Abstract
Background
Postoperative hypocalcemia caused by hypoparathyroidism is one of the most common morbidities of total thyroidectomy. The aim of this study was to analyze the kinetics and factors affecting PTH levels after total thyroidectomy and central neck dissection (CND).
Material/Methods
We performed a retrospective study in 438 consecutive patients who underwent total thyroidectomy between January 2007 and December 2010. No patient had a history of thyroid or neck surgery. PTH and calcium levels were recorded 1 day before the operation, during the first 5 days, and during follow-up (2 weeks and 2, 6, and 12 months).
Results
PTH levels declined to 41.90% of its initial value on the first day after the operation. After surgery, PTH was correlated positively with calcium and inversely with phosphate levels from postoperative day 1 to 14. Based on clinical observation, using a PTH threshold of <7 ng/L on postoperative day 1 was predictive of persistent hypoparathyroidism, with sensitivity and negative predictive value 100%, but poor specificity (70.19%). CND increased the risk of transient hypoparathyroidism compared with total thyroidectomy alone. Patients with thyroiditis had an increased risk of permanent hypoparathyroidism compared with those without thyroiditis. Iatrogenic removal of the parathyroid glands increased the risk of permanent hypoparathyroidism compared with those without iatrogenic parathyroidectomy.
Conclusions
PTH declined on the first day after thyroidectomy. PTH levels <7 ng/L on the first day after surgery might be associated with persistent hypoparathyroidism. CND, thyroiditis, and iatrogenic parathyroidectomy increased the risk of hypoparathyroidism.
Keywords: Hypoparathyroidism, Parathyroid Hormone, Pseudopseudohypoparathyroidism
Background
Hypoparathyroidism is an acquired or inherited condition characterized by decreased levels of circulating parathyroid hormone (PTH) leading to hypocalcemia and slightly higher phosphate levels [1]. The most common cause of acquired hypoparathyroidism is the damaging, removal and/or devascularization of the parathyroid glands, which may be secondary to a trauma or surgery to the thyroid, parathyroids or the neck [1]. Transient hypoparathyroidism is frequent (24.1%) after total thyroidectomy, while chronic hypoparathyroidism is rarer (1.2%) [2]. Hypocalcemia caused by hypoparathyroidism is one of the most common and morbid complications after total thyroidectomy. Hypocalcemia may lead to severe and life-threatening condition, such as nephrocalcinosis, kidney stones, chronic kidney disease and myocardial dysfunction [1].
However, there is an ongoing controversy about the predictive power of post-surgery PTH levels for chronic hypoparathyroidism. Hermann et al. [3] observed that the PTH levels reached a nadir 3h after surgery and that PTH measured 3 h after surgery was superior in predicting the risk of transient or persistent hypocalcemia than measurement at the end of the operation. However, the exact kinetics after surgery is still uncertain.
Predicting the risk of developing hypoparathyroidism is a challenge for the best care offered to patients, and is controversial. Indeed, some studies indicate that PTH levels measured during or shortly after surgery have a high predictive value [4–7], while others advocate daily calcium monitoring [8], calcium monitoring in selected patients only [9], or calcium monitoring only during the first postoperative 24 hours [10–12]. Another study showed that a serum calcium level ≤1.9 mmol/L on postoperative day 2 and PTH <15 pg/mL on postoperative day 1 had high sensitivity and specificity for chronic hypoparathyroidism [2].
Therefore, the present study investigated the kinetics of serum PTH before surgery and during the first 5 days after total thyroidectomy. The objective was to try to identify suitable cut-off levels for PTH in order to predict postoperative chronic hypoparathyroidism, as well as the risk factors for postoperative hypoparathyroidism. In addition, the relationships between PTH secretion and serum calcium and phosphate were evaluated. The results of the present study might lead to a better management of patients undergoing total thyroidectomy.
Material and Methods
Patients
Consecutive patients who underwent a total thyroidectomy for benign or malignant thyroid diseases (n=438) between January 2007 and December 2010 at the Department of Head and Neck Surgery of the Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, were included in the present retrospective study. To be included in the study, patients had: 1) to be diagnosed with a benign thyroid disease or with thyroid cancer, confirmed using imaging, pathology and/or blood biochemistry; 2) to have no history of thyroid or neck surgery; and 3) to have available blood results for the 5 days following surgery. Patients who underwent unilateral lobectomy, subtotal thyroidectomy, or completion thyroidectomy were excluded.
Preoperative evaluation was done in detail in each patient. Thyroid, central and lateral neck lymph nodes were examined by ultrasound. Ultrasound-guided fine needle aspiration was performed to confirm if a malignant or metastatic lesion was suspected. A computed tomographic scan was done from the mastoid of the temporal bone to the innominate artery if the lateral neck lymph node was considered metastatic. Vocal cord function was obtained by direct or indirect laryngoscopy. In addition, thyroid hormone function, parathyroid hormone, calcitonin and serum calcium were also measured.
This study was conducted in accordance with the declaration of Helsinki. This study was approved by the Ethics Committee of the Sir Run Run Shaw Hospital, Zhejiang University School of Medicine. Written informed consent was waived by the Committee.
