Abstract
Background
Direct acting anti-HCV drugs have demonstrated a high cure rate and favorable tolerability. The development of shorter courses of therapy may improve affordability and adherence. Sofosbuvir and ledipasvir together with ribavirin have yielded high efficacy when administered for 8, but not for 6 weeks. We hypothesized that addition of a third potent directly acting antiviral to sofosbuvir and ledipasvir would allow for shortened durations of therapy.
Methods
In this single center, open-label cohort, phase 2 atrial, sixty HCV GT-1 treatment naïve patients were sequentially enrolled onto three arms and treated with 12 weeks of sofosbuvir and ledipasvir (an NS5B nucleotide polymerase inhibitor and an NS5A inhibitor, respectively) (n=20); or 6 weeks with sofosbuvir, ledipasvir, and GS-9669 (a non-nucleoside NS5B inhibitor) (n=20) or 6 weeks with sofosbuvir, ledipasvir and GS-9451 (an NS3/4A protease inhibitor) (n=20). Patients and investigators were unmasked to treatment assignment. The primary efficacy analysis was SVR12 (HCV RNA less than the level of quantitation 12 weeks after treatment completion).
Findings
All subjects treated with sofosbuvir and ledipasvir for 12 weeks achieved SVR12 (95%CI: 83–100%). Nineteen of 20 patients (95% CI: 75–100%) treated with sofosbuvir, ledipasvir and GS-9669 achieved SVR12, with 1 patient relapsing 2 weeks after completion of therapy. Nineteen of 20 patients (95% CI: 75–100%) treated with sofosbuvir, ledipasvir, and GS-9451 for 6 weeks achieved SVR12, one patient was lost to follow up after achieving SVR4. There were no discontinuations of treatment due to adverse events.
Interpretation
In this small proof of concept study, two different three drug regimens administered for 6 weeks resulted in high cure rates for HCV infection with excellent tolerability.
Funding
NIAID, National Cancer Institute and Clinical Center Intramural Program. Clinical Trials.gov number NCT01805882. The study was also supported in part by the German Research Foundation (DFG) by the clinical research unit KFO 129 and a Collaborative Research and Development Agreement between NIH and Gilead Sciences.
INTRODUCTION
An estimated 185 million people in the world are infected with hepatitis C.1 Up to 20% of these patients develop cirrhosis and one-quarter of those will progress to end stage liver disease or hepatocellular carcinoma.2 Until late 2013, the armamentarium for treating hepatitis C genotype-1 (GT-1) has required combination therapy with pegylated interferon, ribavirin and more recently a directly acting agent for upto one year.3–5 These regimens have been difficult to tolerate due to adverse effects associated with each constituent of the triple drug regimens, with efficacy ranging from 56 to 88%.4, 5
In 2013, two new directly acting agents, sofosbuvir and simeprevir, were licensed for the treatment of HCV as part of combination regimens. Recent studies utilizing the combination of sofosbuvir with the antiviral ledipasvir as a one pill per day regimen for 12 weeks, demonstrated high sustained viral response (SVR) rates of 91–100%.6, 7 Regimens that allow for a short duration of therapy with low pill burdens and few adverse effects may improve patient adherence and thus benefit the individual.
An attempt to reduce the duration of therapy to 6 weeks by adding ribavirin to sofosbuvir and ledipasvir resulted in a large numbers of patients who relapsed after therapy.8 We hypothesized that addition of a third potent directly acting antiviral drug instead of ribavirin to the regimen of sofosbuvir and ledipasvir could allow for shorter, efficacious therapy. Thus, we conducted a 3 arm clinical trial, in a predominantly African American population, evaluating a two drug combination of sofosbuvir and ledipasvir for 12 weeks and 3 drug combinations of sofosbuvir and ledipasvir plus either GS-9669, a non-nucleoside NS5B thumb site II inhibitor of the HCV polymerase8, 9 or GS-9451, an inhibitor of the HCV NS3/4A protease10 for 6 weeks.
METHODS
Patients and Study Design
Patients were enrolled at a single center, the Clinical Research Center of the National Institutes of Health (NIH), Bethesda, MD, USA. Enrollment and follow-up data from January 2013 to December 2013 are reported here. Eligible participants were men and women, 18 years of age or older, infected with chronic HCV GT-1 infection (serum HCV RNA ≥ 2000 IU/mL). Patients with cirrhosis were excluded from those treatment groups that received 6 weeks of therapy. The presence or absence of cirrhosis was determined by liver biopsy or by a combination of Fibrosure test plus AST to platelet ratio (APRI). Liver biopsy was required in cases of equivocal Fibrosure results. Full eligibility criteria, are included in the Supplementary Appendix 1. Written or oral informed consent was obtained from all participants.
