Abstract
Infectious agents are increasingly implicated in the development and progression of chronic inflammatory diseases. Several lines of evidence suggest that the common intracellular respiratory pathogen, Chlamydia pneumoniae contributes to the well-established risk factors of atherosclerosis but the exact mechanism is not well understood. It is believed that C. pneumoniae-infected monocytes travel from the lung to the atherosclerotic foci, during which the cells experience mechanical stimuli due to blood flow. In this work, we characterized the effect of physiological levels of shear stress on C. pneumoniae-infected human monocytes in an in vitro flow model. We found that a shear stress of 5 dyn/cm2 enhanced the expression of pro-inflammatory cytokine IL-1β only in infected, but not in uninfected, monocytes. We also found that this enhancement is due to the upregulation of IL-1β gene expression due to shear stress. Our results demonstrate that mechanotransduction is an important, heretofore unaddressed, determinant of inflammatory response to an infection.
Keywords: Chlamydia pneumoniae, Monocytes, Shear stress, IL-1β
Chronic inflammatory response plays a critical role in the development and progression of atherosclerosis, and there is mounting evidence that infection may be a latent, but major, contributor to the pro-inflammatory processes that underlie the disease (Rosenfeld and Campbell 2011; Tufano et al., 2012). Over the past two decades, convincing data suggest that pathogens such as Cytomegalovirus, Porphyromonas gingivalis, Chlamydia pneumoniae, Toxoplasma gondii and Helicobacter cinaedi may act as a source of inflammation that eventually lead, in concert with other risk factors, to the formation of atherosclerotic plaque (Chen et al., 2003; Khan et al., 2012). Of particular interest, several lines of evidence have implicated an important role of C. pneumoniae infection, and the development and progression of atherosclerosis (Campbell and Kuo 2004). However, the causative link and molecular mechanisms of this association are unclear.
Chlamydia pneumoniae is a ubiquitous respiratory intracellular pathogen implicated in the pathogenesis and development of atherosclerosis based on several epidemiological, histopathological, animal studies, and limited clinical trials (Campbell and Kuo 2004; Roulis et al., 2013). Upon C. pneumoniae infection, the infectious elementary bodies (EB) enter the host cell and forms vesicles in the cytoplasm of host cell called inclusions (Krull et al., 2005). Once inside the inclusions, the C. pneumoniae EB differentiates back into non-infectious, replicating reticulate bodies (RB) and the RB subsequently differentiates back to EB. The mature EBs are released to infect other susceptible host cells. In vivo studies suggest that C. pneumoniae infects alveolar macrophages in lung which escape into circulation (Maass et al., 1998), where the bacteria is disseminated and taken up by peripheral blood monocytes (Kalayoglu et al., 2001). These circulating infected monocytes might reach atherosclerotic foci crossing the endothelial barrier (Gieffers et al., 2004). During this transit, the infected monocytes will experience mechanical stresses due to blood flow. Since shear stress plays an important role in vascular homeostasis (Weinbaum et al., 2011), and it may be expected that these forces will also alter the response of infected monocytes. Our preliminary studies on C. pneumoniae infected THP1 monocytic cell lines showed negligible effect of shear stress on cytokines including TNFα, IFN-γ, IL-6, but had a profound influence on IL-1β secretion (Fig. S1). Other cytokines including GM-CSF, G-CSF, IL-10 and IL-12 were below detectable limits (data not shown). Hence in this work, we examined the effect of physiological levels of shear stress on release of IL-1β from primary human monocytes infected with C. pneumoniae. IL-1β is an important cytokine causative in a number of systemic inflammatory diseases including rheumatoid arthritis and atherosclerosis, and is also an important contributor to atherogenesis (Sims and Smith 2010). Activated monocytes/macrophages are considered to be a major source of IL-1β in vivo (Moyer et al., 1991). IL-1β triggers the release of other cytokines and chemokines promoting a chronically pro-inflammatory state, characteristic of atherosclerosis (Yin et al., 2013). Hence, elucidating the role of biophysical forces may help understand the inflammatory response to an infection in the vasculature, and disease progression.