Study design and outcomes
Patients were divided into 2 groups: with central neck dissection (CND) and without CND. Of the 438 patients, 381 underwent total thyroidectomy and CND, and 57 underwent total thyroidectomy alone. The surgical procedure selected was decided according to the American Thyroid Association (ATA) guidelines [13]. Age, gender, pathology results, surgical procedure, parathyroid gland autotransplantation, and iatrogenic removal of the parathyroid glands were analyzed
In the present study, transient hypocalcemia was defined as Ca2+ <2.00 mmol/L or if the patient had hypocalcemia-related symptoms during the first day after surgery [14]. Although the normal Ca2+ range is 2.20–2.70 mmol/L, clinically significant hypocalcemia is uncommon if Ca2+ levels are >2.00 mmol/L [15]. Patients with hypocalcemia-related symptoms received standard treatments for hypocalcemia. These treatments included calcium supplements and, in cases of recalcitrant hypocalcemia, intravenous calcium gluconate.
Transient hypoparathyroidism was defined as serum PTH levels below the normal range during the first day after surgery [1]. In the present study, permanent hypoparathyroidism was diagnosed if: 1) serum PTH levels were below the normal range or Ca2+ <2.00 mmol/L for more than 12 months; or 2) calcium and/or vitamin D supplementation were necessary to treat hypocalcemia-related symptoms for more than 12 months [1,3].
Laboratory measurements
Blood samples were taken from each patient at 6 AM, 1 day before the operation, and on the first, second, third, fourth, and fifth postoperative days. Blood samples were also taken daily after the first 5 days until discharge. Follow-up visits were performed at 2 weeks and at 2, 6, and 12 months for patients whose serum PTH and/or Ca2+ levels remained below the normal or if the patients still needed calcium or vitamin D supplements for hypocalcemia-related symptoms. At 14 days, 113 samples were taken, 78 at 2 months, 69 at 6 months and 53 at 12 months. Follow-up was performed for all patients with hypocalcaemia until levels returned to the normal range.
Serum PTH levels were measured using a Cobas E601 autoanalyzer (Hitachi High-Technologies Corporation, Tokyo, Japan), and the normal range was 15.00–65.00 ng/L. Serum calcium was measured using an automated analyzer (Toshiba Medical Systems Corporation, Tochigi-ken, Japan), and the normal range was 2.20–2.70 mmol/L.
Statistical analysis
Continuous variables are presented as mean ±standard deviation (SD) or median (range), and were analyzed using Friedman’s rank test or Student t-test, as appropriate. Categorical data are presented as frequencies, and were analyzed using the χ2 test. Pearson’s rho was used to test for correlations. P-values <0.05 were considered statistically significant.
Results
Patients’ characteristics
A total of 438 patients were included in the study including 352 women and 86 men. Mean age was 46.7±11.6 years. The indications for surgery and the extent of thyroidectomy are shown in Tables 1 and 2. Autotransplantation of parathyroid tissue was performed in 39 patients (39 glands), at the surgeon’s discretion.
Table 1.
Indications for thyroidectomy in the 438 included patients.
| Indications for surgery | N |
|---|---|
| Euthyroid nodular goiter | 9 |
| Thyroiditis | 3 |
| Graves disease | 45 |
| Follicular thyroid carcinoma | 4 |
| Papillary thyroid microcarcinoma | 172 |
| Papillary thyroid carcinoma | 201 |
| Medullary thyroid carcinoma | 2 |
| Undifferentiated carcinoma of thyroid | 2 |
Table 2.
Patient’s characteristics.
| Variable | Central neck dissection (n=381) | No dissection (n=57) | P-value |
|---|---|---|---|
| Gender (M/F) | 79/302 | 7/50 | 0.134 |
| Age, mean± SD (yr) | 46.3±11.8 | 43.9±15.6 | 0.161 |
| TT alone | 0 | 57(1) | |
| TT + ICND | 177 (4)* | 0 | |
| TT + ICND + LND | 110 (3)* | 0 | |
| TT + BCND | 61 (2)* | 0 | |
| TT + BCND + LND | 33 (2)* | 0 | |
| Parathyroid autotransplantation (n) | 37 | 2 | 0.125 |
TT – total thyroidectomy; ICND – ipsilateral central neck dissection; BCND – bilateral central neck dissection; LND – lateral neck dissection.
Values in parentheses are the number of patients with permanent hypoparathyroidism.