Study Design
Patients were sequentially enrolled into three groups. Patients were contacted for screening visits and start of study drug in the order in which patients initially contacted the NIAID study team with interest and completed eligibility requirements, respectively. A substudy to measure early viral kinetics and pharmacokinetics in 10 patients in each treatment group was offered to all patients at the time of enrollment until substudy enrollment was completed. In the first group, 20 patients were treated for 12 weeks with sofosbuvir and ledipasvir. In the second group, 20 patients were treated for 6 weeks with sofosbuvir, ledipasvir, and GS-9669. In the third group, 20 patients were treated for 6 weeks with sofosbuvir, ledipasvir and GS-9451. Sofosbuvir at a dose of 400 mg and ledipasvir at a dose of 90 mg was administered as a single combination tablet taken once daily. GS-9669 was administered at a dose of 500 mg (two 250mg tablets) once daily and GS-9451 at a dose of 80 mg (single tablet) daily. Criteria for stopping study medications were failure to achieve a >2log10 decline in HCV RNA at week 4). The protocol permitted participants who failed treatment the option of treatment with the current standard of care, which at the time of the study was pegylated-interferon, ribavirin and sofosbuvir. This was later amended with an option for re-treatment with sofobuvir and ledipasvir for 12 weeks for patients who failed the 6 week treatment regimens. Neither patient nor investigators were blinded.
Study Oversight
The study was approved by the institutional review board of the National Institute of Allergy and Infectious Diseases (NIAID) and was conducted in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki and regulatory requirements. The Regulatory Compliance and Human Participants Protection Branch of NIAID served as the study sponsor and medical monitor. Gilead Sciences Inc. provided drug and scientific advice.
Efficacy Assessments
Plasma HCV RNA levels were measured at all time points using the real time HCV Assay (Abbott), with a LLOQ of 12 IU/mL and a lower limit of detection (LLOD) of 3 IU/mL. Serum HCV RNA levels were also measured using the COBAS TaqMan HCV RNA assay, version 2.0 (Roche), with a LLOQ of 43 IU/mL and a LLOD of 15 IU/mL at select time points.
Safety Assessments
Adverse events and clinical laboratory results were recorded throughout the study. Adverse events were graded from 1(mild) to 4 (severe) according to the NIAID Division of AIDS (DAIDS) toxicity table (version 1.0). Pill counts were performed at multiple time points during treatment.
Viral Kinetics
During the first month of treatment, HCV RNA levels were measured at Day 0,1,3,5,7,10,14, 21 and 28 in all patients. Very early viral kinetics (VK) were obtained in a subset of 29 of the 60 participants (ten in group one; ten in group two; nine in group three) by measuring HCV RNA levels at 0, 1, 2, 4, 8, 12, 24, and 36 hours after administration of the first dose of study medications.
IL28B and IFNL4 Genotyping
Whole blood was collected using PAX gene Blood DNA tubes (Qiagen) and stored at −80C until DNA extraction using the Paxgene Blood DNA Kit (PreAnalytiX, a Qiagen/BD Company). IL28B and IFNL4 genotype was determined on DNA specimens using the 5′ nuclease assay with IL28B and IFNL4 allele specific TaqMan probes (ABI TaqMan allelic discrimination kit) and the ABI7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). Genotyping of variants at the rs12979860 (referred to as IL28B genotype) and rs368234815 (IFNL4) loci was performed with custom TaqMan assays as previously described.7
Liver biopsy
An optional research liver biopsy after treatment completion (within two weeks of drug cessation) was offered to all participants who had a pre-treatment liver biopsy for staging and eligibility performed at the NIH Clinical Center. Histopathological assessments of post-treatment liver biopsies were performed by a single pathologist with liver expertise in a non-blinded fashion at the time of biopsy and staged according to the Knodell histological activity index (HAI).11
Clinical End Points
The primary efficacy end point was the proportion of participants with plasma HCV viral load below the level of quantification 12 weeks after treatment completion (SVR12). The primary safety endpoint was the frequency and severity of adverse events. Secondary endpoints that have been completed and included are the proportion of participants with unquantifiable HCV viral load at specified time points during and after treatment discontinuations due to adverse events, safety laboratory changes and evaluation of HCV resistance mutations in the patient who relapsed. Other uncompleted secondary endpoints are not reported. A post-hoc comparison of viral kinetics between treatment arms was also performed. Data through SVR12 is included here with follow up through 48 weeks post-treatment ongoing.
Modeling Viral Kinetics
Viral kinetic (VK) modeling with a multiscale model12, 13 was performed in all participants who participated in the study as described in Supplementary Appendix 1.
Deep Sequencing
Deep sequencing of the HCV NS5A and NS5B genes was performed in samples collected at baseline and time of virologic failure the patient who relapsed by DDL (DDL Diagnostics Laboratory, Rijswijk, Netherlands.