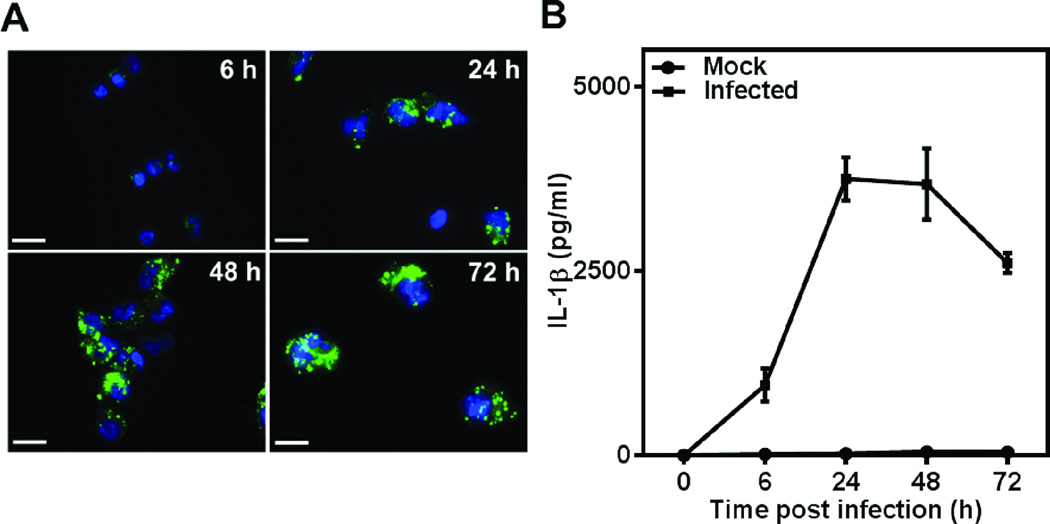
C. pneumoniae has tropism for monocytes/macrophages in vivo (Kaukoranta-Tolvanen et al., 1996; Boman et al., 1998). We monitored the kinetics of C. pneumoniae infection in primary human monocytes in vitro. Our isolation protocol consistently produced a monocyte fraction of high purity (~90%), as measured by CD14 expression by flow cytometry (data not shown). Upon infection, C. pneumoniae forms inclusions in the cytoplasm of primary human monocytes, which grows in size over a period of 72 h, and occupies almost the entire cell volume (Fig. 1A). We observed at a multiplicity of infection (MOI) 1, the infectivity was 80–90%, and the monocytes adhered and spread on tissue culture surfaces, which is characteristic of a macrophage phenotype. The inclusions appeared much bigger and denser compared to those in the THP1 monocytic cell line, described previously by Evani et al. (2013a), reflecting more robust bacterial activity in primary monocytes than in THP1 cells. We observed a steady increase in the production of pro-inflammatory IL-1β during the initial course of infection, which saturates as the infection is established in the host (Fig. 1B).
Figure 1.
C. pneumoniae infection of human monocytes and IL-1β secretion. (A) Human monocytes were infected with C. pneumoniae at MOI 1 and cultured for up to 72 hours. The adherent cells were fixed at different time points and stained with anti-Chlamydia antibody and DAPI. Cell nuclei (blue), and Chlamydial inclusions (green) are shown. Magnification is 1000×; Scale bar is 12 µm. (B) Kinetics of IL-1β release from C. pneumoniae- infected human monocytes. The supernatants from infected human monocytes were collected at 6, 24, 48 and 72 h and analyzed for IL-1β. The results are expressed as mean ± SD of a representative experiment performed in triplicates for one donor.