Perioperative parathyroid hormone and calcium levels
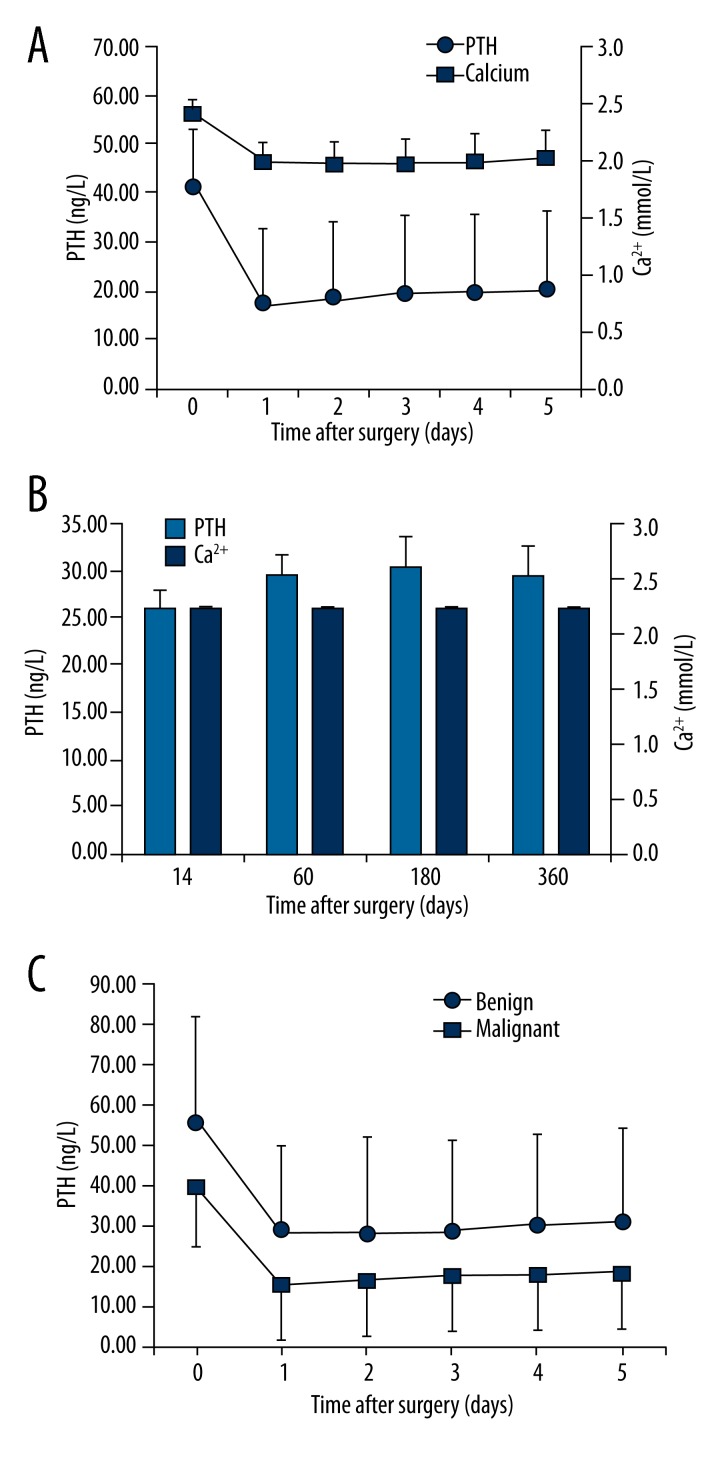
Figure 1 shows the perioperative kinetics of serum PTH and Ca2+ in the first 5 days and for up to 12 months after surgery in all 438 patients who underwent total thyroidectomy. Perioperative PTH levels declined from 41.89 ng/L (100%) before operation to 17.55 ng/L (41.90% of the initial value) on the first day morning after operation (P<0.01). During the first 5 days after surgery, there was a small but statistically significant increase compared with baseline values (P<0.01), up to a value of 20.49 ng/L (48.90% of baseline). At the first follow-up outpatient visit, PTH levels reached 26.03ng/L (62.14% of baseline) (Figure 1B). We also analyzed the data according to whether the patients had benign or malignant tumors and the serum PTH levels are presented in Figure 1C. The benign tumor group had a higher preoperative level of serum PTH, at 55.90 ng/L, than the malignant tumor group, at 39.91 ng/L, and these remained separated by a similar amount but the overall trend of the decline in PTH then small increase as described above for the total cohort was followed by both groups.
Figure 1.
Serum levels of calcium and parathyroid hormone (PTH) (A) during the perioperative period, and (B) during follow-up. (C) PTH levels during the perioperative period according to tumor malignancy. Time point 0 corresponds to the day before the surgery.
The 39 patients who had parathyroid autotransplantation were analyzed separately. These patients had lower PTH levels (14.85 ng/L on the first day after surgery). One patient developed permanent hypoparathyroidism. The others recovered with kinetics similar to those depicted in Figure 1.
The preoperative levels of PTH in the groups treated with different surgical methods were 40.04±13.95 ng/L in the CND group and 39.67±16.17 ng/L in the lateral neck dissection (LND) group. There was no significant difference between them (P=0.427).
Serum Ca2+ values declined from 2.41 mmo/L (100%) before operation to 1.99 mmol/L (82.8% of baseline) on the first day after surgery, and reached a nadir on the second day after operation (1.97 mmol/L, 81.8% of baseline) (P<0.01). During the first 5 days after surgery, a slight increase was observed, up to 2.03 mmol/L (84.27% of baseline) (P<0.01) (Figure 1A). At the first follow-up outpatient visit, the Ca2+ values reached 2.22 mmol/L (92.0% of baseline) (Figure 1B), a value similar to baseline. However, at this time a number of patients were receiving calcium supplements, which probably interfered with the results. Mean serum PTH and Ca2+ levels were lower than the initial value at follow-up examinations, up to 12 months (Figure 1B). This subsequent decline may be due to a negative selection bias: patients who did not feel ill tended to drop out. In contrast, patients with persistent symptoms were motivated to attend follow-up. In fact, patient drop-out at 12 months was 88%.