Statistical Analysis
The primary efficacy and safety analyses were based on an intention to treat population (all patients who received at least one dose of study medication). Sample size was calculated to provide both a sufficiently high probability of observing at least one adverse event of probability ≥10% and with pre-specified (CI) confidence intervals for estimates of efficacy assuming 20 patients in each treatment group. With 20 patients in each treatment group, if the true probability of an adverse event due to a regimen is 10% or more, a sample size of 20 allows an 88% chance of observing at least one such adverse event. With a sample size of 20 if all patients achieved SVR12 the 95% confidence interval for that estimate is 83–100% and if 19 patients achieved SVR 12 the 95% confidence interval for that estimate is 75–100%. The proportion of patients with an SVR 12 weeks after completion of therapy was calculated. Baseline demographics were compared using Kruskall-Wallis test for continuous outcomes and Chi-squared tests for binary outcomes. Estimated decline in HCV viral load between arms was compared using a Kruskal-Wallis test and for significant values multiple comparisons were made between samples using the Conover-Inman14 procedure including correction for multiple tests. Analyses were performed using BiAS, PRISM 6.0, SAS, STAT-CRUNCH, and S-Plus 8.0.
Role of the funding source
Data collection, review and analysis were performed by NIH investigators. All sponsors participated in the study design and writing of the report. NIH affiliated investigators had full access to all data in the study, and A.K. and the corresponding author had final responsibility for the decision to submit for publication.
RESULTS
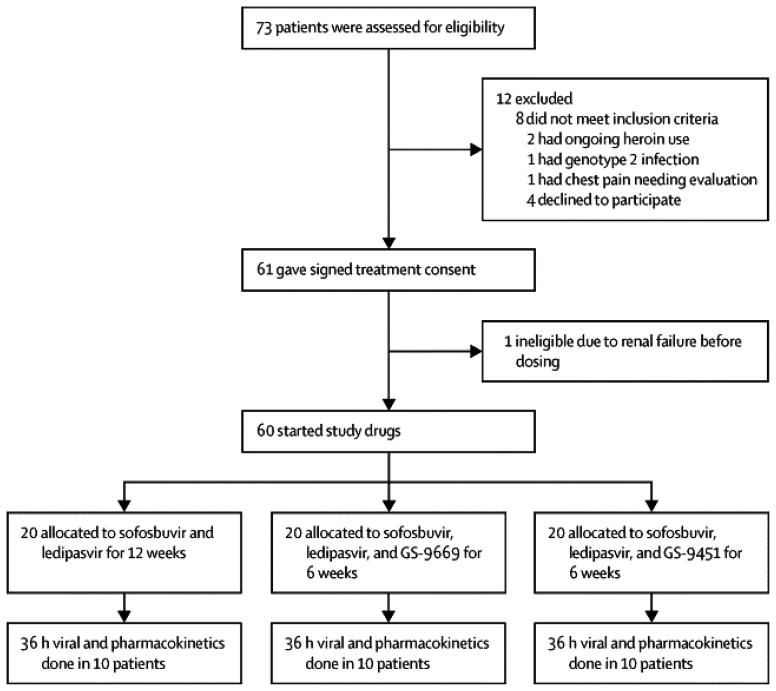
Seventy-three participants were screened and 60 were enrolled in this study (Figure 1).
Figure 1. Patient Disposition.
Screening and enrollment of the study. No subjects discontinued the study after starting study drugs. One patient had renal insufficiency due to NSAID use for an injury and did not receive study drugs.
BASELINE CHARACTERISTICS OF PARTICIPANTS
Baseline characteristics were similar among treatment groups in the study. Participants were predominantly black (88%; 53/60), male (72%; 43/60), had IL28 non-CC genotype (80%; 48/60), were infected with HCV GT-1a (70%; 42/60), and had high baseline plasma HCV RNA levels (>800,000 IU/mL) (70%; 42/60). Twenty-five percent (15/60) of patients had Stage 3 liver disease. Fifteen percent (3/20) treated with sofosbuvir and ledipasvir had Stage 4 disease.(Table 1)
Table 1.
Baseline Demographics and Clinical Characteristics
| Sofosbuvir + Ledipasvir n=20 |
Sofosbuvir + Ledipasvir + GS-9669 n=20 |
Sofosbuvir + Ledipasvir + GS-9451 n=20 |
p-value | |
|---|---|---|---|---|
|
| ||||
| 12 wk | 6 wk | 6 wk | ||
| Age – mean ± standard deviation | 57 ± 8 | 54 ± 7 | 54 ± 9 | 0.28 |
| Male – n(%) | 14 (70) | 13 (65) | 16 (80) | 0.56 |
| Race+ – n(%) | ||||
| Black | 16 (80) | 19 (95) | 18 (90) | 0.32 |
| White | 4 (20) | 1 (5) | 2 (10) | |
| Ethnicity+ – n(%) | ||||
| Hispanic | 1 (5) | 0 | 0 | 0.36 |
| Non-Hispanic | 19 (95) | 20 (100) | 20 (100) | |
| BMI – mean ± standard deviation | 25 ± 4 | 28 ± 7 | 28 ± 6 | 0.16 |
| HCV genotype – n(%) | ||||
| 1a | 11 (55) | 14 (70) | 17 (85) | 0.12 |
| 1b | 9 (45) | 6 (30) | 3 (15) | |
| HCV RNA >800,000 IU/mL – n(%) | 15 (75) | 13 (65) | 14 (70) | 0.79 |
| IL28B genotype – n(%) | ||||
| CC | 5 (25) | 2 (10) | 5 (25) | 0.66 |
| CT | 9 (45) | 10 (50) | 7 (35) | |
| TT | 6 (30) | 8 (40) | 8 (40) | |
| IFNL4 genotype – n(%) | ||||
| TT/TT | 3 (15) | 3 (15) | 5 (25) | 0.68 |
| ΔG/TT | 10 (50) | 10 (50) | 6 (30) | |
| ΔG/ΔG | 7 (35) | 7 (35) | 9 (45) | |
| Knodell HAI, Metavir or Fibrosure Fibrosis Score* – n(%) | ||||
| 0–2 | 12(60) | 15 (75) | 15 (75) | 0.16 |
| 3 | 5 (25) | 5 (25) | 5 (25) | |
| 4 | 3 (15) | 0 | 0 | |
For eligibility 2 (3%) patients had a Fibrosure/APRI, 9 (15%) patients had biopsy scored using Metavir system and 49 (82%) patients had biopsy scored using Knodell HAI system.