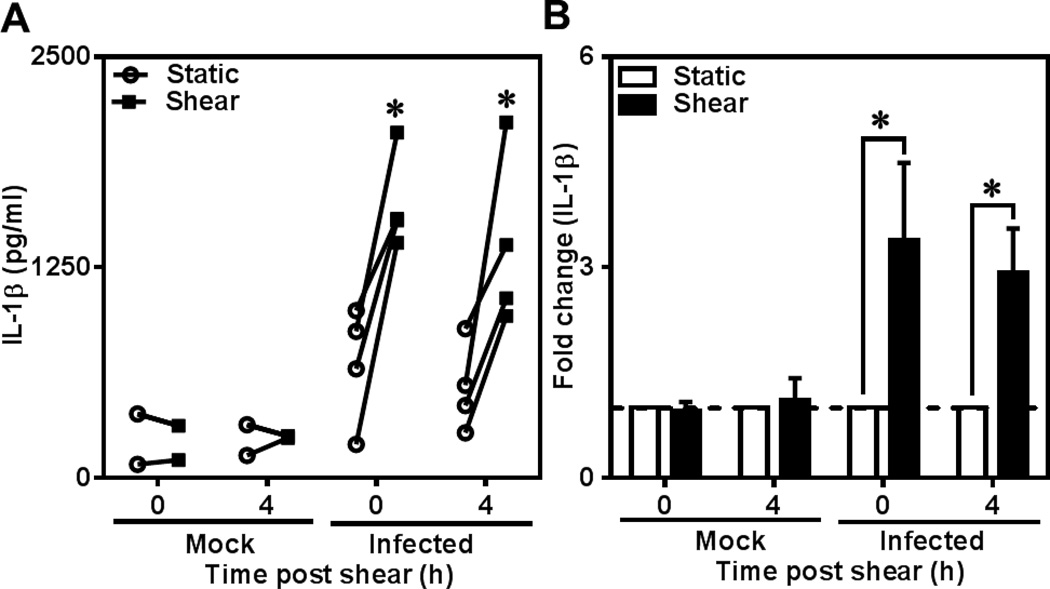
Having established that C. pneumoniae infection triggers a pro-inflammatory response with high levels of IL-1β, we sought to examine the effect of shear stress on IL-1β production by Chlamydia- infected monocytes. Monocytes have been shown to be the vectors for the transport of C. pneumoniae from the lungs to atheroprone sites. As the infected monocytes escape into circulation, they experience hydrodynamic shear stress due to blood flow. To simulate the biological events in vitro, we subjected human monocytes infected for 48 h to shear stress of 5 dyn/cm2 for 1 h as representative of physiological levels of bulk shear stress experienced by the cells in circulation. We chose 48 h post infection as a representative of late time point during chlamydial infection cycle, where the bacterium is well established and takes control of the host cell machinery. Uninfected monocytes exposed to shear, and infected monocytes under static conditions were used as controls. We measured freshly expressed IL-1β levels at 0 h and 4 h after exposure to shear stress so as to capture the effect of shear stress on both the release of existing and de novo synthesized IL-1β, respectively. As shown in Fig. 2A, the uninfected controls showed very low levels of IL-1β production under both static and shear conditions. Interestingly, we observed that shear stress did not have any effect on IL-1β release from uninfected cells but only infected cells responded to applied shear stress with the release of IL-1β. The IL-1β production increased 3–4 fold due to shear exposure (Fig. 2B). There was a slight decrease in the cytokine level after 4 h shear compared to immediately after shear exposure. The increase in IL-1β secretion due to shear stress in infected cells may contribute to the increase in chemokine production and subsequent monocyte chemotaxis as reported in our previous study on effect of shear stress on chemokine production by Chlamydia pneumoniae -infected monocytes (Evani et al., 2013a). We also analyzed the secretion of IL-1β from uninfected and infected cells subjected to a shear stress of 15 dyn/cm2, which is at the higher end of the physiological shear stress range. We observed that 15 dyn/cm2 resulted in an increase in IL-1β production only from infected monocytes and this increase was quantitatively similar to that of 5 dyn/cm2 (Fig. S2).
Figure 2.
Effect of shear stress on IL-1β secretion from C. pneumoniae-infected monocytes. Human monocytes were infected with mock (media only) or C. pneumoniae EB (MOI 1) for 2.5 h. 48 h post infection, uninfected and infected cells at a concentration of 6×106 cells/ml were sheared for 1 hour at 0 (static) and 5 dyn/cm2 (shear) using a cone-and-plate viscometer. After shear exposure, the cells were incubated for further 0 or 4 h, and supernatants and cell lysates were analyzed for IL-1β by ELISA. The results are expressed as actual levels (A) and fold increase (B) from one representative experiment performed for four donors and statistical difference was evaluated using two-way ANOVA (*p<0.05).
