Abstract
Background
Skin cancer is the most common cancer in the United States, and ultraviolet B (UVB) radiation-induced DNA damage is the major environmental factor underlying skin cancer development. p21, a p53-inducible protein, plays a key role in the cellular response to UVB-induced DNA damage.
Material/Methods
Through p21 silencing and overexpression, we investigated the role of p21 in apoptosis, proliferation, cell cycle arrest, and oxidative stress in UVB-irradiated HaCaT keratinocytes.
Results
We found that UVB exposure induced significant p21 downregulation (p<0.05) and was associated with significantly increased apoptosis, significantly decreased proliferation, and significantly increased G2 phase arrest (p<0.05) in UVB-irradiated HaCaT keratinocytes. p21 silencing significantly promoted apoptosis, significantly inhibited G2 phase arrest, and significantly inhibited proliferation (p<0.05), but after UVB irradiation, p21 silencing demonstrated a less significant pro-apoptotic effect and a more significant inhibition of G2 phase arrest (p<0.05), which was reflected in significantly higher proliferative activity (p<0.05). p21 overexpression acted in an anti-apoptotic manner in the absence of UVB-induced DNA damage but acted in a pro-apoptotic manner in the presence of UVB-induced DNA damage, displaying an “antagonistic duality” similar to other growth-promoting oncoproteins. p53 expression mirrored p21 expression, suggesting a regulatory feedback mechanism between p21 and p53 expression. p21 overexpression significantly downregulated glutathione peroxidase and superoxide dismutase antioxidant activity (p<0.05) while significantly upregulating hydrogen peroxide and malondialdehyde content (p<0.05), suggesting a role in decreasing antioxidant defense capabilities in UVB-irradiated HaCaT keratinocytes.
Conclusions
These findings reveal that p21 may play a key role in HaCaT keratinocytes’ response to UVB exposure.
Keywords: Keratinocytes, Skin Neoplasms, Ultraviolet Rays
Background
Skin cancer is the most common cancer affecting fair-skinned persons and has been experiencing an increase in global incidence [1]. Skin cells in unprotected parts of the body are frequently exposed to the detrimental effects of ultraviolet (UV) radiation (especially UVB), which can lead to DNA damage and a higher risk of skin cancer development [2,3]. Because carcinogenesis is a complex multistage process associated with the accumulation of critical genetic alterations, effective defense mechanisms are necessary to prevent the manifestations of DNA damage [4–6].
p21 (also termed p21WAF1/Cip1) is a key cell cycle regulator that arrests cells in the G1 and G2 phases [7,8]. Also, p21 is involved in cellular repair processes following radiation-induced DNA damage [9,10]. Specifically, UVB exposure induces p21 to bind to proliferating cell nuclear antigen (PCNA) and inhibit DNA replication, thereby impeding entry into mitosis [10,11].
UVB-induced oxidative events induce the production of reactive oxygen species (ROS), resulting in lipid peroxidation and additional types of DNA damage [12,13]. Skin cells defend against this oxidative damage via the cooperation of chemical and enzymatic antioxidants [13]. Hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) are recognized as markers of oxidative stress. H2O2 plays a role in the immune system, as intracellular H2O2 increases after tissue damage and acts as a signal to white blood cells (WBCs) to converge on the site and initiate the healing process [14,15]. When the genes required to produce H2O2 are silenced, WBCs do not accumulate at the site of tissue damage. MDA is a reactive aldehyde and one of several reactive electrophile species that produce toxic stress; as a result, this aldehyde is used as a biomarker of oxidative stress levels [16]. SOD has a key antioxidant role, illustrated by the severe pathologies evident in SOD-knockout mice, including hepatocellular carcinoma, an acceleration of age-related muscle mass loss, an earlier incidence of cataracts, and a reduced lifespan [17]. Mice lacking SOD do not show any obvious defects and have a normal lifespan, but they are more sensitive to hyperoxic injury. GSH-Px protects organisms from oxidative damage by reducing lipid hydroperoxides to their corresponding alcohols and reducing free H2O2 to water [18,19].
In this study, in order to better elucidate the role of p21 in response in UVB exposure, we first investigated p21 expression levels and apoptotic, proliferative, and cell cycle effects in HaCaT keratinocytes in response to UVB radiation. The HaCaT keratinocyte cell line is the first normally-differentiated, non-tumorigenic permanent epithelial cell line developed from adult human skin, making it a useful in vitro platform for investigating the effects of UVB radiation upon human skin [20]. We then investigated the apoptotic, proliferative, and cell cycle effects, as well as p53 expression, in response to p21 silencing (p21–) and overexpression (p21+) both before and in response to UVB radiation. Finally, we tested the levels of 2 oxidizing species (H2O2 and MDA) and the activity of 2 markers of antioxidant defense capabilities (GSH-Px and SOD) both before and in response to UVB radiation.