Risk factors for hypoparathyroidism after total thyroidectomy
The risk of postoperative hypoparathyroidism in relation to neck dissection, thyroiditis, and iatrogenic removal of parathyroid glands were analyzed (Tables 3 and 4). CND increased the risk of transient hypoparathyroidism compared with total thyroidectomy alone (P<0.001), but did not increase the risk of permanent hypoparathyroidism (P=0.733). Total thyroidectomy and combined CND and lateral neck dissection (LND) also increased the risk of transient hypoparathyroidism compared with total thyroidectomy alone (P=0.002), but did not increase the risk of permanent hypoparathyroidism (P=0.514). However, LND in addition to CND did not increase the postoperative transient or permanent hypoparathyroidism risk compared with total thyroidectomy and CND (P=0.699 and P=0.582, respectively). Patients with thyroiditis had increased risk of permanent hypoparathyroidism compared with those without thyroiditis (P=0.042), but not an increased risk of transient hypoparathyroidism (P=0.814). Iatrogenic removal of parathyroid glands increased the risk of chronic hypoparathyroidism compared with those without iatrogenic parathyroidectomy (P=0.018), but did not increase the rate of transient hypoparathyroidism (P=0.183).
Table 3.
Risk factors for transient hypoparathyroidism after thyroidectomy.
| Variable | N | Transient hypoparathyroidism | |||
|---|---|---|---|---|---|
| No. (%) | P-values | OR | 95% CI | ||
| TT alone vs. | 57 | 17 (29.82%) | <0.001 | 2.980 | 1.599–5.544 |
| TT + CND | 238 | 133 (55.88%) | |||
| TT alone vs. | 57 | 17 (29.82%) | 0.002 | 2.745 | 1.425–5.289 |
| TT + CND+LND | 143 | 77 (53.85%) | |||
| TT + CND vs. | 238 | 133 (55.88%) | 0.699 | 0.921 | 0.607–1.397 |
| TT + CND+LND | 143 | 77 (53.85%) | |||
| Thyroiditis vs. | 38 | 19 (50.00%) | 0.814 | 0.923 | 0.474–1.796 |
| Without thyroiditis | 400 | 208 (52.00%) | |||
| Iatrogenic parathy-roid removal vs. | 123 | 70 (56.91%) | 0.183 | 1.329 | 0.874–2.022 |
| Without incidental parathyroid excision | 315 | 157 (49.84%) | |||
OR – odds ratio; CI – confidence interval; TT – total thyroidectomy; CND – central neck dissection; LND – lateral neck dissection.
Table 4.
Risk factors for permanent hypoparathyroidism after thyroidectomy.
| Variable | N | Permanent hypoparathyroidism | |||
|---|---|---|---|---|---|
| N (%) | P-values | OR | 95% CI | ||
| TT alone vs. | 57 | 1 (1.75%) | 0.733 | 1.448 | 0.171–12.273 |
| TT + CND | 238 | 6 (2.52%) | |||
| TT alone vs. | 57 | 1 (1.75%) | 0.514 | 2.029 | 0.232–17.759 |
| TT + CND+LND | 143 | 5 (3.50%) | |||
| TT + CND vs. | 238 | 6 (2.52%) | 0.582 | 1.401 | 0.420–4.676 |
| TT + CND+LND | 143 | 5 (3.50%) | |||
| Thyroiditis vs. | 38 | 3 (7.89%) | 0.042 | 3.724 | 0.964–14.388 |
| Without thyroiditis | 400 | 9 (2.24%) | |||
| Iatrogenic parathy-roid excision vs. | 123 | 7 (5.69%) | 0.018 | 3.741 | 1.164–12.022 |
| Without iatrogenic parathyroid excision | 315 | 5 (1.59%) | |||
OR – odds ratio; CI – confidence interval; TT – total thyroidectomy; CND – central neck dissection; LND – lateral neck dissection.
Postoperative loss of the physiological correlation
On preoperative sampling in patients with no abnormality in PTH or calcium homeostasis, there was no correlation between PTH and serum calcium levels (r=−0.024, P=0.524) (Table 5). However, 1 day after surgery, a positive correlation was observed (r=0.345, P<0.001) (Table 5). After surgery, this significant (P<0.01) positive correlation was maintained for up to 14 days (Table 5). As can be seen from Table 5, the correlation was stronger on postoperative day 4 and subsequently gradually weakened.
Table 5.
Correlations between PTH and calcium pre- and post-operation in patients without permanent hypoparathyroidism.
| Time | Before operation | POD1 | POD2 | POD3 | POD4 | POD5 | POD14 |
|---|---|---|---|---|---|---|---|
| Variable | Correlation between PTH and calcium | ||||||
| Calcium | |||||||
| rho | −0.024 | 0.345 | 0.632 | 0.697 | 0.699 | 0.662 | 0.301 |
| P-values | 0.524 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 |
| Variable | Correlation between PTH and phosphate | ||||||
| Phosphate | |||||||
| rho | −0.062 | −0.236 | −0.421 | −0.579 | −0.693 | −0.726 | −0.522 |
| P-values | 0.093 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
POD – postoperative day.