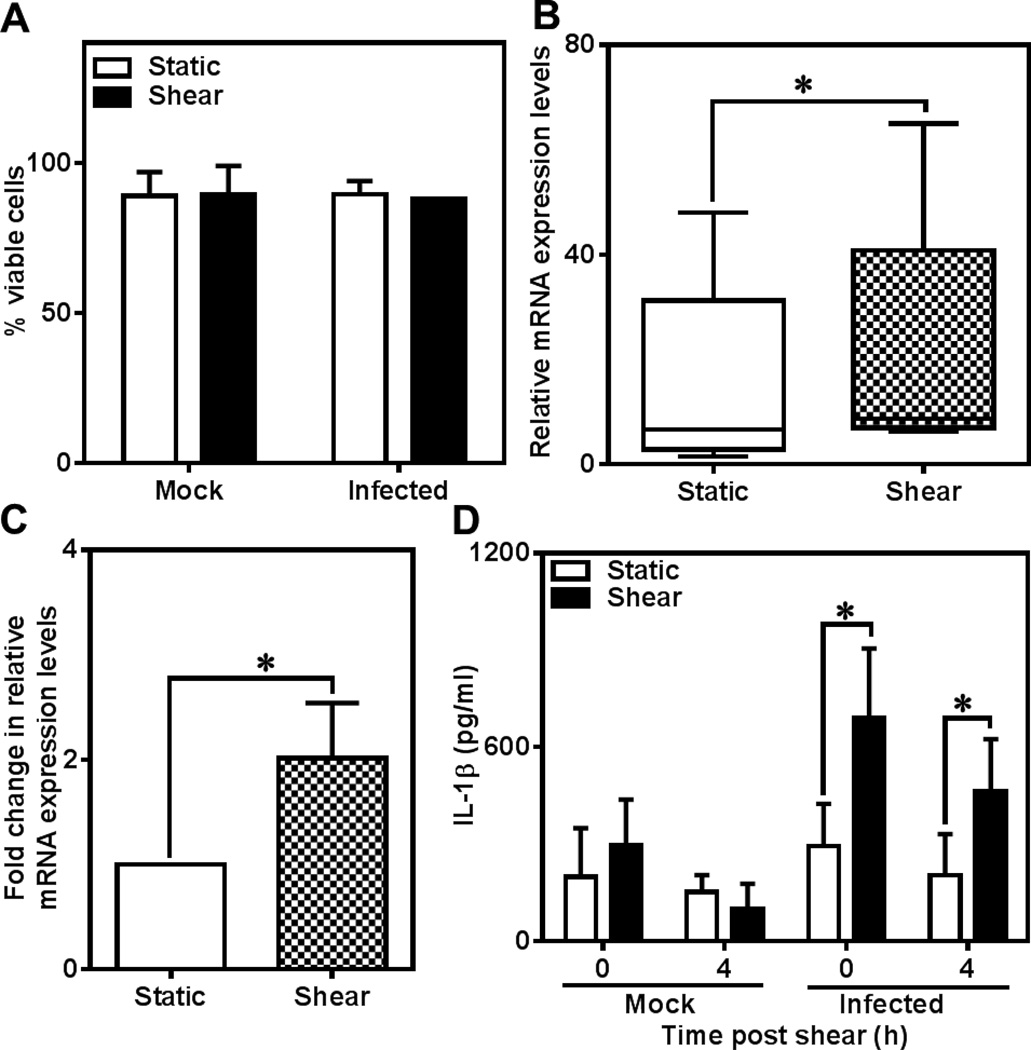
Next, we investigated the source of increased IL-1β secretion due to shear stress. We ruled out the possibility of passive leakage of IL-1β due to compromised membrane integrity during shear exposure by trypan blue exclusion since cells remained viable and intact (Fig. 3A). Next, we analyzed changes in the IL-1β mRNA levels by reverse transcription polymerase chain reaction (RT-PCR). IL-1β is synthesized as pro-IL-1β which is cleaved by inflammasome before being released as functional IL-1β. As expected, we found that infection itself triggered the expression of IL-1β gene when compared to uninfected cells, which served as baseline with measurable mRNA levels (Fig. 3B). We observed that the actual levels of mRNA showed considerable variability possibly due to the differences in the susceptibility of the donor monocytes to infection. We also found that shear stress upregulated IL-1β gene expression by 2-fold only in infected cells (Fig. 3B and C) but uninfected cells showed no measurable IL-1β gene expression. This increase directly translated to intracellular IL-1β levels: while uninfected cells had only baseline levels, shear stress increased the levels of the cytokines inside the cells by 2–3 fold (Fig. 3D). The mRNA and intracellular IL-1β levels demonstrate that shear stress upregulates IL-1β at the genetic level in infected monocytes.