Material and Methods
Cell culture
Human HaCaT keratinocytes (American Type Culture Collection, ATCC, Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, 5% CO2, 37°C) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 mg/ml streptomycin (Hyclone Laboratories, Logan, UT, USA) [10]. Cells were passaged when they reached 95% confluence by trypsinization. Briefly, the passaging method involved washing the cell monolayer twice with 5.0 ml of phosphate buffer saline (PBS) (1×) followed by a 2.0-ml 0.25% trypsin-EDTA (1×) rinse (Hyclone Laboratories, Logan, UT, USA). Thereafter, the cells were incubated at 37°C for 1 min to allow cell detachment. Then, an equal volume of fresh growth medium was added to suspend the detached cells in solution as well as to stop trypsin action.
Experimental group construction
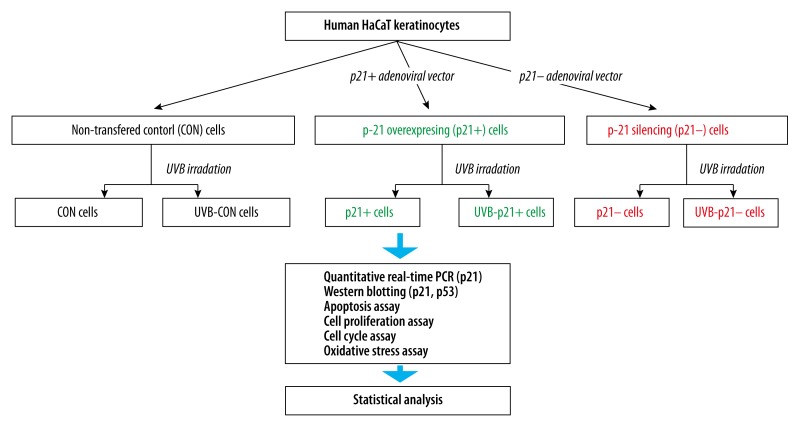
Cells were divided into 6 groups as follows: (i) unexposed, untreated control cells transfected with an empty adenoviral vector (CON cells); (ii) CON cells irradiated once with 30 mJ/cm2 UVB (UVB-CON cells); (iii) cells transfected with p21-overexpression adenoviral vector (50 nM) for 6 h (p21+ cells); (iv) p21+ cells irradiated once with 30 mJ/cm2 UVB (UVB-p21+ cells); (v) cells transfected with p21-interference adenoviral vector (50 nM) for 6 h (p21– cells); and (vi) p21– cells irradiated once with 30 mJ/cm2 UVB (UVB-p21– cells). The cells were then subjected to the assays described below.
P21-adenovirus transfection
The day following cell seeding, Polybrene (8 μg/ml, Sigma, St. Louis, MO, USA) was applied to p21-overexpression and p21-interference adenoviral vectors expressing green fluorescent protein (GFP) (Invitrogen, Carlsbad, CA, USA) at multiplicities of infection (MOI) of 2.5, 5, 10, and 20 [10]. Post-transfection, cells were harvested, and the transfection efficiency was determined by biochemical detection of p21 expression using anti-human p21 antibodies (Santa Cruz Biotechnologies, Inc., Santa Cruz, CA, USA). After incubating for 14 h, fresh medium was replaced after rinsing the cells, and the HaCaT keratinocytes were then cultured to ~60–80% confluence in 100×20 mm dishes in 0.1% FBS-containing medium until UVB irradiation [10].
UVB irradiation
At 36 h post-transfection, UVB irradiation was performed as previously described with minor modifications [10]. After rinsing with PBS twice, the UVB-irradiated groups (i.e., UVB-CON, UVB-p21+, and UVB-p21-) in PBS were exposed to UVB (dose: 30 mJ/cm2, flux: 1.0 mW/cm2, distance: 13 cm) using the CL1000 Ultraviolet Crosslinker (UVP, San Gabriel, CA, USA). A spectroradiometer (Luzchem Research, Inc., Ontario, Canada) was used to assess spectral outputs, and a Goldilux UV meter equipped with a UVB detector (Oriel Instruments, Stratford, CT, USA) was used to measure UVB doses. Under identical conditions, the control groups (i.e., CON, p21+, and p21–) were sham-irradiated. Cells were cultured under standard conditions and then collected at particular endpoints.
Quantitative real-time PCR
Total mRNA was then extracted using a mRNA Isolation Kit (Invitrogen Corp., San Diego, CA, USA), and first-strand synthesis was performed using a cDNA Synthesis Kit (Promega, Madison, WI, USA) in accordance with the manufacturer’s instructions. p21 and p53 mRNA levels were estimated by quantitative real-time PCR using the following specific primers: p21 5′-TAGCAGCGGAACAAGGAG-3′ (forward), p21 5′-AAACGGGAACCAGGACAC-3′ (reverse), p53 5′-CCAC CATCCACTACAACTAC-3′ (forward), and p53 5′-AAACACGC ACCTCAAAGC-3′ (reverse). The CT values were normalized to GAPDH.
Western blotting
Western blotting was performed as previously described [10]. A bicinchoninic acid (BCA) assay was used to assess protein concentrations for equivalent loading (Pierce, Rockford, IL, USA). At 24 h after the last UVB exposure, cells were washed once with PBS and lysed on ice. After homogenization of the lysates, 30 mg of proteins were electrophoresed on a 10% sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE). The proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was incubated with the primary antibodies (i.e., anti-p53, anti-p21, and anti-GAPDH (Abcam, Cambridge, MA, USA) in 2% bovine serum albumin (BSA) in Tris-buffered saline (TBST) overnight at 41°C, and then incubated with the appropriate secondary antibody (Abcam, Cambridge, MA, USA) for 45 min at room temperature. The bands were then visualized by chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA).