Race and Ethnicity was self-reported.
Abbreviations: BMI: Body mass index, SD: standard deviation, HCV: hepatitis C virus
VIROLOGICRESPONSE
All patients (20/20; 95%CI: 83–100%) treated with sofosbuvir and ledipasvir had an unquantifiable HCV RNA (Roche Cobas Taqman Assay) 12 weeks after the completion of therapy. Ninety-five percent (19/20; 95%CI: 75–100%)) of patients treated with sofosbuvir, ledipasvir and GS-9669 or GS-9451 had an unquantifiable HCV RNA 12 weeks after completion of therapy. Five percent of patients (1/20) treated with sofosbuvir, ledipasvir and GS-9669 for 6 weeks relapsed 2 weeks after completion of therapy. This patient was infected with HCV genotype 1a, had stage 3 liver disease, a baseline HCV viral load of 1,922,287 IU/ml and IL28B CT genotype. In addition, this patient had 7% of M28T and 13% of Q30H NS5A resistance variants at baseline. At relapse, a double mutant M28T+Q30H was detected, which is associated with >1000-fold reduced susceptibility to ledipasvir. In contrast, neither sofosbuvir resistance variant S282T nor GS-9669 resistance variants were detected at relapse.
One patient (1/20) treated with sofosbuvir, ledipasvir and GS-9451 was incarcerated after having achieved SVR4. A special session of the NIAID IRB deemed that data obtained after incarceration could not be included for publication. Eighty-five percent of (17/20) participants treated with sofosbuvir and ledipasvir had an unquantifiable level of HCV RNA by week 4 of treatment. All participants treated with the three drug combination of sofosbuvir, ledipasvir and GS-9669 or sofosbuvir, ledipasvir and GS-9451 had an unquantifiable level of HCV RNA by week 4 of treatment (Table 2).
Table 2.
Patients With Hepatitis C Virus RNA Lower than the Level of Quantification*
| Time Point | Sofosbuvir + Ledipasvir n=20 |
Sofosbuvir + Ledipasvir + GS-9669 n=20 |
Sofosbuvir + Ledipasvir + GS-9451 n=20 |
|---|---|---|---|
|
| |||
| 12 wk | 6 wk | 6 wk | |
| During Treatment –n (% [95% CI]) | |||
| Day 7 | 2 (10[1–31]) | 0 0 |
5 (25[9–49]) |
| Week 2 | 6 (30[12–54]) | 6 (30[12–54]) | 9 (45[23–68]) |
| Week 4 | 17 (85[62–97]) | 20 (100[83–100]) | 20 (100[83–100]) |
| Week 6 | – | 20 (100[83–100]) | 20 (100[83–100]) |
| Week 8 | 20 (100[83–100]) | – | – |
| Week 12 | 20 (100[83–100]) | – | – |
| Post Treatment Period –n (%[95% CI]) | |||
| Week 4 | 20 (100[83–100]) | 19 (95[75–100]) | 20 (100[83–100]) |
| Week 12 | 20 (100[83–100]) | 19 (95[75–100]) | 19** (95[75–100]) |
The limit of HCV RNA quantification was 43 IU/mL
one patient who achieved SVR4 was incarcerated subsequent to this visit. Although records were available at 12 weeks post-therapy, a special session of the NIAID IRB including a prisoner representative decided that data from subsequent time points could not be reported.
