Abstract
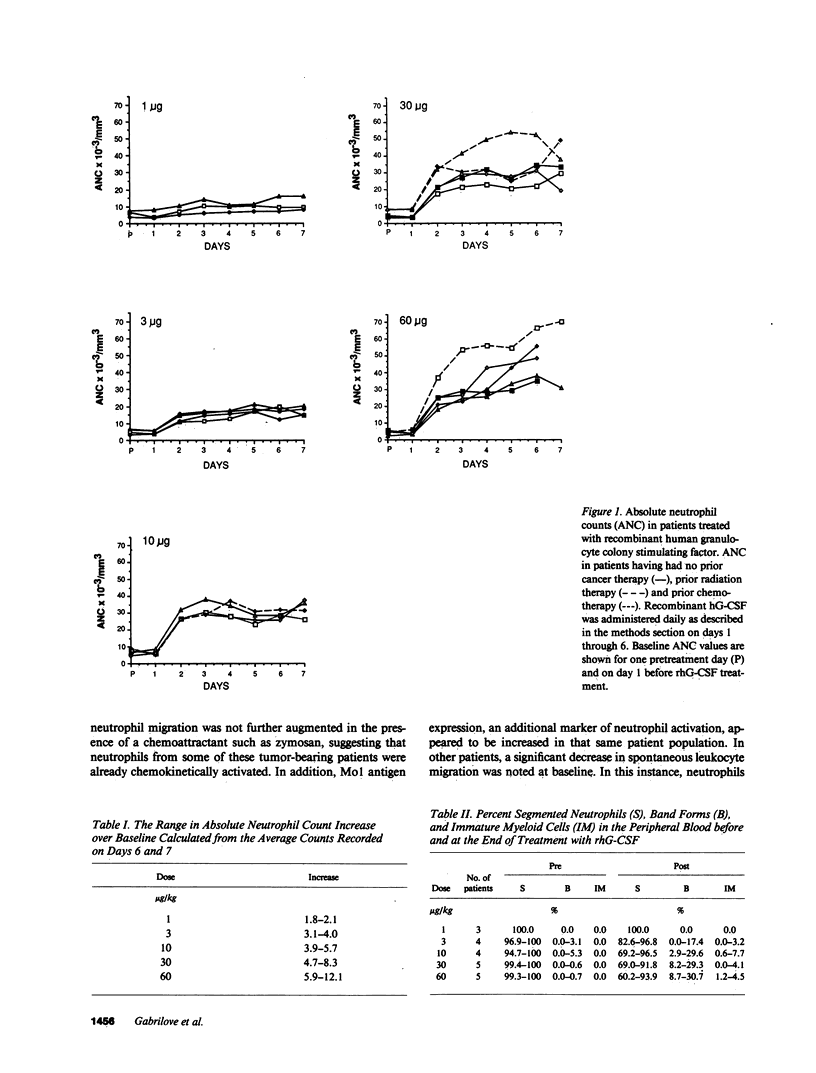
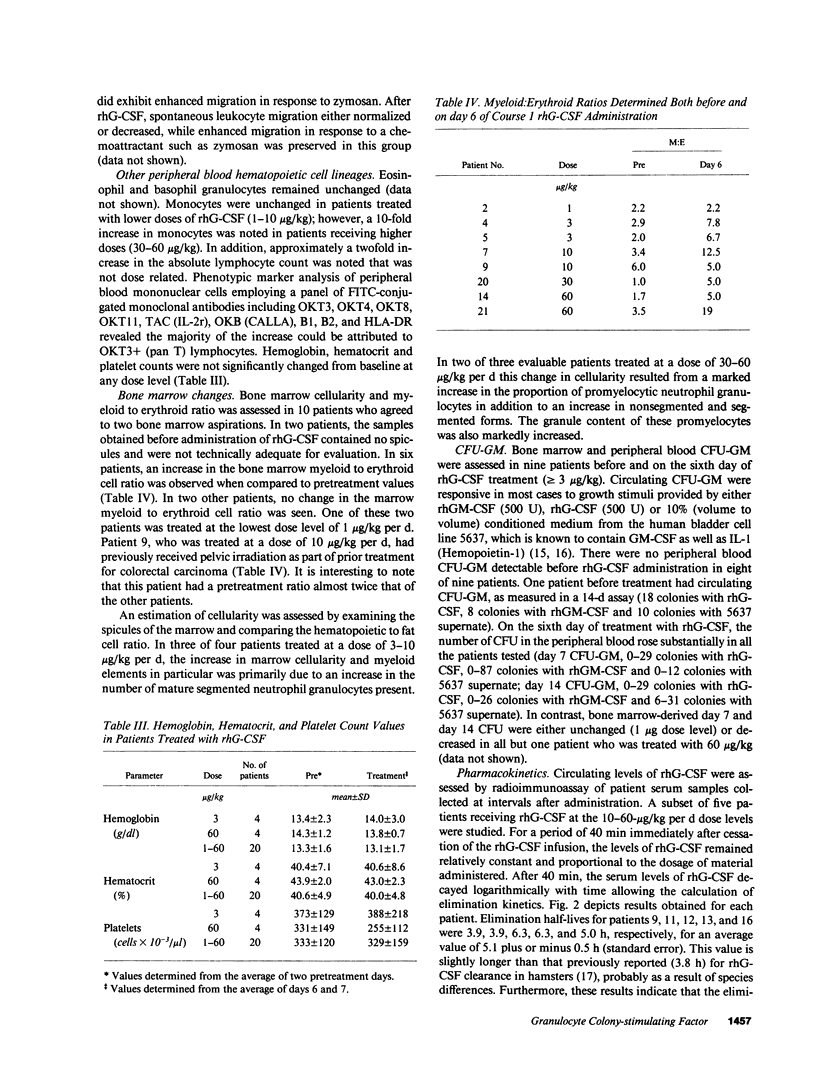
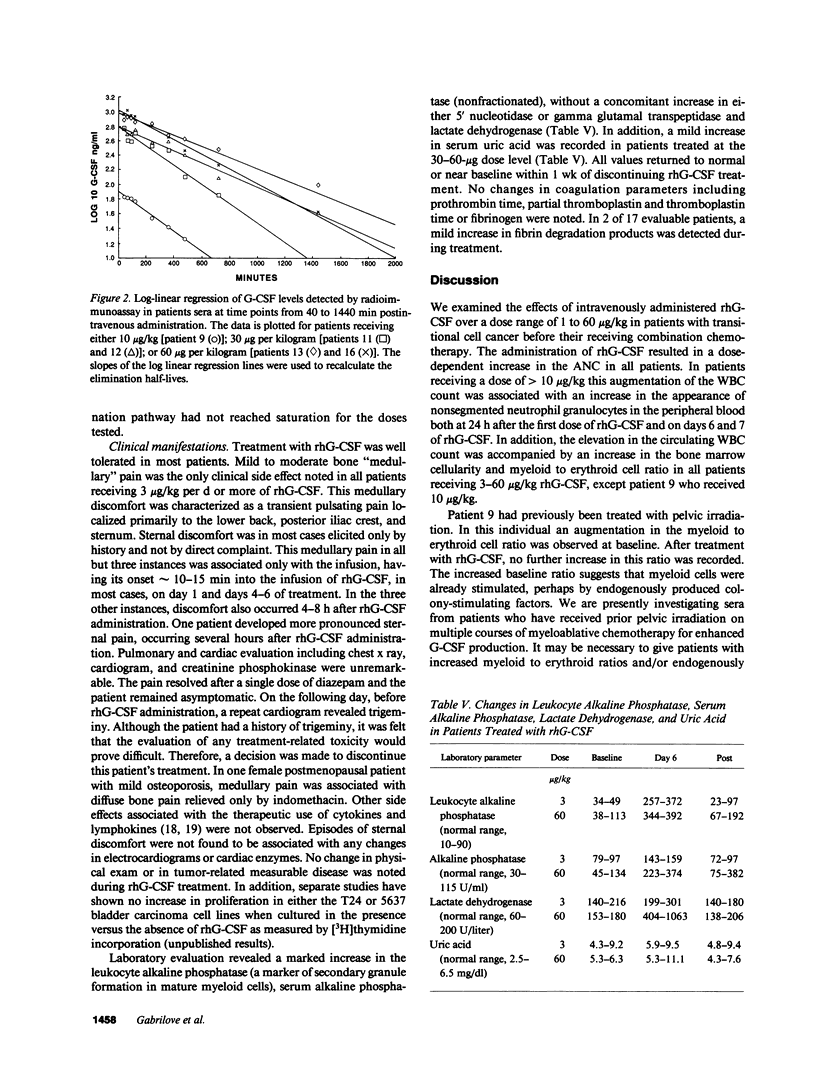
Recombinant human granulocyte colony-stimulating factor (rhG-CSF) was administered at a dose of 1-60 micrograms/kg of body weight to 22 patients with transitional cell carcinoma before chemotherapy as part of a Phase I/II study. In all patients, a specific dose-dependent increase in the absolute neutrophil count (ANC) of 1.8-12 fold was seen. In addition, this augmentation in the ANC was accompanied by an increase in leukocyte alkaline phosphatase, a marker of secondary granule formation. In six of eight patients analyzed, an increase in bone marrow myeloid to erythroid cell ratio was seen. Day 14 peripheral blood cell derived colony forming unit granulocyte macrophage were also increased by day 6 of rhG-CSF treatment. Circulating levels of eosinophils and basophils were unchanged; however, a 10-fold increase in monocytes was observed in patients treated at the highest doses. There was also a small increase in CD3+ lymphocytes that was not dose dependent. Hemoglobin, hematocrit, and platelet count remained near baseline throughout the period of rhG-CSF administration. These findings demonstrate that rhG-CSF is a potent stimulus for normal neutrophil proliferation and maturation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Wang E. A., Clark S. C., Sieff C. A. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986 Aug;78(2):597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Lopez A. F., Nicola N. A., Warren D. J., Vadas M. A., Sanderson C. J., Metcalf D. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood. 1986 Jul;68(1):162–166. [PubMed] [Google Scholar]