Apoptosis assay by flow cytometry
Apoptosis was assessed with an In Situ Cell Apoptosis Detection Kit III (FITC) according to the kit’s instructions (Boster, Wuhan, USA). As previously described [10], apoptotic cells positive for annexin V-FITC were quantified by flow cytometry using a BD Calibur (BD Biosciences).
Cell proliferation assay
The cell growth inhibition rate (IR) was measured using the cell counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) following the manufacturer’s instructions. The 450-nm absorbance of the solutions was measured using a microplate reader (MDS Analytical Technologies, Sunnyvale, CA, USA) with SoftMaxPro software (Molecular Devices, Sunnyvale, CA, USA). After UVB irradiation, cell counts were indirectly estimated from a standard curve generated using solutions of known cell counts: 0, 1×104, 1×105, 5×105, and 1×106 cells/well. The CCK-8 ratio of HaCaT keratinocytes according to the passage number was defined as the ratio of OD 450 nm in 5% PL at 1×1012/l to that in 5% FBS.
Cell cycle analysis by flow cytometry
Cell cycle analysis was performed as described previously [10]. Briefly, at particular time points post-treatment, HaCaT keratinocytes were harvested and 5 ml ice cold 70% ethanol was used for fixation. Then, the fixed HaCaT keratinocytes were treated with propidium iodide (PI, 20 mg/ml) and RNase A (1 mg/ml) (Sigma Chemical Co., St. Louis, MO, USA). Flow cytometry and the software package ModFitLT (Becton-Dickinson, San Jose, CA, USA) were used to determine DNA content.
Oxidative stress assay
The MDA concentration in HaCaT keratinocytes was determined based on thiobarbituric acid (TBA) reactivity (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, after mixing trichloroacetic acid with HaCaT keratinocytes and centrifuging, a supernatant was obtained, and TBA was added. The developed red color of the resulting reaction was measured at 532 nm with a spectrophotometer. Other procedures were carried out following the manufacturer’s protocols. The H2O2 content in the cells was assessed using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); specifically, H2O2 binds to molybdenic acid to form a complex, which was measured at 405 nm, and the H2O2 content was then calculated. SOD activity in gastric submucosal arteries was assessed using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the auto-oxidation of hydroxylamine. The developed blue color was measured at 550 nm. GSH-Px activity was determined by the velocity method using a GSH-Px kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The reaction was initiated by the addition of H2O2. A series of enzymatic reactions was activated by GSH-Px in the cells, which subsequently led to the conversion of GSH (reduced glutathione) to oxidized glutathione (GSSG). The change in absorbance during the conversion of GSH to GSSG was recorded spectrophotometrically at 412 nm.
Statistical analysis
Means from three independent experiments were reported. Two-way analysis of variance (ANOVA) and Student’s t-testing were applied for comparative analyses. A two-sided p-value of less than 0.05 was deemed significant.
Results
Successful transfection of HaCaT keratinocytes with adenoviral vectors
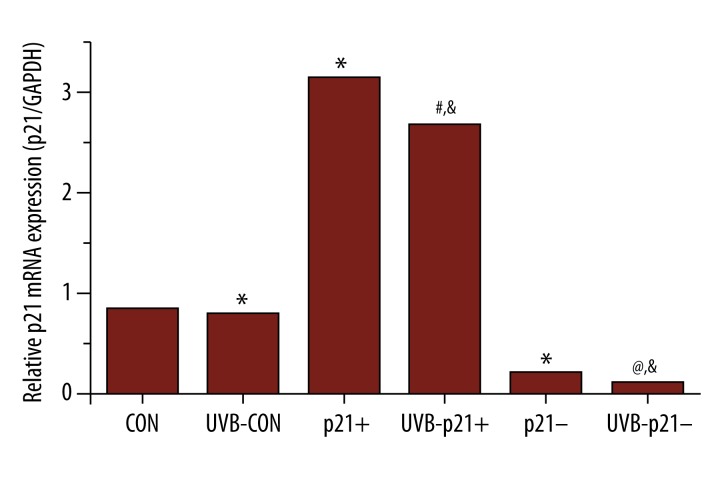
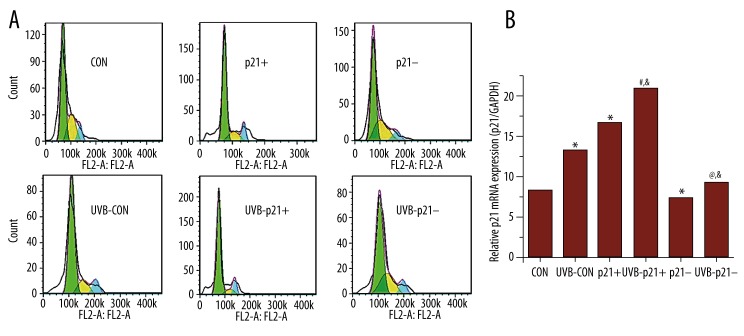
A schematic of the experimental workflow is depicted in Figure 1. In order to further probe the relationship between p21 expression level and UVB irradiation on HaCaT keratinocytes, p21-overexpression (p21+), p21-interference (p21–), and empty adenoviral vectors (CON) were transfected into HaCaT keratinocytes [10]. All adenoviral vectors were successful transfected and performed their function; as expected, the p21 mRNA and protein levels of p21+ cells were significantly upregulated compared to that of CON cells (p21+ vs. CON, p<0.05), while the p21 mRNA and protein levels of p21– cells were significantly downregulated compared to that of CON cells (p21– vs. CON, p<0.05; Figures 2, 3C).