On preoperative sampling in patients with no abnormality in parathyroid secretion or serum phosphate homeostasis, there was no correlation between PTH and phosphate levels (r=−0.062, P=0.093) (Table 5). However, 1 day after surgery, a negative correlation was found (r=−0.236, P<0.001) (Table 6). After surgery, this significant (P<0.01) negative correlation was maintained for up to 14 days (Table 5). As can be seen from Table 5, the correlation become stronger in the first 5 days after surgery and subsequently weakened on day 14.
Table 6.
Correlations between PTH and calcium and phosphate pre- and post-operation in patients with PTH in the normal range.
| Time | Before operation | POD1 | POD2 | POD3 | POD4 | POD5 | POD14 |
|---|---|---|---|---|---|---|---|
| Variable | Correlation between PTH and calcium | ||||||
| Calcium | |||||||
| rho | −0.079 | −0.050 | 0.011 | −0.065 | 0.005 | −0.072 | 0.301 |
| P-values | 0.308 | 0.482 | 0.875 | 0.373 | 0.945 | 0.316 | 0.007 |
| Variable | Correlation between PTH and phosphate | ||||||
| Phosphate | |||||||
| rho | 0.014 | −0.251 | −0.275 | −0.150 | −0.109 | −0.195 | −0.522 |
| P-values | 0.854 | <0.001 | <0.001 | 0.039 | 0.133 | 0.006 | <0.001 |
POD – postoperative day.
Chronic hypoparathyroidism
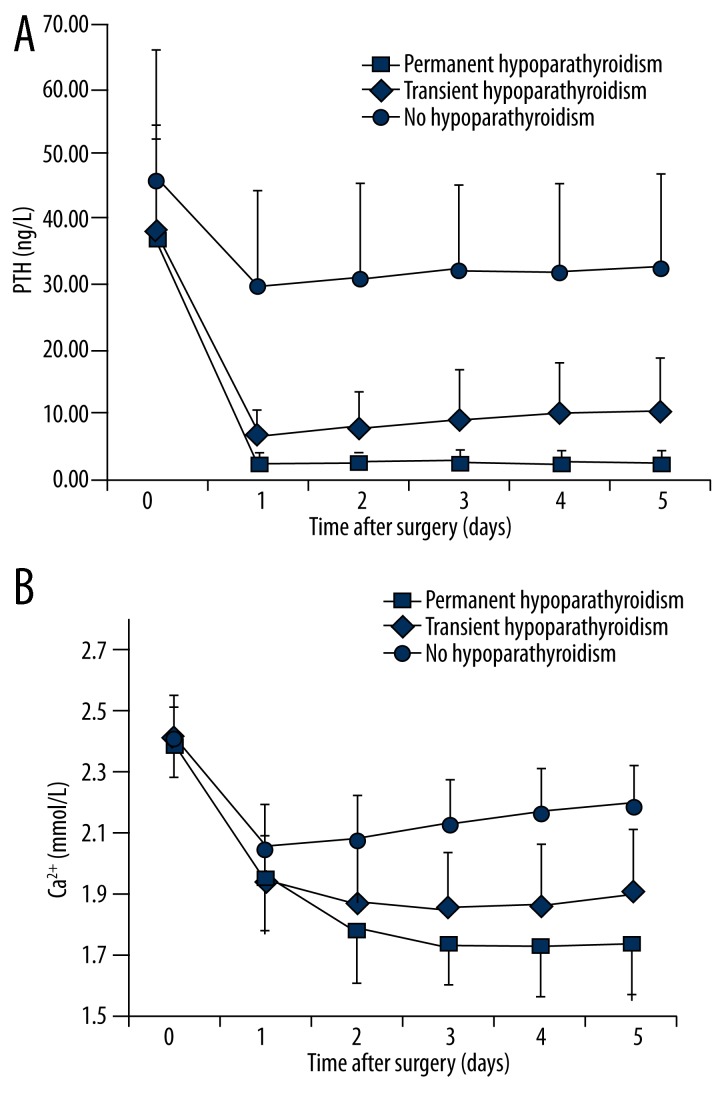
Twelve (2.74%) of the 438 patients who underwent total thyroidectomy and neck dissection developed chronic hypoparathyroidism. Patients with chronic hypoparathyroidism had a pronounced perioperative decline in PTH levels, which was more important than in patients with transient hypoparathyroidism (Figure 2). However, there was also a smaller decline in PTH levels in patients with PTH levels within the normal range. Accordingly, the correlations shown in Tables 5 and 6 were recalculated after excluding patients with transient or permanent hypoparathyroidism. This reanalysis showed that the correlations between PTH and serum Ca2+ disappeared and that the correlations between PTH and serum phosphate were still present during the first 3 days after surgery (Table 6).
Figure 2.
Perioperative serum levels of (A) parathyroid hormone (PTH) and (B) calcium. Patients were grouped according to outcome: no hypoparathyroidism (n = 199), transient hypoparathyroidism (n = 227) and persistent hypoparathyroidism (n = 12). Time point 0 corresponds to the day before the surgery.