Figure 3.
Regulation of IL-1β expression in C. pneumoniae-infected monocytes. Human monocytes were infected with C. pneumoniae EB (MOI 1) for 2.5h. 48 h post infection, 500 µl of cells at a concentration of 6×106 cells/ml was sheared for 1 h at 0 and 5 dyn/cm2 in a cone-and-plate viscometer. The cells were counted and checked for viability using trypan blue assay (A). The results are expressed as mean ± SD of one representative experiment performed for three donors. The cells were collected immediately post shear and intracellular IL-1β and RNA levels were quantified using ELISA and RT-PCR, respectively. The IL-1β levels were below detectable limits for uninfected cells. The results are expressed as actual levels (B) and fold increase (C) of IL-1β mRNA with respect to static, infected and intracellular IL-1β (D) for 5 donors and statistical difference was evaluated using paired t-test.
Our observations suggest that the mechanical stimuli on the infected cell surface are transduced to a secretory inflammatory response only in the presence of an additional operative. A likely candidate may be autocrine/paracrine signaling due to cytokines released from infected monocytes. It has previously been shown that cytokines such as TNFα, IL-1β or IL-13 act in concert with mechanical forces to modulate gene expression patterns in endothelial cells (Sheikh et al., 2005; Huang, et al., 2013a), airway epithelial cells (Huang et al., 2012b), and smooth muscle cells (Desai et al., 2011). On the other hand, uninfected monocytes are unresponsive to shear stress contrasts with another leukocyte subtype, naïve neutrophils, which upon isolation from blood remain unactivated in the presence of shear stress but get activated under static conditions.
In summary, our results show that C. pneumoniae infection of human monocytes creates a pro-inflammatory microenvironment by the secretion of IL-1β which is further elevated by exposure to fluid shear stress created due to blood flow. We also show that this exacerbated production of IL-1β cytokine is due to mechanotransduction at the gene level with marked increase in IL-1β mRNA expression. Taken together, our study addresses the potent role of biomechanical factors in modulating cellular and molecular responses of inflammatory cells in disease progression in the vasculature. While there is enough evidence that infection can be an etiologic factor for cardiovascular events, understanding the underlying mechanisms may lead to novel anti-infective treatments. In a larger context, our results tempt us to speculate that fluid shear stress, i.e., blood flow, may be an important regulator of systemic acute and chronic diseases by controlling local concentrations not only by transport but also their secretion.
Materials and Methods
Human monocytes
Primary human monocytes from buffy coat were isolated as described previously in Evani et al. (2013b). The monocytes were then resuspended in RPMI (ATCC, Manassas, VA) complete media supplemented with 10% FBS (Life Technologies, Grand Island, NY) at a concentration of 1×106 cells/ml and kept at 37°C. The cell viability was estimated using trypan blue exclusion assay (Countess automated cell counter, Life Technologies, Grand Island, NY). Monocyte purity was measured by flow cytometry (BD-FACS Caliber, Becton-Dickinson, San Jose, CA) after staining with FITC- conjugated CD14 antibody with corresponding isotype control (Miltenyi, Auburn, CA).