Rapid HCV viral kinetic response to sofosbuvir, ledipasvir and GS-9451
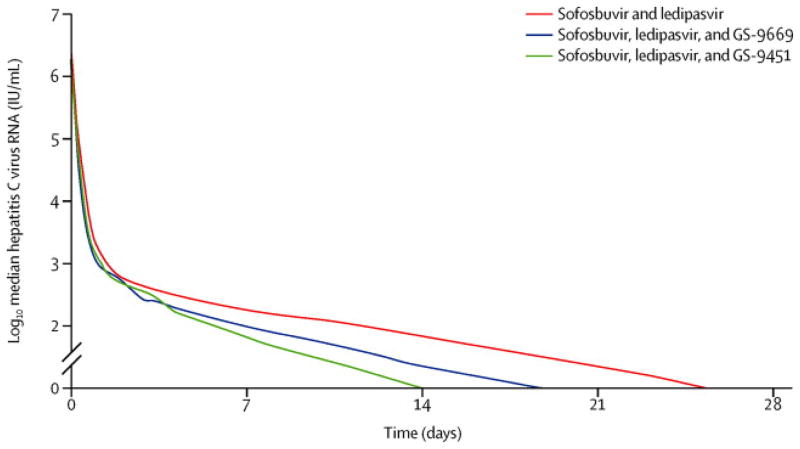
There was a rapid, sustained decline in HCV RNA among all three groups. Viral kinetic modeling shows a three-phase decline, with a moderate decay during an intermediate phase between a rapid first phase and a slower third phase (Figure 2). This form of decay can be modeled and fitted in each patient (Supplemental Figure S1). We examined whether there was a significantly faster HCV viral decline with three drugs versus two drugs. In particular, fitted HCV RNA was significantly lower at early time points in patients treated with sofosbuvir, ledipasvir and GS-9451 compared to the two other regimens (p<0.05 HCV RNA at days 7, 14 and 21) (exact p vales in Supplemental Table S2). Our model estimated the effect of overall treatment on HCV clearance by taking into account the effectiveness of the regimen in blocking HCV production (ε1 and ε2) and intracellular decay (κ). Median overall treatment effect observed in all three regimens were not significantly different (p>0.05; sofusbuvir and ledipasvir - 99.977%, sofosbuvir, ledipasvir and GS-9669 – 99.954%, sofosbuvir, ledipasvir and GS-9451–99.984%). Nevertheless, there were more patients in the group treated with sofosbuvir, ledipasvir and GS-9451 who achieved a maximum threshold effect of 99.95% or higher in clearing plasma HCV, than there were in patients treated with the other regimens (p=0.09).
Figure 2. Decline in Median HCV Viral Load Day 0–28.
Decline in HCV VL after initiation of study drugs is shown. There was a significant difference in HCV RNA at Day 7, 14 and 28 for subjects who received sofosbuvir, ledipasvir and GS-9451 (green) compared to those who received sofosbuvir, ledipasvir and GS-9669 (blue) or sofosbuvir and ledipasvir (red) (p<0.05).
SAFETY
All 60 patients completed treatment. The most common adverse events were diarrhea, headache and fatigue (Table 3). Most adverse events were mild in severity. The two grade 3 adverse events that occurred were pain related to a post-treatment research liver biopsy and an episode of vertigo requiring hospitalization in a patient with a history of severe intermittent episodes of vertigo. No grade 4 laboratory abnormalities occurred. Eleven grade 3 laboratory abnormalities occurred in nine patients. Grade 3 hyperglycemia and hypoglycemia occurred in one patient with a history of insulin-dependent diabetes mellitus. Two patients had asymptomatic hypophosphatemia. One patient with a history of anemia had transient decreased hemoglobin to 8.9 g/dL which improved to baseline (≥10 g/dL) prior to completion therapy. Three patients treated had transiently elevated serum creatinine levels. Two elevations occurred after completion of study drugs, one in a patient with baseline renal insufficiency who reported dehydration at week 8 (baseline GFR: 56 mL/min/1.73m2; week 8: 25 mL/min/1.73m2; week 10 without intervention: 68 mL/min/1.73m2) and another in a patient who initiated 1600 mg/day of ibuprofen for arthritis (baseline GFR: 96 mL/min/1.73m2; Week 6: 35 mL/min/1.73m2; Week 7: 90 mL/min/1.73m2). A third patient with baseline renal insufficiency had transient worsening of renal function on therapy that resolved without intervention (baseline GFR: 66 mL/min/1.73m2; Day 7: 35 mL/min/1.73m2; Day 9: 59 mL/min/1.73m2). One patient treated with sofosbuvir and ledipasvir developed an isolated elevation of ALT and AST at week 4, which peaked at 94 and 230 respectively at week 5 with a normal bilirubin. Work up for autoimmune, infectious and toxin induced causes of elevated transaminases was unremarkable; however at week 5, the patient revealed that she had been eating multiple grapefruit daily for the previous four weeks, which has a potential interaction with the atypical antipsychotic lurasidone the patient was taking concomitantly. The patient discontinued grapefruit and ALT and AST declined to pre-therapy levels by week 6 without interruption of study drug.
Table 3.