As could be predicted from the decline in PTH level, the postoperative decrease in serum Ca2+ levels was more important in patients with chronic hypoparathyroidism. In addition, PTH and serum Ca2+ levels were maintained at the lowest levels without increasing in the first 5 days after surgery in patients with persistent hypoparathyroidism (Figure 2).
Predictive value of postoperative Ca2+ and parathyroid hormone levels
Serum Ca2+ <2.0 mmol/l on day 1 after surgery had a sensitivity of 58.33% and a negative predictive value of 97.70% for permanent hypoparathyroidism. The specificity (49.77%) was limited and the positive predictive value (3.16%) was poor. Thus, a low postoperative Ca2+ level predicts neither that a patient will develop persistent hypoparathyroidism, nor a normal Ca2+ concentration excludes it.
When PTH levels on the first day after surgery were examined, all twelve patients who eventually developed persistent hypoparathyroidism had levels <7 ng/L. PTH levels <7ng/L on the first after surgery had a sensitivity of 100% and a negative predictive value of 100% for permanent hypoparathyroidism. The specificity (70.19%) was limited and the positive predictive value (8.63%) was poor. Thus, although a postoperative PTH level below 7 ng/L does not predict that a patient will develop persistent hypoparathyroidism, PTH level above 7 ng/L on the first day after thyroidectomy excludes it.
Discussion
The aim of the present study was to analyze the kinetics and factors affecting PTH levels after total thyroidectomy and CND. Results showed that PTH levels declined to 41.90% of its initial value on the first day after operation. After surgery, PTH was correlated positively with calcium and inversely with phosphate levels from postoperative day 1 to 14. PTH below 7 ng/L on postoperative day 1 was predictive of persistent hypoparathyroidism, with sensitivity and negative predictive value 100%, but poor specificity (70.19%). CND increased the risk of transient hypoparathyroidism compared with total thyroidectomy alone. Patients with thyroiditis had an increased risk of permanent hypoparathyroidism compared with those without thyroiditis. Iatrogenic removal of the parathyroid glands increased the risk of permanent hypoparathyroidism compared with those without iatrogenic parathyroidectomy.
Considerable effort has been spent on the prevention of recurrent laryngeal nerve palsy after thyroidectomy [16–19], but postoperative hypoparathyroidism and its consequences remain widely underrated. Total thyroidectomy has been shown to be a significant risk factor for hypoparathyroidism and it occurs more frequently in total thyroidectomy than in near-total or subtotal thyroidectomy [20]. Permanent loss of parathyroid function still occurs in 1.2% after total thyroidectomy plus bilateral CND [2], and up to 16.2% according to some studies [21]. In the present study, 12 of 438 patients (2.74%) developed persistent hypoparathyroidism. Transient hypoparathyroidism was observed in 227 (51.83%) of the 438 patients.
During surgery, manipulations in the vicinity of the parathyroid gland may transiently compromise the blood flow to the gland or impair the venous efflux. These changes in blood supply are predicted to reduce the systemic availability of PTH. Conversely, the gland may be subjected to mechanical stress, which can result in the transient release of PTH. These opposite changes may cancel each other and thus the mean PTH levels at the end of surgery may not reflect the real function of the reserved parathyroid. Hermann et al. [3] observed that PTH levels reached a nadir 3 h after surgery and that PTH measured 3 h after surgery was superior in predicting the risk of transient or persistent hypocalcemia to measurement at the end of the operation. In the present study, the PTH levels reached the nadir on the first day morning after surgery (10 to 16 hours after surgery), and in the first 5 days after surgery, there was a small but significant increase. From these results, it appears that the PTH levels on the first day morning after surgery could really reflect the function of the preserved parathyroid glands.
Several studies have addressed the question of when serum PTH should be measured to identify patients at risk of developing persistent hypocalcemia, but the early studies were not large enough [4,6,7,22–24]. One recent large prospective study of 523 patients concluded that subnormal PTH values 4 h after surgery did not predict postoperative hypocalcemia [25]. Another recent large prospective study of 402 patients concluded that normal PTH levels 3 h after surgery and normal serum calcium levels on the first postoperative day ruled out persistent hypoparathyroidism [3]. In the present study, the observation period was sufficiently long (more than 12 months) to differentiate between patients with transient hypocalcemia-related symptoms and those with persistent hypocalcaemia. Based on these observations, determination of PTH levels on the first day morning after surgery could be used to rule out the possibility of persistent hypocalcemia.
In the present study, CND increased the risk of transient hypoparathyroidism. This conclusion is supported by the findings from Roh et al. [26] and Cavicchi et al. [27]. However, in the present study, CND did not increase the risk of chronic hypoparathyroidism. This phenomenon may be due to the small number of patients who underwent bilateral CND, which weakened the impact of CND on parathyroid glands function. Roh et al. [28] reported that LND increased the rate of hypocalcemia after surgery. However, in the present study, LND did not increase the risk of transient or permanent hypoparathyroidism. This phenomenon may be due to the efforts spent to preserve the superior thyroid vessel and the middle thyroid veins during lateral neck dissection. Indeed, the blood supply of the superior parathyroid glands comes from the superior thyroid vessel, and the middle thyroid veins also play a role in blood efflux of the superior and inferior parathyroid glands [29,30].