Chlamydia pneumoniae culture and monocyte infection
Chlamydia pneumoniae TW183 EB was obtained from University of Washington (Seattle, WA), and aliquoted and stored at −80°C. C. pneumoniae stock titer was established and the cells propagated following established protocols (Campbell and Kuo 2009; Evani et al., 2013a). Monocytes were infected with C. pneumoniae EB at multiplicity of infection (MOI) of 1 as described previously by Evani et al. (2013a). To quantify infectivity, the adherent cells at 72 h post infection were fixed in freshly prepared 2% paraformaldehyde, permeabilized with 1× Permwash (BD Biosciences, San Jose, CA), and labeled with C. pneumoniae-specific murine primary antibody TT401 and FITC-conjugated anti-mouse secondary antibody (Abcam, Cambridge, MA). The nuclei were counter-stained with DAPI (Life Technologies, Grand Island, NY) and analyzed using a fluorescence microscope (1000x, Leica DMI6000).
Exposure to shear stress
Human monocytes were either infected with PBS (Mock) or C. pneumoniae EB (Infected) at MOI 1 for 48 h, after which, the wells were placed on ice for 5 min, gently scraped, and separated from the supernatant by centrifugation. The cells were resuspended in PBS containing 20 ug/ml gentamicin at a concentration of 6×106 cells and equilibrated for 1 h at 37°C. 500 µl of this cell suspension was sheared for 1 h at 0 (static) or 5 dyn/cm2, and cells and supernatant were collected either immediately after shear exposure or after incubation for another 4 h at 37°C (Evani et al., 2013a). We used a cone-and-plate viscometer with an angle of 0.5° (DVII+, Brookfield Instruments). The shear stress values are average shear stress experienced by over 500,000 cells in a cone-and-plate viscometer over a 1 h duration. The supernatant was supplemented with the recommended 1× concentration of Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and stored at −80°C for further analysis. The static controls were maintained at conditions similar to shear treatment except for the rotation of the cone. Cell viability was estimated as described above, and for intracellular ELISA assays, the cell lysates were prepared as described below.
IL-1β assay
Supernatant from mock- or C. pneumoniae-infected monocytes was collected, supplemented with the recommended 1× concentration of Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and stored at −80°C for further analysis. For intracellular ELISA, the flasks/wells were incubated on ice for 5 min to detach the adherent cells, gently scraped, separated by centrifugation (10 min, 300×g) and counted. The cells were lysed for intracellular ELISA using RIPA buffer (Thermo Scientific, Rockford, IL) with 1× concentration of Halt protease inhibitor cocktail (Thermo Scientific).The cell lysate was collected by spinning down at 10,000 rpm at 4°C for 10 min and stored at −80°C for further analysis. The supernatant or cell lysates were assayed for IL-1β using Human IL-1β ELISA Set II (BD Biosciences, San Diego, CA) according to manufacturer’s instructions.
Estimation of IL-1β RNA levels
RNA was extracted from the monocytes using the RNeasy Kit (Qiagen, Valencia, CA), and was reverse transcribed to cDNA using TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA) following manufacturer’s instructions. qRT-PCR was performed using SYBR green PCR master mix (Applied Biosystems, Foster City, CA), and the amount of cDNA in the samples for IL-1β gene was determined after normalizing the levels of induction of actin using ABI Prism 7300 system (Applied Biosystems). The expression levels of IL-1β were calculated from the detection threshold (CT) values of IL-1β, and the levels of induction were determined with the ΔΔCT method, where the quantity of each transcript was determined by the equation 2−ΔΔCT. The following primers (Huang et al., 2013b) (Integrated DNA Technologies, Coralville) were used for the experiment: β-actin, sense, 5′-GAGACCTTCAACACCCCAGCC-3′; β-actin, antisense, 5′-GAATCTTCATGAGGTAGTCAG-3′; IL-1β, sense, 5′-AAAAGCTTGGTGATGTCTGG-3′; IL-1β, antisense, 5′-TTTCAACACGCAGGACAGG-3′.
Statistics
All the experiments were performed in either triplicates or at least from three donors under independent conditions. The results are represented as mean ± SD from one representative experiment/ experiments from different donors in the plots. Statistical differences between treatments were evaluated as paired tests either using one-tailed Student’s t-test, or two-way ANOVA (GraphPad Prism, La Jolla, CA), and the results were considered significant if p<0.05.