Figure 1.
Schematic of experimental workflow.
Figure 2.
p21 mRNA Levels by RT-PCR. RT-PCR confirms significant p21 mRNA upregulation in p21-overexpressing (p21+) cells and significant p21 mRNA silencing in p21-silenced (p21–) cells. UVB exposure significantly decreases p21 mRNA expression across all experimental groups. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
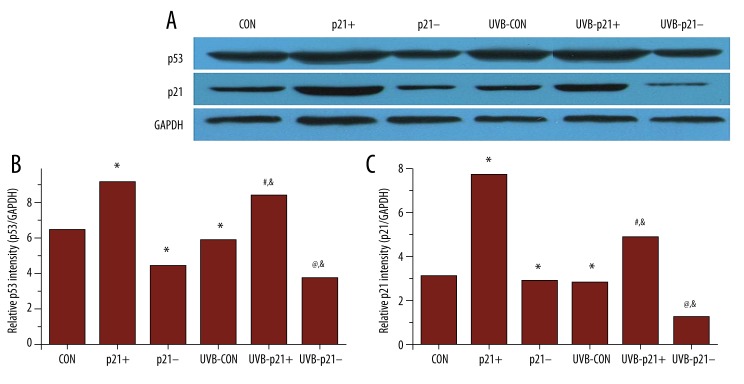
Figure 3.
p21 and p53 protein expression by western blotting. (A) Western blots of p21 and p53 expression with GAPDH as a reference standard. (B) p53 protein expression mirrored p21 protein expression. (C) As expected, p21 protein expression was significantly higher and lower in p21-overexpressing (p21+) and p21-silenced (p21–) cells, respectively. After UVB irradiation, p21 expression levels displayed the same trend but were significantly lower. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
Effects of UVB irradiation on p21 expression in HaCaT keratinocytes
To assess p21 expression after UVB radiation, HaCaT keratinocytes were treated with 0.2% serum for 24 h to reach quiescence (i.e., in actively-dividing cells, p21 is destabilized with a reduced 20–60 min half-life [10,21]). UVB (30 mJ/cm2) was then applied to these HaCaT keratinocytes, which were harvested 1.5 h after exposure. Levels of p21 mRNA and protein expression were both found to be significantly downregulated after UVB exposure across all experimental groups (UVB-CON vs. CON, UVB-p21+ vs. p21+, UVB-p21– vs. p21–, p<0.05; Figures 2, 3C). Moreover, after irradiation, p21 expression in UVB-p21+ cells remained significantly upregulated compared to UVB-CON cells (UVB-p21+ vs. UVB-CON, p<0.05; Figure 2), and p21 expression in UVB-p21– cells remained significantly downregulated compared to UVB-CON cells (UVB-p21– vs. UVB-CON, p<0.05; Figure 2).
Relationship between p21 and p53 protein levels in HaCaT keratinocytes
Following UVB irradiation, the expression levels of p21 and p53 displayed the same trend across all experimental groups with UVB irradiation significantly reducing both p21 and p53 expression (UVB-CON vs. CON, UVB-p21+ vs. p21+, UVB-p21– vs. p21–, p<0.05; Figure 3B, 3C). p53 expression mirrored p21 expression; i.e., p53 expression in p21+ cells was significantly upregulated compared to CON cells (p21+ vs. CON, p<0.05; Figure 3B), while p53 expression in p21– cells was significantly downregulated compared to CON cells (p21– vs. CON, p<0.05; Figure 3B). After irradiation, p53 expression in UVB-p21+ cells remained significantly upregulated compared to UVB-CON cells (UVB-p21+ vs. UVB-CON, p<0.05; Figure 3B), and p53 expression in UVB-p21– cells remained significantly downregulated compared to UVB-CON cells (UVB-p21– vs. UVB-CON, p<0.05; Figure 3B).