Adverse Events and Laboratory Abnormalities During Treatment Period
| Sofosbuvir + Ledipasvir for 12 weeks (n=20) | Sofosbuvir + Ledipasvir + GS-9669 for 6 weeks (n=20) | Sofosbuvir + Ledipasvir + GS-9451 for 6 weeks (n=20) | |
|---|---|---|---|
| Any adverse event during treatment –n(%) | 20 (100) | 20 (100) | 20 (100) |
| Any serious adverse event during treatment - n(%) | 0 | 0 | 2(10) |
| Common adverse events** - n(%) | |||
| Night sweats | 0 | 2 (10) | 0 |
| Constipation | 0 | 2 (10) | 1 (5) |
| Vomiting | 1 (5) | 2 (10) | 0 |
| Nausea | 1 (5) | 2 (10) | 1 (5) |
| Shoulder pain | 2 (10) | 0 | 0 |
| Common cold | 4 (20) | 1 (5) | 1 (5) |
| Fatigue | 2 (10) | 2 (10) | 4 (20) |
| Diarrhea | 1 (5) | 5 (25) | 3 (15) |
| Headache | 5 (25) | 5 (25) | 0 |
| Rash | 3 (15) | 1 (5) | 0 |
| Abdominal pain | 2 (10) | 2 (10) | 0 |
| Back Pain | 2 (10) | 0 | 1 (5) |
| Laboratory abnormalities | |||
| Any Grade 3 abnormality during treatment - n (%) | 4 (20) | 2 (10) | 4 (20) |
| Hypophosphatemia | |||
| Grade 3 | 0 | 2 (10) | 0 |
| Elevated serum creatinine | |||
| Grade 3 | 0 | 0 | 3 (15) |
| Decreased hemoglobin | |||
| Grade 3 | 0 | 0 | 1 (5) |
| Elevated ALT | |||
| Grade 3 | 1 (5) | 0 | 0 |
| Elevated AST | |||
| Grade 3 | 1 (5) | 0 | 0 |
| Elevated LDL | |||
| Grade 3 | 1 (5) | 0 | 0 |
| Hyperglycemia | |||
| Grade 3 | 1 (5) | 0 | 0 |
| Hypoglycemia | |||
| Grade 3 | 1 (5) | 0 | 0 |
Treatment period includes time on study medication and 30 days after discontinuation
Occurring in ≥10% of patients
DISCUSSION
In the present study, chronic HCV genotype 1 infection in patients without cirrhosis was successfully treated with a 6 week course of a combination of three oral directly acting antiviral agents. The regimens were well tolerated, rapidly suppressed HCV viremia in this patient population, and resulted in high rates of SVR12.
Treatment for HCV infection is changing rapidly to obviate the need for parenteral interferon and oral ribavirin, both associated with numerous toxicities including teratogenicity in the case of ribavirin. Single directly acting antiviral drugs, including sofosbuvir, were initially used in combination with pegylated interferon and ribavirin for the treatment of HCV genotype 1 infection, resulting in improved efficacy.3, 15, 16 Recently, the prospect of efficacious interferon- and ribavirin-free regimens has been realized6, 17–20. In this regard, certain interferon-free regimens have been remarkably well tolerated, but the long duration of therapy (12–24 weeks) has raised concerns about adherence and expense21. Attempts to shorten the duration of therapy to 6 weeks with the combination of sofosbuvir, ledipasvir and ribavirin resulted in SVR rates of 68% (95% CI: 47–85) in a small sample size study, considerably less than that observed with 8 (95% CI: 90–97) or 12(95% CI: 92–98) weeks of sofosbuvir and ledipasvir alone.22 Two studies have examined regimens of 8 weeks duration. In the first, 8 weeks of sofosbuvir and ledipasvir was found this to be non-inferior to 12 weeks of sofosbuvir and ledipasvir.22 In the second smaller study, 8 weeks of ABT-450/ritonavir/ABT-267/ABT-333 and ribavirin resulted in high SVR rates of 86% for patients with HCV GT-1b and 96% for patients with HCV GT-1a infection.23 No formal comparison of 8 versus 12 weeks of this combination was performed in that study. While SVR rates were high in both trials, more relapsers were seen in the groups treated for 8 weeks than for 12 suggesting that efficacy may diminish with these regimens at shorter durations.22, 23 It has not been clear what combinations of antiviral agents and what duration of therapy would be most efficacious, tolerable and cost-effective for patients with various host factors and viral factors.
In the present proof of concept study, the use of three direct acting agents with different mechanisms of action in two separate therapeutic arms of the protocol tested in a monoinfected urban population allowed for a shorter duration of suppressive therapy and resulted in high cure rates and excellent tolerability. Furthermore, viral kinetic modeling suggests that the three drug regimen of sofosbuvir, ledipasvir and GS-9451, targeting three different stages of the HCV lifecycle, resulted in enhanced HCV clearance compared to the other regimens which target only two stages.
We anticipated that detectable plasma HCV RNA at the end of treatment would be predictive of relapse; however, 6 patients with quantifiable HCV RNA at the end of treatment (range: 14–64 IU/mL), using an HCV RNA assay with a lower limit of quantification of 12 IU/mL went on to achieve SVR. The exact mechanism of how NS5A inhibitors, such as ledipasvir, suppress HCV virus is unknown; however, it has been postulated that the drug leads to the production of non-infectious virus particles13. This could possibly explain the detection of quantifiable HCV at end of treatment in patients who subsequently achieve SVR without continued therapy. Another plausible explanation would be a role played by the host innate and/or adaptive immune system in eliminating residual HCV in vivo after completion of therapy.