Qasaimeh et al. [31] reported that malignant thyroid diseases were a risk factor for the iatrogenic removal of the parathyroid glands, and that iatrogenic parathyroidectomy increased the rate of transient hypocalcaemia after thyroidectomy. In the present study, the iatrogenic removal of the parathyroid glands increased the risk of permanent hypoparathyroidism. Careful examination of the surgical specimen intraoperatively decreased the incidence of inadvertent parathyroidectomy during thyroidectomy [32]. Therefore, it is important to examine the resected specimen for overlooked parathyroid tissue that could be returned by autotransplantation.
In the present study, there was a positive correlation between calcium levels and circulating PTH concentrations after surgery. Therefore, in the majority of the patients, PTH secretion became the driving force for maintaining serum calcium levels. The low serum calcium levels did not trigger an appropriate increase in PTH secretion, presumably because the secretory capacity of the parathyroid glands was insufficient. This impaired function was seen for up to 14 days in the entire study population, but was not seen in patients without postoperative hypoparathyroidism. This impaired function was aggravated in the first 4 days after surgery, and attenuated from the fifth day.
In addition to its effects on serum calcium, PTH is also the predominant regulator of serum phosphate. In humans, the change in serum phosphate levels in response to a change in PTH concentration is almost immediately detected, whereas the alteration in serum calcium levels may be delayed [33]. In the present study, there was a negative correlation between phosphate levels and circulating PTH concentrations after surgery. This correlation was still observed for up to 3 days in patients without postoperative hypoparathyroidism, and for 14 days if extended to the entire study population. The correlation was enhanced in the first 5 days after surgery. This temporal difference in the response of serum calcium and phosphate to circulating PTH makes serum phosphate a potentially valuable predictor of hypocalcaemia post-thyroidectomy.
Sensitivity analyses showed that PTH below 7 ng/L on postoperative day 1 was predictive of persistent hypoparathyroidism, with sensitivity and negative predictive value 100%, but poor specificity of 70.19%. However, more markers should be measured to achieve a model with a high predictive value. Indeed, a previous study showed that combining PTH levels (≤15 pg/mL) on postoperative day 2 with calcium levels (≤1.9 mmol/L) on day 1 resulted in a sensitivity of 96.3%, specificity of 96.1%, positive predictive value of 86% and negative predictive value of 99%. In the present study, calcium had no predictive value for hypoparathyroidism. More research is necessary to address this issue.
The present study suffered from some limitations. Even if the sample size was large, the number of patients who developed persistent hypoparathyroidism was small. In addition, the follow-up study suffered from a selection bias: patients feeling well and with good calcium levels mostly dropped out, leaving the uncontrolled patients. In the present study, ionized calcium was not determined separately because this measurement requires sophisticated instrumentation that was not available routinely. Finally, the PTH threshold <7ng/L was determined based on clinical observation since the number of patients was too small to perform reliable ROC curve analyses. Further prospective multicenter studies are necessary to correctly assess the role of PTH levels after thyroidectomy.
Conclusions
PTH declined on the first day after thyroidectomy. PTH levels <7 ng/L on the first day after surgery might be associated with persistent hypoparathyroidism. CND, thyroiditis, and iatrogenic parathyroidectomy increased the risk of hypoparathyroidism.
Footnotes
Source of support: This research was supported by the Major Science and Technology Project of Zhejiang Province (grant no. 2012C13020-1)
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–37. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asari R, Passler C, Kaczirek K, et al. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg. 2008;143:132–37. doi: 10.1001/archsurg.2007.55. discussion 38. [DOI] [PubMed] [Google Scholar]
- 3.Hermann M, Ott J, Promberger R, et al. Kinetics of serum parathyroid hormone during and after thyroid surgery. Br J Surg. 2008;95:1480–87. doi: 10.1002/bjs.6410. [DOI] [PubMed] [Google Scholar]
- 4.Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002;131:515–20. doi: 10.1067/msy.2002.123005. [DOI] [PubMed] [Google Scholar]
- 5.Lombardi CP, Raffaelli M, Princi P, et al. Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136:1236–41. doi: 10.1016/j.surg.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Higgins KM, Mandell DL, Govindaraj S, et al. The role of intraoperative rapid parathyroid hormone monitoring for predicting thyroidectomy-related hypocalcemia. Arch Otolaryngol Head Neck Surg. 2004;130:63–67. doi: 10.1001/archotol.130.1.63. [DOI] [PubMed] [Google Scholar]
- 7.Richards ML, Bingener-Casey J, Pierce D, et al. Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg. 2003;138:632–5. doi: 10.1001/archsurg.138.6.632. discussion 35–36. [DOI] [PubMed] [Google Scholar]