p21’s effect on apoptosis rates in HaCaT keratinocytes
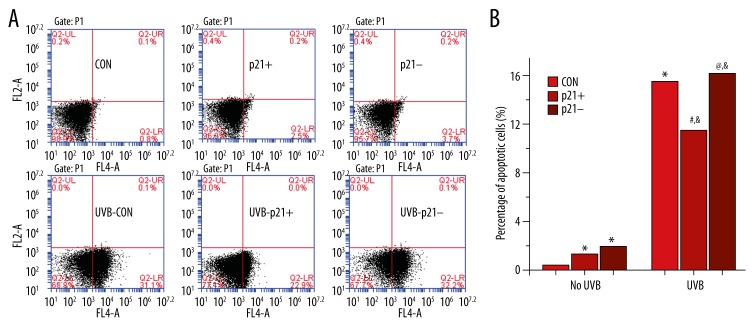
In non-irradiated HaCaT keratinocytes, p21 dysregulation (i.e., both p21 overexpression and p21 silencing) significantly increased apoptosis rates (p21+ vs. CON, p21– vs. CON, p<0.05; Figure 4B). After UVB exposure, apoptosis was significantly increased across all experimental groups (UVB-CON vs. CON, UVB-p21+ vs. p21+, UVB-p21– vs. p21–, p<0.05; Figure 4B). However, the UVB-p21+ apoptosis rate was significantly downregulated compared to that of UVB-CON (UVB-p21+ vs. UVB-CON, p<0.05; Figure 4B), while the apoptosis rate in UVB-p21– cells was significant upregulated compared to UVB-CON cells (UVB-p21– vs. UVB-CON, p<0.05; Figure 4B). These findings suggest that p21 overexpression suppresses apoptosis in UVB-irradiated HaCaT keratinocytes but promotes apoptosis in non-irradiated HaCaT keratinocytes.
Figure 4.
Apoptotic activity by flow cytometry. (A) Flow cytometric analysis of apoptosis. (B) Percentages of apoptotic cells before and after UVB irradiation. With no irradiation, both p21 overexpression (p21+) and p21 silencing (p21–) had a pro-apoptotic effect, but p21 silencing showed a more profound pro-apoptotic effect than p21 overexpression. After UVB irradiation, apoptosis increased in all irradiated groups. In contrast to non-irradiated p21-overexpressing (p21+) cells, p21 overexpression in irradiated p21-overexpressing (UVB-p21+) cells displayed an anti-apoptotic effect. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
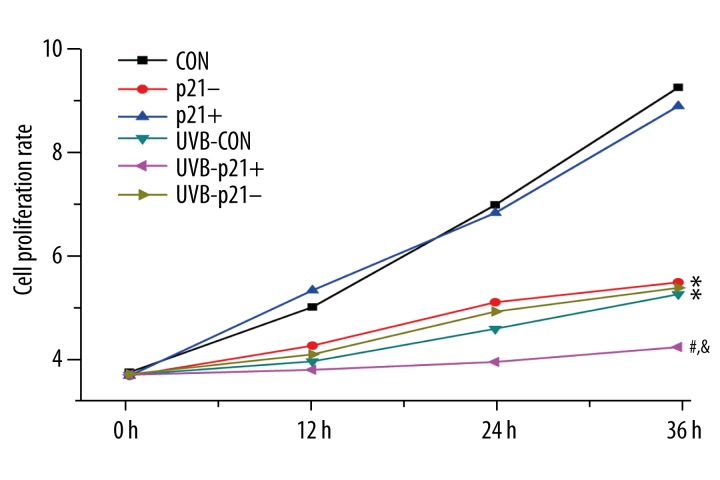
p21’s effect on HaCaT keratinocyte proliferation
In non-irradiated cells, loss of p21 resulted in suppression of proliferation rates (p21– vs. CON, p<0.05, Figure 5). The proliferation rates of CON and p21+ cells were significantly suppressed after irradiation (UVB-CON vs. CON, UVB-p21+ vs. p21+, p<0.05; Figure 5); however, there was no significant differences in proliferation rates of p21– cells before and after UVB irradiation (UVB-p21– vs. p21–, p>0.05; Figure 5). Proliferation of UVB-p21+ cells was more obviously suppressed compared to UVB-CON cells (UVB-p21+ vs. UVB-CON, p<0.05; Figure 5). These findings dualistically suggest that p21 overexpression suppresses proliferation in UVB-irradiated HaCaT keratinocytes while loss of p21 suppresses proliferation in non-irradiated HaCaT keratinocytes.
Figure 5.
Cell proliferation assay. The proliferation rates of non-irradiated control (CON) cells and non-irradiated p21-overexpressing (p21+) cells were significantly higher than UVB-irradiated cells. The proliferation rates of all UVB-exposed groups (UVB-CON, UVB-p21+, and UVB-p21– cells) were significantly suppressed. Proliferation of UVB-p21+ cells was more obviously suppressed compared to those of both UVB-p21– and UVB-CON cells, but there are no significant differences in proliferation between p21– and UVB-p21– cells. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
p21’s effects on HaCaT keratinocyte cell cycle arrest
UVB exposure raises G2 phase arrest in HaCaT keratinocytes (UVB-CON vs. CON, p<0.05; Figure 6B). p21 overexpression displays the ability to potentiate G2 phase arrest both before and after UVB irritation (p21+ vs. CON and UVB-p21+ vs. UVB-CON, p<0.05; Figure 6B). Conversely, p21 silencing displays the ability to diminish G2 phase arrest both before and after UVB irritation (p21– vs. CON and UVB-p21– vs. UVB-CON, p<0.05; Figure 6B).
Figure 6.
