Abstract
Background
A rapid increase in multidrug-resistant Gram-negative infections has led to a reemergence of colistin use globally. Although it is well described among adults, colistin use and its associated toxicities in children are poorly understood. We report findings from the largest case series of pediatric colistin use to date.
Methods
We queried pediatric infectious diseases specialists from the Emerging Infections Network to identify members who had prescribed intravenous colistin within the past 7 years. We collected relevant demographic and clinical data. Bivariate analyses and multivariable logistic regression were performed.
Results
Two hundred twenty-nine pediatric infectious diseases specialists completed the survey (84% response); 22% had prescribed colistin to children. Among respondents, 92 cases of colistin use from 25 institutions were submitted. The most commonly targeted organisms were multidrug-resistant Pseudomonas (67.4%), multidrug-resistant Acinetobacter baumanii (11.9%), carbapenemase-producing Enterobacteriaceae (13.0%) and extended-spectrum β-lactamase producing Enterobacteriaceae (5.4%). Development of resistance to colistin was observed in 20.5% of patients. Additional antimicrobial therapy was administered to 84% of patients, and 22% of children experienced nephrotoxicity (not associated with dosage or interval of colistin prescribed). Renal function returned to baseline in all patients. Children aged ≥13 years had approximately 7 times the odds of developing nephrotoxicity than younger children, even after controlling for receipt of additional nephrotoxic agents (odds ratio 7.16; 95% confidence interval: 1.51–14.06; P = 0.013). Four children exhibited reversible neurotoxicity.
Conclusions
Most pediatric infectious diseases specialists have no experience prescribing colistin. Colistin use in children has been associated primarily with nephrotoxicity and, to a lesser extent, neurotoxicity, both of which are reversible. Emergence of resistance to colistin is concerning.
Keywords: colistin, pediatrics, nephrotoxicity, multidrug-resistant Gram-negative organisms, antibiotics
The emergence of multidrug-resistant (MDR) Gram-negative organisms coupled with a lack of antimicrobials with activity against these bacteria has led to a renewed interest in the antibiotic colistin.1–6 The use of colistin was abandoned in the 1980s in favor of newer antibiotics that were perceived to have improved side-effect profiles; however, the ever-growing problem of bacterial resistance has led to a reemergence of colistin use.7 Most Gram-negative pathogens remain susceptible to colistin, including MDR Acinetobacter baumannii, Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae.8
The efficacy and safety of colistin has been extensively studied in adults; nephrotoxicity and neurotoxicity have been reported to range between 3.5–58% and 0–7%, respectively.9–16 Little is known about colistin use and its associated toxicities in children in the United States. Furthermore, optimal administration strategies (eg, dosing, interval) remain unclear. The objectives of this study were to describe current practices of prescribing colistin to children in the United States and to understand toxicities associated with colistin use in children.
METHODS
Study Design
This is a retrospective case series of colistin use among pediatric patients. To identify cases, we queried pediatric infectious diseases physician members of the Emerging Infections Network in January 2012 to identify members who have cared for children aged ≤18 years who received intravenous colistin within the past 7 years. The Emerging Infections Network consists of 258 pediatric infectious diseases physicians in the United States, practicing in 44 states (at the time of survey distribution). Members who indicated that they had prescribed colistin were requested to complete an electronic data collection form with patient-specific data. No patient identifiers were provided. The study was approved by the Johns Hopkins Hospital Institutional Review Board.
Data Collection
Patients were excluded if they received ≤72 hours of parenteral colistin. Data regarding demographic characteristics of the children receiving colistin, preexisting medical conditions, type of infections, isolated pathogens and travel to or from another country within 30 days before hospital admission were obtained. Additionally, characteristics of colistin treatment (dose, interval and duration), concomitant antibiotic treatment, use of additional potentially nephrotoxic agents, the emergence of colistin resistance, observed adverse events and clinical outcomes were recorded.
