusano A, Rigano R, Ortona E, et al. Immunomodulatory mechanisms during Echinococcus granulosus infection. Exp Parasitol. 2008;119(4):483–89. doi: 10.1016/j.exppara.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an Update. Acta Trop. 2006;98(1):74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol Med Microbiol. 2006;47(1):24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16(1):18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankenfeld-Enkvist GV, Brannback M. Technological trends and needs in food diagnostics. Technol Review. 2002;2:132–35. [Google Scholar]
- 6.Fung DYC. Rapid methods and automation in microbiology. Compreh Rev Food Sci Food Safety. 2002;1:3–21. doi: 10.1111/j.1541-4337.2002.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 7.Jayarao BM, Oliver SP, Matthews KR, King SH. Comparative evaluation of Vitek gram-positive identification system and API Rapid Strep system for identification of Streptococcus species of bovine origin. Vet Microbiol. 1991;26(3):301–8. doi: 10.1016/0378-1135(91)90023-9. [DOI] [PubMed] [Google Scholar]
- 8.Van Doorn HR, Koelewijn R, Hofwegen H, et al. Use of enzyme-linked immunosorbent assay and dipstick assay fordetection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45(2):438–42. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uçan US, Aras Z, Semacan A. Serodiagnosis of Brucella canis infection in dogs by a dipstick enzyme immunoassay. Eurasian J Vet Sci. 2010;26(2):109–12. [Google Scholar]
- 10.Sako Y, Fukuda K, Kobayashi Y, Ito A. Development of an immunochromatographic test to detect antibodies against recombinant Em18 for diagnosis of alveolar echinococcosis. J Clin Microbiol. 2009;47:252–54. doi: 10.1128/JCM.01476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Sherbiny MM, Farrag MA, Fayad MH, et al. Application andassessment of a dipstick assay in the diagnosis of hydatidosis and trichinosis. Parasitol Res. 2004;93(2):87–95. doi: 10.1007/s00436-004-1076-x. [DOI] [PubMed] [Google Scholar]
- 12.Liance M, Janin V, Bresson-Hadni S, et al. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial Western Blot. J Clin Microbiol. 2000;38:3718–21. doi: 10.1128/jcm.38.10.3718-3721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X. Dot immunogold filtration assay (DIGFA) with multiple native antigens for rapid serodiagnosis of human cystic and alveolar echinococcosis. Acta Trop. 2010;113:114–20. doi: 10.1016/j.actatropica.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Wen H, Li J, Lin R, McManus DP. Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Dev Immunol. 2012;2012:101895. doi: 10.1155/2012/101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sako Y, Tappe D, Fukud K, et al. Immunochromatographic Test with Recombinant Em18 Antigen for the Follow-Up Study of Alveolar Echinococcosis. Clin Vaccine Immunol. 2011;18(8):1302–5. doi: 10.1128/CVI.05156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Gao C, Steverding D, et al. Differential diagnosis of cystic and alveolar echinococcosis using an immunochromatographic test based on the detection of specific antibodies. Parasitol Res. 2013;112:3627–33. doi: 10.1007/s00436-013-3550-9. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto Y. Usefulness of recombinant Em18-ELISA to evaluate efficacy of treatment in patients with alveolar echinococcosis. J Gastroenterol. 2005;40:426–31. doi: 10.1007/s00535-004-1559-7. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa Y. Serological monitoring of progression of alveolar echinococcosis with multiorgan involvement by use of recombinant Em18. J Clin Microbiol. 2009;47:3191–96. doi: 10.1128/JCM.01111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tappe D. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am J Trop Med Hyg. 2009;80:792–97. [PubMed] [Google Scholar]
- 20.Tappe D, Sako Y, Itoh S. Immunoglobulin G subclass responses to recombinant Em18 in the follow-up of patients with alveolar echinococcosis in differentclinical stages. Clin Vaccine Immunol. 2010;17:944–48. doi: 10.1128/CVI.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Ito A, Chen X, et al. Specific IgG responses to recombinant antigen B and Em18 in cystic and alveolar echinococcosis in China. Clin Vaccine Immunol. 2010;17:470–75. doi: 10.1128/CVI.00466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao GH, Wang JY, Yang YT, et al. Serological evaluation of five Echinococcus granulosus antigens for detecting cystic echinococcosis by ELISA. Chin J Zoonoses. 2012;28:811–14. [Google Scholar]
- 23.Abuseir S, Nagel-Kohl, Wolken S, Strube C. An immunoblot for detection of Taenia saginata cysticercosis. Parasitol Res. 2013;112:2069–73. doi: 10.1007/s00436-013-3368-5. [DOI] [PubMed] [Google Scholar]