- Chapman P. B., Lester T. J., Casper E. S., Gabrilove J. L., Wong G. Y., Kempin S. J., Gold P. J., Welt S., Warren R. S., Starnes H. F. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987 Dec;5(12):1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- Charbord P., Tippens D., Wight T. S., Gown A. M., Singer J. W. Stromal cells from human long-term marrow cultures, but not cultured marrow fibroblasts, phagocytose horse serum constituents: studies with a monoclonal antibody that reacts with a species-specific epitope common to multiple horse serum proteins. Exp Hematol. 1987 Jan;15(1):72–77. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. In vitro growth of granulocytic and mononuclear cell colonies from blood of normal individuals. Blood. 1971 Feb;37(2):131–135. [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilove J. L., Welte K., Harris P., Platzer E., Lu L., Levi E., Mertelsmann R., Moore M. A. Pluripoietin alpha: a second human hematopoietic colony-stimulating factor produced by the human bladder carcinoma cell line 5637. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2478–2482. doi: 10.1073/pnas.83.8.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillio A. P., Bonilla M. A., Potter G. K., Gabrilove J. L., O'Reilly R. J., Souza L. M., Welte K. Effects of recombinant human granulocyte-colony stimulating factor on hematopoietic reconstitution after autologous bone marrow transplantation in primates. Transplant Proc. 1987 Dec;19(6 Suppl 7):153–156. [PubMed] [Google Scholar]
- Hartmann D., Entringer M. A., Vasil M., Robinson W. A. The effect of bacterial infection on granulopoiesis. Proc Soc Exp Biol Med. 1981 May;167(1):6–11. doi: 10.3181/00379727-167-41115. [DOI] [PubMed] [Google Scholar]
- Hirano T. Changes in polymorphonuclear leukocyte motility under agarose and luminol-dependent chemiluminescence response in patients with gastric cancer. Gastroenterol Jpn. 1984 Oct;19(5):447–456. doi: 10.1007/BF02807257. [DOI] [PubMed] [Google Scholar]