Definitions
Nephrotoxicity was defined as a creatinine clearance ≤60 mL/min if creatinine clearance was normal at baseline or a decrease in the category of clearance (mild 50–60 mL/min; moderate 10–50 mL/min; severe <10 mL/min). Use of additional nephrotoxic agents that were queried included amphotericin, aminoglycosides, cyclosporine, acyclovir, vancomycin, cidofovir, cyclophosphamide, methotrexate, cisplatin, nonsteroidal anti-inflammatory medications and intravenous contrast. The emergence of resistance to colistin was defined as a >2-fold increase in the minimum inhibitory concentration of colistin. Clinical cure was defined as resolution of signs and symptoms of infection at the discretion of the treating physician.
Statistical Analysis
Descriptive analyses included median and interquartile range for continuous data and proportions for categorical data. Bivariate analyses were performed using the Fisher exact test for categorical variables. The outcome of nephrotoxicity was assessed using regression analyses adjusting for receipt of additional nephrotoxins and age (selected as confounders a priori). Age was assessed as both a continuous variable and categorical variable (age greater than or less than 13 years). This cutoff was chosen as the constant values used to calculate creatinine clearance changes at age 13 years. Children with and without cystic fibrosis (CF) were compared because of anticipated differing dosing techniques, sites of infection and underlying medical conditions. The number of cases of colistin were regressed over time to determine if there was a significant change in prescription of colistin from 2005 to 2011. Data were analyzed using STATA 11 (STATA corp., College Station, TX) and a 2-sided P value <0.05 was considered statistically significant.
RESULTS
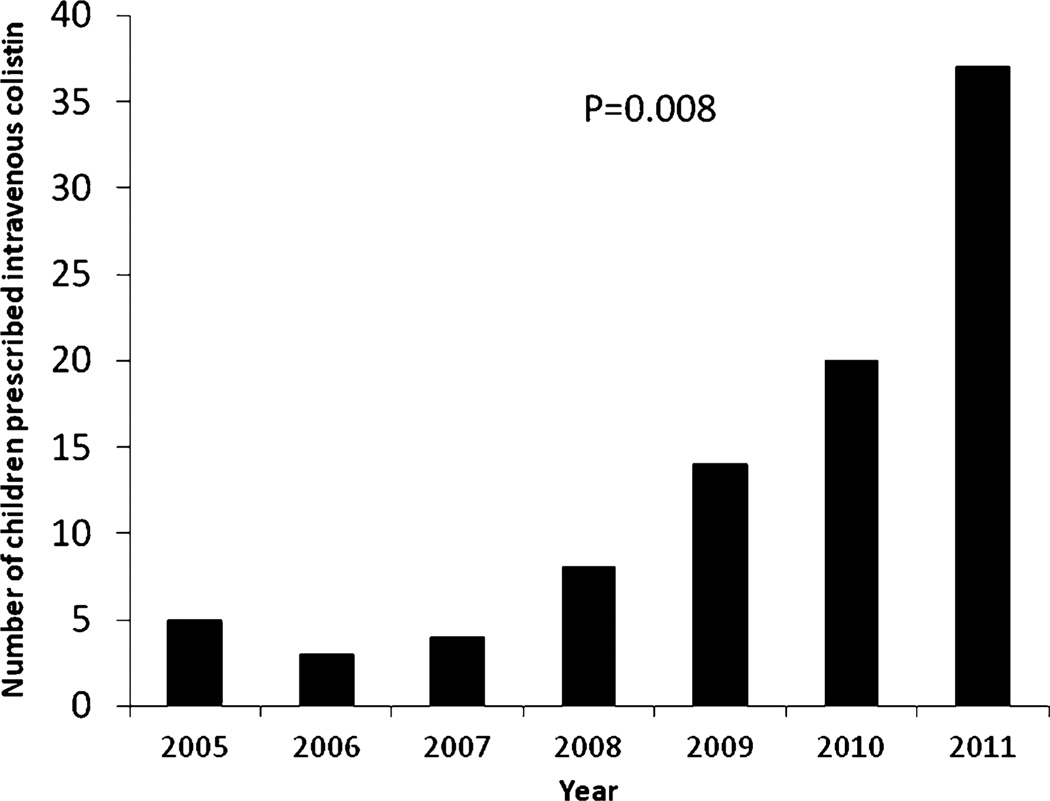
Of the 217 pediatric infectious diseases specialists completing the survey (84% response), 22% had prescribed colistin to children. A total of 92 unique cases of colistin use between 2005 and 2011 from 25 institutions were submitted. Of the 92 cases reported, the majority (62%) were between 2010 and 2011 (Fig. 1).