Supplementary Material
Figure S1. Effect of shear stress on C. pneumoniae infection of THP1 monocytic cell line. THP1 cells were infected with mock (media only) or C. pneumoniae EB (MOI 2) for 2.5 h. 36 h post infection, uninfected and infected cells at a concentration of 6×106 cells/ml were sheared for 1 hour at 0 (static) and 7.5 dyn/cm2 (shear) using a cone-and-plate viscometer. After shear exposure, the cells were spun down and supernatants were analyzed for cytokines by Bioplex assay as described in Evani et al., 2013a. The results are expressed as actual levels of cytokine responses from one representative experiment performed in triplicates and statistical difference was evaluated using two-way ANOVA (*p<0.05).
Figure S2. Effect of shear stress (15 dyn/cm2) on IL-1β secretion from C. pneumoniae-infected monocytes. Human monocytes were infected with mock (media only) or C. pneumoniae EB (MOI 1) for 2.5 h. 48 h post infection, uninfected and infected cells at a concentration of 6×106 cells/ml were sheared for 1 hour at 0 and 15 dyn/cm2 using a cone-and-plate viscometer. After shear exposure, the cells were spun down and supernatants & cell lysates were analyzed for IL-1β by ELISA. The results are expressed as fold increase from experiments performed for four donors and statistical difference was evaluated using two-way ANOVA (*p<0.05).
Acknowledgments
This work was supported by grant HL112629 from the NIH.
Footnotes
Conflict of interest: none
References
- Boman J, Soderberg S, Forsberg J, Birgander LS, Allard A, Persson K, Jidell E, Kumlin U, Juto P, Waldenstrom A, Wadell G. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis. 1998;178(1):274–277. doi: 10.1086/517452. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC. Chlamydia pneumoniae-an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2(1):23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC. Cultivation and laboratory maintenance of Chlamydia pneumoniae. Curr Protoc Microbiol. 2009;Chapter 11(Unit11B):11. doi: 10.1002/9780471729259.mc11b01s12. [DOI] [PubMed] [Google Scholar]
- Chen R, Xiong S, Yang Y, Fu W, Wang Y, Ge J. The relationship between human cytomegalovirus infection and atherosclerosis development. Mol Cell Biochem. 2003;249(1–2):91–96. [PubMed] [Google Scholar]
- Desai LP, Wu Y, Tepper RS, Gunst SJ. Mechanical stimuli and IL-13 interact at integrin adhesion complexes to regulate expression of smooth muscle myosin heavy chain in airway smooth muscle tissue. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L275–L284. doi: 10.1152/ajplung.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evani SJ, Dallo SF, Murthy AK, Ramasubramanian AK. Shear Stress Enhances Chemokine Secretion from Chlamydia pneumoniae-infected Monocytes. Cell Mol Bioeng. 2013a;6(3):326–334. doi: 10.1007/s12195-013-0291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013b;27(8):3017–3029. doi: 10.1096/fj.12-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieffers J, G. van Zandbergen G, Rupp J, Sayk F, Kruger S, Ehlers S, Solbach W, Maass M. Phagocytes transmit Chlamydia pneumoniae from the lungs to the vasculature. Eur Respir J. 2004;23(4):506–510. doi: 10.1183/09031936.04.00093304. [DOI] [PubMed] [Google Scholar]
- Huang RB, Gonzalez AL, Eniola-Adefeso O. Laminar shear stress elicit distinct endothelial cell E-selectin expression pattern via TNFα and IL-1β activation. Biotechnol Bioeng. 2013a;110(3):999–1003. doi: 10.1002/bit.24746. [DOI] [PubMed] [Google Scholar]
- Huang TT, Chong KY, Ojcius DM, Wu YH, Ko YF, Wu CY, Martel J, Lu CC, Lai HC, Young JD. Hirsutella sinensis mycelium suppresses interleukin-1β and interleukin-18 secretion by inhibiting both canonical and non-canonical inflammasomes. Sci Rep. 2013b;3:1374. doi: 10.1038/srep01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Crawford M, Higuita-Castro N, Nana-Sinkam P, Ghadiali SN. miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J. 2012;26(8):3351–3364. doi: 10.1096/fj.11-199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayoglu MV, Perkins BN, Byrne GI. Chlamydia pneumoniae-infected monocytes exhibit increased adherence to human aortic endothelial cells. Microbes Infect. 2001;3:963–969. doi: 10.1016/s1286-4579(01)01458-7. [DOI] [PubMed] [Google Scholar]