- Juttner C. A., To L. B., Haylock D. N., Branford A., Kimber R. J. Circulating autologous stem cells collected in very early remission from acute non-lymphoblastic leukaemia produce prompt but incomplete haemopoietic reconstitution after high dose melphalan or supralethal chemoradiotherapy. Br J Haematol. 1985 Dec;61(4):739–745. doi: 10.1111/j.1365-2141.1985.tb02888.x. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Schreml W., Fliedner T. M., Heimpel H. Reaction of human granulopoiesis to high-dose cyclophosphamide therapy. Blut. 1979 Jan 22;38(1):9–16. doi: 10.1007/BF01082923. [DOI] [PubMed] [Google Scholar]
- MECHANIC R. C., FREI E., 3rd, LANDY M., SMITH W. W. Quantitative studies of human leukocytic and febrile response to single and repeated doses of purified bacterial endotoxin. J Clin Invest. 1962 Jan;41:162–172. doi: 10.1172/JCI104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCredie K. B., Hersh E. M., Freireich E. J. Cells capable of colony formation in the peripheral blood of man. Science. 1971 Jan 22;171(3968):293–294. doi: 10.1126/science.171.3968.293. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Gabrilove J., Sheridan A. P. Therapeutic implications of serum factors inhibiting proliferation and inducing differentiation of myeloid leukemic cells. Blood Cells. 1983;9(1):125–144. [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Platzer E., Oez S., Welte K., Sendler A., Gabrilove J. L., Mertelsmann R., Moore M. A., Kalden J. R. Human pluripotent hemopoietic colony stimulating factor: activities on human and murine cells. Immunobiology. 1986 Sep;172(3-5):185–193. doi: 10.1016/S0171-2985(86)80098-5. [DOI] [PubMed] [Google Scholar]
- Platzer E., Welte K., Gabrilove J. L., Lu L., Harris P., Mertelsmann R., Moore M. A. Biological activities of a human pluripotent hemopoietic colony stimulating factor on normal and leukemic cells. J Exp Med. 1985 Dec 1;162(6):1788–1801. doi: 10.1084/jem.162.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Richman C. M., Weiner R. S., Yankee R. A. Increase in circulating stem cells following chemotherapy in man. Blood. 1976 Jun;47(6):1031–1039. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Strife A., Lambek C., Wisniewski D., Gulati S., Gasson J. C., Golde D. W., Welte K., Gabrilove J. L., Clarkson B. Activities of four purified growth factors on highly enriched human hematopoietic progenitor cells. Blood. 1987 May;69(5):1508–1523. [PubMed] [Google Scholar]