FIGURE 1.
Number of children prescribed intravenous colistin from 2005 to 2011 in the United States based on response to survey of pediatric infectious diseases physicians with P value representing trend over time.
Patient Characteristics
The median age of children receiving intravenous colistin was 16 years and ranged between 2 months and 18 years. Children with CF receiving colistin tended to be older than children without CF (Table 1). The cases included in this study represent children from geographically diverse regions across the United States. Most (54%) patients resided in the Northeast region. Ten patients (11%) had a history of travel to or residence in another country within 30 days of admission. These countries included Greece, Haiti, Liberia, Mexico (3), Qatar, the United Arab Emirates (2) and Venezuela. CF was the most frequent underlying condition of children prescribed colistin (47%). Other common underlying medical conditions included tracheostomy dependency (14%), trauma (11%) and malignancy (10%) (Table 1). Eight (18%) children with CF and 46 (96%) children without CF were admitted to the intensive care unit during the time colistin was being administered.
TABLE 1.
Baseline Characteristics and Clinical Outcomes of 92 Children Receiving Intravenous Colistin Therapy
| Overall (n = 92; %) |
Cystic Fibrosis (n = 44; 48%) |
No Cystic Fibrosis (n = 48; 52%) |
P* | |
|---|---|---|---|---|
| Age (yr) | 16 (11–17.5) | 17 (15.5–18) | 12.2 (3–17) | <0.01 |
| Dose in mg/kg (median, IQR) | — | 2.5 (2.2–2.5) | 2.5 (1.625–2.5) | 0.21 |
| Interval (h; median, IQR) | — | 8 (8–8) | 12 (8–12) | <0.01 |
| Travel to or from foreign country ≤30 d of current admission† | 10 (10.9) | 4 (9.1) | 6 (12.5) | 0.60 |
| Intensive care unit admission | 54 (58.7) | 8 (18.2) | 46 (95.8) | <0.01 |
| Underlying medical conditions | ||||
| Burns | 3 (3.3) | — | 3 (6.8) | 0.11 |
| Immunocompromised | 17 (18.5) | 6 (12.5) | 11(25) | 0.18 |
| Solid organ transplant‡ (lung, liver, kidney) | 11 (12.0) | 6 (12.5) | 5 (11.4) | 1.00 |
| Malignancy | 9 (9.8) | — | 9 (20.5) | <0.01 |
| Postoperative surgical site infection | 4 (4.3) | — | 4 (9.1) | 0.05 |
| Previously healthy child with recent trauma | 10 (10.9) | — | 10 (22.7) | <0.01 |
| Tracheostomy dependency | 13 (14.1) | — | 13 (29.5) | <0.01 |
| Adverse drug events | ||||
| Clostridium difficile infection | 1 (1.1) | 0 | 1 (2.1) | 1.00 |
| Gastrointestinal complains | 2 (2.2) | 1 (2.3) | 1 (2.1) | 1.00 |
| Nephrotoxicity | 21 (22.3) | 9 (20.5) | 12 (48) | 0.63 |
| Neurotoxicity | 4 (4.3) | 4 (9.1) | — | 0.05 |
| Rash | 1 (1.1) | 0 | 1 (2.1) | 1.00 |
| Respiratory | 2 (2.2) | 1 (2.3) | 1 (2.1) | 1.00 |
| Outcome | ||||
| Clinical cure§ | — | — | 22 (45.9) | |
| Died while receiving intravenous colistin | 15 (16.3) | 3 (6.8) | 12 (25) | 0.02 |
Comparing children with and without cystic fibrosis.