Cell cycle analysis by flow cytometry. (A) Flow cytometric analysis of cell cycle proportions. (B) UVB exposure raises G2 phase arrest in HaCaT keratinocytes. p21 overexpression (p21+) potentiated G2 phase arrest both before and after UVB irritation, while p21 silencing (p21–) diminished G2 phase arrest both before and after UVB irritation. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
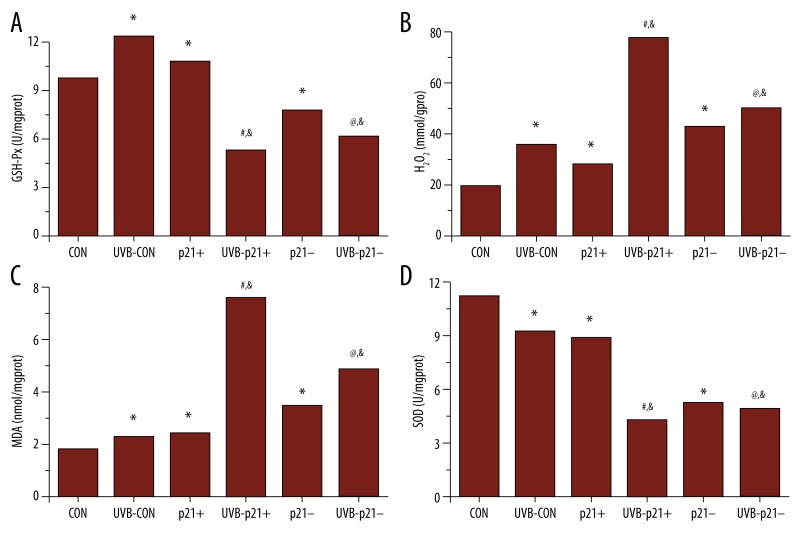
Changes in oxidative stress markers
To determine whether changes in p21 expression are associated with oxidative stress, we measured MDA and H2O2 content as well as GSH-Px and SOD activity in all six experimental groups (Figure 7). In non-irradiated cells, p21 overexpression resulted in significant increases in GSH-Px activity, H2O2 content, and MDA content and a significant decrease in SOD activity (p21+ vs. CON, p<0.05, Figure 7). Loss of p21 resulted in significant increases in H2O2 content and MDA content and significant decreases in GSH-Px and SOD (p21– vs. CON, p<0.05, Figure 7). UVB irradiation of CON, p21+, and p21– cells resulted in significant increases in H2O2 and MDA content and a significant decrease in SOD (UVB-CON vs. CON, UVB-p21+ vs. p21+, UVB-p21– vs. p21–, p<0.05; Figure 7). Interestingly, UVB irradiation in CON cells resulted in a significant increase in GSH-Px (UVB-CON vs. CON, p<0.05; Figure 7), while UVB irradiation in p21+ and p21– cells resulted in significant decreases in GSH-Px (UVB-p21+ vs. p21+, UVB-p21– vs. p21–, p<0.05; Figure 7). These results suggest that GSH-Px expression is sensitive to p21 dysfunction in both non-irradiated and UVB-irradiated HaCaT keratinocytes.
Figure 7.
Oxidative stress assays. (A) p21 overexpression (p21+) significantly upregulated GSH-Px activity, while p21 silencing (p21–) significantly downregulated GSH-Px activity. UVB irradiation significantly upregulated GSH-Px activity in control (CON) cells but significantly downregulated GSH-Px activity in UVB-irradiated p21-overexpressing (UVB-p21+) and UVB-irradiated p21-silenced (UVB-p21–) cells. (B) Both p21 overexpression (p21+) and p21 silencing (p21–) significantly raised H2O2 content. UVB irradiation significantly raised H2O2 content in UVB-irradiated control (UVB-CON), UVB-irradiated p21-overexpressing (UVB-p21+) and UVB-irradiated p21-silenced (UVB-p21–) cells. (C) Both p21 overexpression (p21+) and p21 silencing (p21–) significantly raised MDA content. UVB irradiation significantly raised MDA content in UVB-irradiated control (UVB-CON), UVB-irradiated p21-overexpressing (UVB-p21+) and UVB-irradiated p21-silenced (UVB-p21–) cells. (D) Both p21 overexpression (p21+) and p21 silencing (p21–) significantly reduced SOD activity. UVB irradiation significantly reduced SOD activity in UVB-irradiated control (UVB-CON), UVB-irradiated p21-overexpressing (UVB-p21+) and UVB-irradiated p21-silenced (UVB-p21–) cells. * p<0.05 vs. CON, # p<0.05 vs. p21+, @ p<0.05 vs. p21–, & p<0.05 vs. UVB-CON.
Discussion
In this study, we investigated p21 expression levels and apoptotic, proliferative, and cell cycle effects in HaCaT keratinocytes after UVB irradiation. In agreement with a previous study [10], we showed that UVB exposure induces significant p21 mRNA and protein downregulation in HaCaT keratinocytes (UVB-CON vs. CON, p<0.05; Figures 2, 3C). This p21 downregulation in UVB-irradiated cells was associated with significantly increased apoptosis, decreased proliferation, and increased G2 phase arrest (UVB-CON vs. CON, p<0.05; Figures 4–6), likely to promote the elimination of DNA-damaged cells. These findings are consistent with a previous study that demonstrated p21 protein degradation in a variety of mammalian cell types after low-dose UV irradiation (<40 mJ/m2) through a ubiquitin/Skp2-dependent proteasomal mechanism [22].