While 12 weeks of therapy was effective in all patients in this trial, one patient in this trial treated for 6 weeks relapsed two weeks after the completion of therapy. The patient had advanced, stage 3 liver disease and a high baseline HCV viral load, (1,922,287 IU/mL), both of which are predictors of poor response to some therapeutic regimens that contain only direct acting agents.12, 18 In addition this patient was found to have both a double M28T and Q30H mutation in the NS5A region after therapy which is associated with >1000 fold reduced susceptibility to ledipasvir respective compared to wild-type HCV virus. The patient did take 98 percent of his medications as determined by pill counts. Patients with cirrhosis were not included in the 6 week, triple combination arms of the study at this time since these patients may be more difficult to treat18. Given the small numbers of patients with stage 3 liver disease in this study and exclusion of patients with cirrhosis from the 6 week treatment groups, further studies are required to determine whether the 6 week regimens evaluated here can be used as successfully in patients with cirrhosis.
While treatment regimens were generally well tolerated, one patient developed elevated ALT/AST while on sofosbuvir and ledipasvir. The patient was eating multiple grape fruits, which can lead to CYP3A4 inhibition, while also taking lurasidone, an atypical antipsychotic for chronic bipolar disorder. Inhibition of CYP3A4 has been described to increase levels of lurasid one and is thought to have led to this toxicity, given that neither sofosbuvir or ledipasvir are metabolized by CYP3A4.24 Toxicity due to study drug however, cannot completely be excluded without study drug levels which are not available.
This study suggests that use of combination direct acting agent regimens for 6 weeks leads to reasonable treatment outcomes, findings that should be validated in larger trials. Whether therapeutic regimens could be further shortened for patients with specific host or viral parameters and comorbidities merits testing. Limitations of the study include sequential, non-randomized enrollment and that it is a single site trial.. Confidence in the estimates of efficacy are limited by the small numbers of patients included and ability to use only historical comparisons for efficacy. Additionally, patients received intensive nursing support and monitoring which may be hard to replicate in community-based treatment programs for hepatitis C.
In conclusion, 6 week regimens of oral combination direct acting agent therapy appears to be effective in treating patients with early stage chronic HCV. Given the confines of the population studied, we can now speculate that a six week course of a well-tolerated regimen of three directly acting oral antiviral agents may achieve a SVR12 in at least 75%, and perhaps close to 100%, of persons infected with HCV. This short duration, simple therapy for HCV may prove relevant for the global elimination of hepatitis C, where simple, well-tolerated, therapy of short duration is required to ensure adherence.
Supplementary Material
Research in context.
Systematic review
We searched PubMed on May 27, 2014 with a combination of the medical subject heading (MeSH) search terms “HCV treatment” and “antiviral agent” and consulted the HCV treatment guidelines for phase II and III clinical trials evaluating therapies for hepatitis C for patients with genotype-1 hepatitis C virus (HCV). We also searched the reference list of included articles for additional articles meeting inclusion criteria.
Interpretation
Many direct-acting agents are in development, and 16 clinical trials of interferon-free regimens for patients with HCV GT-1 have been published. These trials have shown promising safety and efficacy using combination DAA therapy with or without ribavirin for 8–24 weeks for the treatment of HCV genotype 17, 8, 17–20, 22, 23, 25–32. One other study evaluated a regimen of 6 weeks duration and found an SVR12 rate of 68%8. While this current study is small, we found high SVR12 rates using the regimens included for only 6 weeks which supports the possibility that a short, six week treatment duration may be effective for select patients.
Acknowledgments
We would like to acknowledge the contributions of the following individuals: Katie Watkins BS, Erin Rudzinski BS, and Susan Vogel RN, BSN (clinical monitoring support), Judith Starling PharmD and Lori Gordon (pharmacy), Michelle Chakrabarti BS, Jerome Pierson PhD, John Tierney BSN, MPM (Regulatory support); William Ronnenberg JD/MIP, MS, Richard Williams PhD and Mike Mowatt PhD (technology transfer support), Marc Teitelbaum MD, CPI (sponsor medical monitor), John Powers MD (oversight), Mary Hall (protocol support), Cathy Rehm, Sarah Jones, David Wu B.S., Leighton Daigh B.S. and Jessica Johl B.S. (laboratory support), Senora Mitchell (clinic support).
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. This research was supported in part by the National Institute of Allergy and Infectious Diseases. The study was also supported in part by the German Research Foundation (DFG) by the clinical research unit KFO 129. Study medications were provided by Gilead Sciences, Inc. These entities did not have a role in the writing of the manuscript or decision to submit for publication Study medications were provided by Gilead Sciences, Inc. and the study was partially funded by a Collaborative Research and Development Agreement between NIH and Gilead Sciences. PP, WS, JM, and GMS, employees of Gilead Sciences, participated in the writing of the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
SK and AK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AK and SK performed the literature search. AK, SK, AN, MP, ASF, MAP, and HM contributed to the study design. AK, ZS, MM, SS, TP, KT, EP, AN, DE, RS, CK, CH, TJ, RK, GD, AO, LLB, EGM, RK, MS, GT, RT, JC, SA, DK, and BJW collected data. AK, ZS, MM, SS, DB, EH, and MP analyzed data. AK, AO, LLB, EGM, RK, MS, DB, EH, DK, BJW, ASF, MAP, and HM interpreted data. ZS, MM, SS, TP, KT, DB, and EH contributed to figure design. AK wrote the first draft of the manuscript and all authors participated in the review and critique of the manuscript.