- 8.Del Rio P, Arcuri MF, Ferreri G, et al. The utility of serum PTH assessment 24 hours after total thyroidectomy. Otolaryngol Head Neck Surg. 2005;132:584–86. doi: 10.1016/j.otohns.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 9.McHenry CR. “Same-day” thyroid surgery: an analysis of safety, cost savings, and outcome”. Am Surg. 1997;63:586–89. discussion 89–90. [PubMed] [Google Scholar]
- 10.Luu Q, Andersen PE, Adams J, et al. The predictive value of perioperative calcium levels after thyroid/parathyroid surgery. Head Neck. 2002;24:63–67. doi: 10.1002/hed.10013. [DOI] [PubMed] [Google Scholar]
- 11.Bentrem DJ, Rademaker A, Angelos P. Evaluation of serum calcium levels in predicting hypoparathyroidism after total/near-total thyroidectomy or parathyroidectomy. Am Surg. 2001;67:249–51. discussion 51–52. [PubMed] [Google Scholar]
- 12.Marohn MR, LaCivita KA. Evaluation of total/near-total thyroidectomy in a short-stay hospitalization: safe and cost-effective. Surgery. 1995;118:943–47. doi: 10.1016/s0039-6060(05)80098-4. discussion 47–48. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 14.Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336:1298–302. doi: 10.1136/bmj.39582.589433.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group AESG. Australian Endocrine Surgeons Guidelines AES06/01. Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: analysis of Australian data and management recommendations. ANZ J Surg. 2007;77:199–202. doi: 10.1111/j.1445-2197.2007.04018.x. [DOI] [PubMed] [Google Scholar]
- 16.Sancho JJ, Pascual-Damieta M, Pereira JA, et al. Risk factors for transient vocal cord palsy after thyroidectomy. Br J Surg. 2008;95:961–67. doi: 10.1002/bjs.6173. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekhar SS, Randolph GW, Seidman MD, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013;148:S1–37. doi: 10.1177/0194599813487301. [DOI] [PubMed] [Google Scholar]
- 18.Hayward NJ, Grodski S, Yeung M, et al. Recurrent laryngeal nerve injury in thyroid surgery: a review. ANZ J Surg. 2013;83:15–21. doi: 10.1111/j.1445-2197.2012.06247.x. [DOI] [PubMed] [Google Scholar]
- 19.Randolph GW, Dralle H, et al. International Intraoperative Monitoring Study Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(Suppl 1):S1–16. doi: 10.1002/lary.21119. [DOI] [PubMed] [Google Scholar]
- 20.Nawrot I, Pragacz A, Pragacz K, et al. Total thyroidectomy is associated with increased prevalence of permanent hypoparathyroidism. Med Sci Monit. 2014;20:1675–81. doi: 10.12659/MSM.890988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid. 2012;22:911–17. doi: 10.1089/thy.2012.0011. [DOI] [PubMed] [Google Scholar]
- 22.Warren FM, Andersen PE, Wax MK, Cohen JI. Intraoperative parathyroid hormone levels in thyroid and parathyroid surgery. Laryngoscope. 2002;112:1866–70. doi: 10.1097/00005537-200210000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Lam A, Kerr PD. Parathyroid hormone: an early predictor of postthyroidectomy hypocalcemia. Laryngoscope. 2003;113:2196–200. doi: 10.1097/00005537-200312000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Payne RJ, Hier MP, Tamilia M, et al. Postoperative parathyroid hormone level as a predictor of post-thyroidectomy hypocalcemia. J Otolaryngol. 2003;32:362–67. doi: 10.2310/7070.2003.13985. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi CP, Raffaelli M, Princi P, et al. Parathyroid hormone levels 4 hours after surgery do not accurately predict post-thyroidectomy hypocalcemia. Surgery. 2006;140:1016–23. doi: 10.1016/j.surg.2006.08.009. discussion 23–25. [DOI] [PubMed] [Google Scholar]
- 26.Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009;115:251–58. doi: 10.1002/cncr.24027. [DOI] [PubMed] [Google Scholar]
- 27.Cavicchi O, Piccin O, Caliceti U, et al. Transient hypoparathyroidism following thyroidectomy: a prospective study and multivariate analysis of 604 consecutive patients. Otolaryngol Head Neck Surg. 2007;137:654–58. doi: 10.1016/j.otohns.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245:604–10. doi: 10.1097/01.sla.0000250451.59685.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobori M, Saiki S, Tanaka N, et al. Blood supply of the parathyroid gland from the superior thyroid artery. Surgery. 1994;115:417–23. [PubMed] [Google Scholar]
- 30.Johansson K, Ander S, Lennquist S, Smeds S. Human parathyroid blood supply determined by laser-Doppler flowmetry. World J Surg. 1994;18:417–20. doi: 10.1007/BF00316825. discussion 20–21. [DOI] [PubMed] [Google Scholar]
- 31.Qasaimeh GR, Al Nemri S, Al Omari AK. Incidental extirpation of the parathyroid glands at thyroid surgery: risk factors and post-operative hypocalcemia. Eur Arch Otorhinolaryngol. 2011;268:1047–51. doi: 10.1007/s00405-010-1413-x. [DOI] [PubMed] [Google Scholar]
- 32.Abboud B, Sleilaty G, Braidy C, et al. Careful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg. 2007;133:1105–10. doi: 10.1001/archotol.133.11.1105. [DOI] [PubMed] [Google Scholar]
- 33.Ellsworth R. Studies on the Physiology of the Parathyroid Glands: V. Action of Parathyroid Extract on the Renal Threshold for Phosphorus. J Clin Invest. 1932;11:1011–17. doi: 10.1172/JCI100455. [DOI] [PMC free article] [PubMed] [Google Scholar]