We also investigated the effects of p21 silencing and overexpression on p53 expression both before and in response to UVB radiation. There is a complex regulatory interplay between p21 and p53 expression in mammalian cells. p21 expression is transcriptionally regulated by both p53-dependent and -independent mechanisms; namely, the p21 promoter contains two conserved p53-binding sites, and at least one of these is required for p53 responsiveness after DNA damage [23]. In turn, the negative regulation of several p53-repressed genes is p21-dependent, and p21 overexpression represses a similar set of genes [24]. In this study, p53 expression mirrored p21 expression (i.e., p21+ cells displayed higher p53 expression, while p21– cells displayed lower p53 expression) (Figure 3). As there is no previous evidence that p21 directly induces p53 expression, it is more likely that p21 overexpression over-repressed p53-regulated genes, and this over-repression resulted in regulatory feedback that increased p53 expression. Further investigation is required to determine the precise mechanism(s) at play.
We also investigated the effects of p21 silencing (p21–) on HaCaT keratinocytes both before and in response to UVB radiation. In non-irradiated HaCaT keratinocytes, p21 silencing significantly promoted apoptosis while significantly inhibiting proliferation and G2 phase arrest (p21– vs. CON, p<0.05; Figures 4B, 5, 6). This finding is consistent with previous studies that show suppressing p21 expression by anti-sense technology and homologous recombination shifts cells from cell-cycle arrest to apoptosis [25]. In contrast, after UVB irradiation, p21 silencing demonstrated a less profound pro-apoptotic effect and a more profound inhibition of G2 phase arrest (UVB-p21– vs. UVB-CON, p<0.05; Figures 4B, 6), which is reflected in significantly higher proliferative activity in p21-silenced cells after irradiation (UVB-p21– vs. UVB-CON, p<0.05; Figure 5). This set of findings is consistent with studies of p21 knockout mice that demonstrate that UV-induced cell cycle arrest is partially dependent on p21 [26].
We also investigated the effects of p21 overexpression (p21+) on HaCaT keratinocytes both before and in response to UVB radiation. In non-irradiated HaCaT keratinocytes, p21 overexpression significantly inhibited apoptosis and promoted G2 phase arrest while not significantly effecting proliferation (p21+ vs. CON, p<0.05, p>0.05; Figures 4B, 5, 6). However, after UVB exposure, the opposite effect was observed – p21 overexpression significantly promoted apoptosis and G2 phase arrest while significantly inhibiting cell proliferation (UVB-p21+ vs. UVB-CON, p<0.05; Figures 4B, 5, 6). These findings indicate that, in HaCaT keratinocytes, p21 overexpression acts in an anti-apoptotic manner in the absence of UVB-induced DNA damage but acts in a pro-apoptotic manner in the presence of UVB-induced DNA damage. This conclusion coincides with previous studies that show p21, in addition to being an inhibitor of cell proliferation, can also inhibit apoptosis in a number of systems, counteracting its tumor-suppressive functions; this “antagonistic duality” of p21 is also present in other growth-promoting oncoproteins such as Myc and E2F1 [27,28]. However, regardless of UVB-induced DNA damage, p21 overexpression promoted G2 phase arrest in HaCaT keratinocytes. This is consistent with a previous study in human colon cancer cells that demonstrated p21 overexpression reliably induces a stable G2 phase arrest, likely through a PCNA-based mechanism [29].
Oxidative stress is associated with increased production of oxidizing species or decreased antioxidant defense capabilities [30]. In this study, we tested two oxidizing species (H2O2, MDA) and two markers of antioxidant defense capabilities (GSH-Px and SOD). As expected, both H2O2 (the primary ROS produced by mitochondrial respiration) and MDA (a marker of free radical species-related injury) significantly increased after UVB exposure (UVB-CON vs. CON, p<0.05; Figure 7B, 7C). Interestingly, increases in these oxidative stress markers were the most potentiated in UVB-irradiated p21-overexpressing cells (UVB-p21+ vs. UVB-CON, p<0.05; Figure 7B, 7C). In addition, after UVB irradiation, GSH-Px (a free radical scavenging enzyme that converts H2O2 to water) activity significantly increased while SOD (an antioxidant enzyme that destroys ROS) activity significantly decreased (UVB-CON vs. CON, p<0.05; Figure 7A, 7D). Interestingly, both GSH-Px and SOD activity were most potently downregulated in UVB-irradiated p21-overexpressing cells (UVB-p21+ vs. UVB-CON, p<0.05; Figure 7A, 7D). These combined findings suggest that p21 overexpression may play a role in decreasing antioxidant defense capabilities in UVB-irradiated HaCaT keratinocytes. Further research is required to determine the mechanism(s) at play.