- Sulowicz W. Random migration of leukocytes and phagocytic activity of neutrophils in patients with cancer of gastrointestinal tract at different clinical stages. Rev Esp Oncol. 1983;30(3):317–321. [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983 Aug;72(2):441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly H., Bastit D., Lucet J. C., Esperou H., Monconduit M., Piguet H. Haemopoietic reconstitution after autologous peripheral blood stem cell transplantation in acute leukaemia. Lancet. 1986 Jul 19;2(8499):154–155. doi: 10.1016/s0140-6736(86)91962-8. [DOI] [PubMed] [Google Scholar]
- To L. B., Haylock D. N., Kimber R. J., Juttner C. A. High levels of circulating haemopoietic stem cells in very early remission from acute non-lymphoblastic leukaemia and their collection and cryopreservation. Br J Haematol. 1984 Nov;58(3):399–410. doi: 10.1111/j.1365-2141.1984.tb03987.x. [DOI] [PubMed] [Google Scholar]
- WOLFF S. M., RUBENSTEIN M., MULHOLLAND J. H., ALLING D. W. COMPARISON OF HEMATOLOGIC AND FEBRILE RESPONSE TO ENDOTOXIN IN MAN. Blood. 1965 Aug;26:190–201. [PubMed] [Google Scholar]
- Walter R. J., Danielson J. R. Defective monocyte chemotaxis in patients with epidermoid tumors of the head and neck. Arch Otolaryngol. 1985 Aug;111(8):538–540. doi: 10.1001/archotol.1985.00800100086013. [DOI] [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Cohen A. M., Murdock D. C., Boone T. C., Inoue H., Chazin V. R., Hines D., Souza L. M. Recombinant human granulocyte colony stimulating factor: molecular and biological characterization. Immunobiology. 1986 Sep;172(3-5):175–184. doi: 10.1016/S0171-2985(86)80097-3. [DOI] [PubMed] [Google Scholar]