ROLE OF THE SPONSOR
The Regulatory Compliance and Human Participants Protection Branch of the National Institute of Allergy and Infectious Diseases (NIAID) served as the study sponsor and was involved in the review and approval of the study via the usual peer-review process as well as the study management. The Regulatory Compliance and Human Participants Protection Branch did not play a role in the design of the study, data collection and analysis, interpretation of the data, preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLAIMER
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The data from this study have been partially presented at the 64rd Annual Meeting of the American Association for the Study of Liver Diseases: The Liver meeting, Washington D.C., Massachusetts, USA 2013 and Hepdart 2013: Frontiers in Drug Development for Hepatitis C, Big Island, USA Hawaii 2013. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
CONFLICT OF INTEREST STATEMENT
William T. Symonds, Phillip Pang, G. Mani Subramanian and John G. McHutchison are employees of Gilead Pharmaceuticals. Rohit Talwani has served as a speaker for Merck and performs research funded by Vertex pharmaceuticals. Jose Chavez is a Member of the Regional Advisory Boards for Abbott, Bristol-Myers Squibb and Gilead. Gebeyehu Teferi serves on the Gilead and Merck Advisory Boards and as a speaker for Gilead.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Seminars in liver disease. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 3.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, Demicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;13(5):401–8. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 4.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. The New England journal of medicine. 2011;365(11):1014–24. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–16. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski M, Gardiner DF, Rodriguez-Torres M, Reddy R, et al. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. NEJM. 2014;370:211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 7.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–23. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 8.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Subramanian GM, et al. Efficacy of Nucleotide Polymerase Inhibitor Sofosbuvir plus the NS5A Inhibitor Ledipasvir or the NS5B Non-nucleoside Inhibitor GS-9669 Against HCV Genotype 1 Infection. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Gruener LHD, et al. A novel NS5B non-nucleoside thumb site II inhibitor, demonstrates potent antiviral activity, favorable safety profile and potential for once-daily dosing. J Hepatol. 2012;52(Suppl 2):S471. [Google Scholar]
- 10.Lawitz EJ, Hill JM, Marbury T, Demicco MP, Delaney W, Yang J, et al. A Phase I, randomized, placebo-controlled, 3-day, ascending-dose study of GS-9451, an NS3/4A protease inhibitor, in genotype 1 hepatitis C patients. Antiviral therapy. 2013;18(3):311–9. doi: 10.3851/IMP2415. [DOI] [PubMed] [Google Scholar]
- 11.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 12.Guedj J, Neumann AU. Understanding hepatitis C viral dynamics with direct-acting antiviral agents due to the interplay between intracellular replication and cellular infection dynamics. Journal of theoretical biology. 2010;267(3):330–40. doi: 10.1016/j.jtbi.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Guedj J, Dahari H, Rong L, Sansone ND, Nettles RE, Cotler SJ, et al. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):3991–6. doi: 10.1073/pnas.1203110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conover WJ. Practical Nonparametric Statistics. 3. 1999. [Google Scholar]
- 15.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 16.Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: The randomized PILLAR study. Hepatology. 2013;58(6):1918–29. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. The New England journal of medicine. 2013;368(1):34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 18.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;310(8):804–11. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawitz E, Poordad F, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, et al. A phase 2a trial of 12-week interferon-free therapy with two direct-acting antivirals (ABT-450/r, ABT-072) and ribavirin in IL28B C/C patients with chronic hepatitis C genotype 1. J Hepatol. 2013;59(1):18–23. doi: 10.1016/j.jhep.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Everson GT, Sims KD, Rodriguez-Torres M, Hezode C, Lawitz E, Bourliere M, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146(2):420–9. doi: 10.1053/j.gastro.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing Hepatitis C Direct Acting Antivirals, for use in large-scale treatment access programs in developing countries. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. The New England journal of medicine. 2014;370(20):1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 23.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. The New England journal of medicine. 2014;370(3):222–32. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 24.Sunovion. Latuda (lurasidone): package insert. Marlborough: Sunovion; 2010. [Google Scholar]
- 25.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England journal of medicine. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 26.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 27.Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55(3):742–8. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. The New England journal of medicine. 2012;366(3):216–24. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 29.Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. The New England journal of medicine. 2013;368(1):45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Ikeda K, Suzuki F, Toyota J, Karino Y, Chayama K, et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58(4):655–62. doi: 10.1016/j.jhep.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 31.Zeuzem S, Buggisch P, Agarwal K, Marcellin P, Sereni D, Klinker H, et al. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology. 2012;55(3):749–58. doi: 10.1002/hep.24744. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Mullhaupt B, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. The New England journal of medicine. 2013;369(7):630–9. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.