Clinically, these results confirm that exposure to UVB radiation produces oxidative damage in human keratinocytes that adversely affects their viability and can lead to skin aging and malignancy [31]. In vivo, human UVB-induced erythema occurs ~4 h post-exposure, peaks at ~8–24 h, and declines thereafter; this UVB erythema may last for weeks in fair-skinned and older persons and has been positively associated with the development of apoptotic keratinocytes [32]. There are four prescriptive measures that can be taken to reduce these adverse effects: (i) minimization of sunlight exposure through remaining indoors or using protective clothing, coverings, sunglasses, umbrellas, and/or parasols [33]; (ii) the application of sun protection factor (SPF) sunscreen on the skin to reduce photodamage [34], (iii) the induction or transdermal delivery of antioxidant enzymes (e.g., GSH-Px, catalase, and SOD) [35]; and (iv) supplementation with a combination of non-enzymatic antioxidants, including glutathione, α-tocopherol, ascorbate, and β-carotene [35].
Conclusions
UVB exposure induced p21 downregulation in HaCaT keratinocytes and was associated with significantly increased apoptosis, decreased proliferation, and increased G2 phase arrest. Secondly, p53 expression mirrored p21 expression, suggesting a regulatory feedback mechanism between p21 and p53 expression. Thirdly, p21 silencing promoted apoptosis and inhibited G2 phase arrest and proliferation, but after UVB irradiation, demonstrated a less profound pro-apoptotic effect and a more profound inhibition of G2 phase arrest, which was reflected in higher proliferative activity. Fourthly, p21 overexpression acted in an anti-apoptotic manner in the absence of UVB-induced DNA damage but acted in a pro-apoptotic manner in the presence of UVB-induced DNA damage, displaying an “antagonistic duality” similar to other growth-promoting oncoproteins. Finally, p21 overexpression may play a role in decreasing antioxidant defense capabilities in UVB-irradiated HaCaT keratinocytes. Further research into the molecular mechanism(s) underlying the complex role of p21 in UVB-irradiated keratinocytes is still needed.
Footnotes
Source of support: Departmental sources
References
- 1.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2013;810:120–40. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 2.de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Choi S-W, Mason JB. Folate and carcinogenesis: An integrated Scheme. J Nutr. 2000;130:129–32. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424:107–15. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 6.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 7.Harper JW, Adami GR, Wei N, et al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 8.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 9.Macleod KF, Sherry N, Hannon G, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–44. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 10.Lei X, Liu B, Han W, et al. UVB-Induced p21 degradation promotes apoptosis of human keratinocytes. Photochem Photobiol Sci. 2010;9:1640–48. doi: 10.1039/c0pp00244e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Waga S, Hannon GJ, et al. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–37. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Kwon H, Jeong H, et al. Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma lucidum. Phytother Res. 2001;15:245–49. doi: 10.1002/ptr.830. [DOI] [PubMed] [Google Scholar]
- 13.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 16.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 17.Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 18.Pacifici RE, Davies K. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37:166–80. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- 19.Sies H, Cadenas E, Symons M, Scott G. Oxidative stress: Damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311(1152):617–31. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 20.Yong W, Peng D, Wang L, et al. Screening of HaCaT clones for CCL20 gene knockout and preliminary exploration of gene-targeting vector transfection approaches in this cell line. Med Sci Monit Basic Res. 2015;21:21–28. doi: 10.12659/MSMBR.893143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendjennat M, Boulaire J, Jascur T, et al. UV irradiation triggers ubiquitin-dependent degradation of p21 WAF1 to promote DNA repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–85. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 24.Löhr K, Möritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507–16. doi: 10.1074/jbc.M212517200. [DOI] [PubMed] [Google Scholar]
- 25.Blundell RA. The biology of p21 Waf1/Cip1 – review paper. American Journal of Biochemistry and Biotechnology. 2006;2:33. [Google Scholar]
- 26.Shannon J, Kefford R, Mann G. Responses to ultraviolet-B in cell lines from hereditary melanoma kindreds. Melanoma Res. 2001;11:1–9. doi: 10.1097/00008390-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–49. [PubMed] [Google Scholar]
- 28.Tyner AL, Gartel AL. Roles of cyclin kinase inhibitors in G1 phase progression G1 phase progression. Kluwer; New York: 2003. pp. 58–76. [Google Scholar]
- 29.Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–20. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 30.Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods 1. Free Radic Biol Med. 1999;27:1173–81. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 31.Cimino F, Cristani M, Saija A, et al. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors. 2007;30:129–38. doi: 10.1002/biof.5520300206. [DOI] [PubMed] [Google Scholar]
- 32.Brenner M, Hearing VJ. The Protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Lian S, Hao Y, et al. Sun-exposure knowledge and protection behavior in a North Chinese population: a questionnaire-based study. Photodermatol Photoimmunol Photomed. 2010;26:177–81. doi: 10.1111/j.1600-0781.2010.00513.x. [DOI] [PubMed] [Google Scholar]
- 34.Gulston M, Knowland J. Illumination of human keratinocytes in the presence of the sunscreen ingredient Padimate-O and through an SPF-15 sunscreen reduces direct photodamage to DNA but increases strand breaks. Mutat Res. 1999;444:49–60. doi: 10.1016/s1383-5718(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 35.Steenvoorden DP, van Henegouwen GMB. The use of endogenous antioxidants to improve photoprotection. J Photochem Photobiol B. 1997;41:1–10. doi: 10.1016/s1011-1344(97)00081-x. [DOI] [PubMed] [Google Scholar]