- Kaukoranta-Tolvanen SS, Teppo AM, Laitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996;21(3):215–221. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- Khan S, Okamoto T, Enomoto K, Sakashita N, Oyama K, Fujii S, Sawa T, Takeya M, Ogawa H, Yamabe H, Akaike T. Potential association of Helicobacter cinaedi with atrial arrhythmias and atherosclerosis. Microbiol Immunol. 2012;56(3):145–154. doi: 10.1111/j.1348-0421.2012.00421.x. [DOI] [PubMed] [Google Scholar]
- Krull M, Maass M, Suttorp N, Rupp J. Chlamydophila pneumoniae Mechanisms of target cell infection and activation. Thromb Haemost. 2005;94(2):319–326. doi: 10.1160/TH05-04-0261. [DOI] [PubMed] [Google Scholar]
- Maass M, Bartels C, Engel PM, Mamat U, Sievers Hans-Hinrich Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J Am Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Moyer CF, Sajuthi D, Tulli H, Williams JK. Synthesis of IL-1α and IL-1β by arterial cells in atherosclerosis. Am J Pathol. 1991;138(4):951–960. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106(5):858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- Roulis E, Polkinghorne A, Timms P. Chlamydia pneumoniae: modern insights into an ancient pathogen. Trends Microbiol. 2013;21(3):120–128. doi: 10.1016/j.tim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Sheikh S, Rahman M, Gale Z, Luu NT, Stone PC, Matharu NM, Rainger GE, Nash GB. Differing mechanisms of leukocyte recruitment and sensitivity to conditioning by shear stress for endothelial cells treated with tumour necrosis factor-α or interleukin-1β. Br J Pharmacol. 2005;145(8):1052–1061. doi: 10.1038/sj.bjp.0706281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Tufano A, Di Capua M, Coppola A, Conca P, Cimino E, Cerbone AM, Di Minno G. The infectious burden in atherothrombosis. Semin Thromb Hemost. 2012;38(5):515–523. doi: 10.1055/s-0032-1315759. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Duan Y, Thi MM, You L. An integrative review of mechanotransduction in endothelial, epithelial (renal) and dendritic Cells (osteocytes) Cell Mol Bioeng. 2011;4(4):510–537. doi: 10.1007/s12195-011-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of shear stress on C. pneumoniae infection of THP1 monocytic cell line. THP1 cells were infected with mock (media only) or C. pneumoniae EB (MOI 2) for 2.5 h. 36 h post infection, uninfected and infected cells at a concentration of 6×106 cells/ml were sheared for 1 hour at 0 (static) and 7.5 dyn/cm2 (shear) using a cone-and-plate viscometer. After shear exposure, the cells were spun down and supernatants were analyzed for cytokines by Bioplex assay as described in Evani et al., 2013a. The results are expressed as actual levels of cytokine responses from one representative experiment performed in triplicates and statistical difference was evaluated using two-way ANOVA (*p<0.05).
Figure S2. Effect of shear stress (15 dyn/cm2) on IL-1β secretion from C. pneumoniae-infected monocytes. Human monocytes were infected with mock (media only) or C. pneumoniae EB (MOI 1) for 2.5 h. 48 h post infection, uninfected and infected cells at a concentration of 6×106 cells/ml were sheared for 1 hour at 0 and 15 dyn/cm2 using a cone-and-plate viscometer. After shear exposure, the cells were spun down and supernatants & cell lysates were analyzed for IL-1β by ELISA. The results are expressed as fold increase from experiments performed for four donors and statistical difference was evaluated using two-way ANOVA (*p<0.05).