Greece, Haiti, Liberia, Mexico (3), Qatar, the United Arab Emirates (2) and Venezuel.
For the cystic fibrosis category, all transplants were lung transplants.
Because all 44 children with cystic fibrosis were being treated for a cystic fibrosis exacerbation with a resistant organism they were chronically colonized with, clinical cure was difficult to determine.
IQR indicates interquartile range.
Microbiology and Clinical Syndromes
The most common organism for which colistin was prescribed was P. aeruginosa. (Table 2) P. aeruginosa was isolated as a pathogen in 65 cases (71%) and in 62 of the cases was resistant to three or more classes of antibiotics. There were 11 cases of MDR A. baumanii (12%), 12 cases of carbapenemase-producing Entero-bacteriaceae (13%), and 5 cases of extended-spectrum β-lactamase producing Enterobacteriaceae (5%). Of note, 82% of children infected with MDR Acinetobacter were being managed in adult intensive care units for non-infectious issues and became infected with the organism after hospitalization.
TABLE 2.
Microbiological Data of Infections in 92 Children Receiving Intravenous Colistin Therapy
| Overall (n = 92; %) |
Cystic Fibrosis (n = 44; 48%) |
No Cystic Fibrosis (n = 48; 52%) |
P* | |
|---|---|---|---|---|
| Resistance profile of microorganisms identified* | ||||
| Extended-spectrum β-lactamase producer | 5 (5.4) | — | 5 (10.4) | 0.06 |
| Carbapenemase-producing Enterobacteriaceae | 12 (13.0) | 3 (6.8) | 9 (18.8) | 0.12 |
| MDR Achromobacter | 2 (2.2) | 2 (4.5) | — | 0.23 |
| MDR Acinetobacter | 11 (12.0) | 1 (2.3) | 10 (22.7) | <0.01 |
| MDR Pseudomonas | 62 (67.4) | 34 (77.3) | 28 (58.3) | 0.04 |
| MDR Stenotrophomonas | 3 (3.3) | 2 (4.5) | 1 (2.1) | 0.61 |
| Site* | ||||
| Blood | 15 (16.3) | — | 15 (31.3) | <0.01 |
| Cerebrospinal fluid | 1 (1.1) | — | 1 (2.1) | 1 |
| Bone/wound | 5 (5.4) | — | 5 (10.4) | 0.36 |
| Intra-abdominal abscess | 6 (6.5) | — | 6 (12.5) | 0.03 |
| Respiratory tract | 68 (74.1) | 44 (100) | 25 (52.1) | <0.01 |
| Urine | 3 (3.3) | — | 3 (6.3) | 0.24 |
More than 1 response may apply to a patient.
Comparing children with and without cystic fibrosis.
Colistin was primarily used to treat ventilator-associated tracheitis and pneumonia (74%) followed by bacteremia (16%) and intra-abdominal abscesses (7%; Table 2). One patient received colistin both parenterally and intrathecally for an MDR P. meningitis. All children with CF included in the cohort were being treated for “CF exacerbations.” In the vast majority of children (91.3%), colistin was prescribed because the infectious pathogen was resistant to multiple antibiotic classes. In 8 (8.7%) cases, it was selected because of multiple patient allergies.
Administration of Colistin
Colistin was administered parenterally to all 92 patients. For children without CF, the initial colistin dose was most frequently 2.5 mg/kg every 12 hours, in accordance with the manufacturer’s insert;17 doses ranged from 1.25 to 5 mg/kg/dose in the absence of preexisting renal insufficiency (Table 1). For children with CF, 81% of patients began with an initial dose of 2.5 mg/kg every 8 hours. No providers administered an initial loading dose of colistin.
Concomitant antimicrobial therapy was administered in 77 (84%) patients, with the most common agents coadministered being aminoglycosides (26%), carbapenems (23%), fluoroquinolones (13%) and ceftazidime (12%); in 51 of these 77 cases (66%), the targeted organism was resistant to the second agent.
Clinical Outcomes
Fifteen patients in the cohort (16%) died while receiving colistin therapy. Mortality was higher in the children without CF (25%). Although numbers were small, there was no difference in mortality between the children without CF receiving colistin monotherapy and combination therapy (P = 0.45). Within the CF and non-CF groups, mortality did not appear related to dose or interval of colistin prescribed (data not shown).
Forty-four children had subsequent cultures growing the same organism. Of these, resistance to colistin emerged in 9 (21%) children during the initial course of colistin therapy. None of the 9 children had CF, and they were all receiving combination therapy. Six of the children had catheter-related bacteremia without catheter removal. The remaining 3 children were being treated for ventilator-associated pneumonia. All 9 children continued to receive colistin therapy because of lack of alternative antibiotic agents and died during therapy.
Adverse Events
Overall, 27 patients (29%) experienced at least 1 adverse event attributed to colistin therapy. The most common adverse outcome reported was nephrotoxicity, which occurred in 21 (22%) children. All cases of nephrotoxicity developed within 1 week of the first administered dose of colistin. One child required hemodialysis for acute renal failure attributed to colistin use. Renal function returned to baseline in all patients who survived after cessation of colistin, with creatinine normalizing a median of 5 days after its discontinuation. There was no association between dose or interval of colistin prescribed and the development of nephrotoxicity (P > 0.80). Age was associated with nephrotoxicity when modeled continuously (P = 0.04) and children aged ≥13 years had approximately 7 times the odds of developing nephrotoxicity than younger children, even after adjusting for the receipt of additional nephrotoxins (odds ratio 7.16; 95% confidence interval: 1.51–14.06; P = 0.01).
Four children (all with underlying CF) exhibited signs of neurotoxicity. Paresthesias were reported in 2 children which reversed in both cases within 2 days of discontinuation of the antibiotic. Two children developed persistent headaches after initiating colistin therapy which subsided after colistin was discontinued. All 4 children were exposed to colistin at a later time and had recurrence of these symptoms.
DISCUSSION
To increase understanding of the current use of colistin in children, we report findings from the largest case series of colistin therapy in children to date. Our results suggest that prescription of colistin may be increasing, likely mirroring the increase in MDR Gram-negative organism infections in children, although clinical experience with this agent is still limited to a minority of pediatric infectious diseases practitioners. Although colistin appears to be prescribed more frequently, the use of colistin appears limited to the treatment of highly resistant organisms or when multiple allergies preclude the use of other classes of antibiotics. Our results indicate that in the United States, colistin is most commonly prescribed to children with CF with infections due to MDR P. aeruginosa.
The optimal dose and interval of colistin administration for children remains uncertain. Recent studies suggest that the manufacturer’s recommended dose of colistin is suboptimal, leading to delays in achieving appropriate target drug concentrations.18–22 In our cohort, most children were prescribed colistin according to the manufacturer’s recommendation (2.5–5 mg/kg per day, divided into 2–4 equal doses, with interval adjustment recommended in renal impairment),17 and no difference in clinical response was observed regardless of the prescribed dose or interval within the CF group and non-CF groups. Because the children without CF were much more ill than children with CF, we did not compare clinical outcomes based on total daily dose or interval between these groups. Children with CF were generally administered higher total daily doses of colistin, typically 7.5 mg/kg/day divided in 8-hour intervals, presumably because of the higher volume of distribution in children with CF.23,24 This higher daily dose appears to be relatively well tolerated. In our cohort, no child received a loading dose of colistin. However, recent pharmacokinetic data suggest that plasma colistin concentrations may be insufficient before steady state, and patients may benefit from a colistin loading dose.22 It is noteworthy that according to recent clinical experience, higher daily doses of intravenous colistin, up to 720 mg per day, administered in large series of patients did not result in greater toxicity.25,26 More studies are needed to better elucidate the appropriate dose and interval of colistin administration and whether a loading dose may be necessary.
In our cohort, 84% of children received combination therapy, despite the majority of their pathogens exhibiting in vitro resistance to the additional agent. Although all children who developed resistance were receiving combination therapy, our study did not have adequate power to determine if combination therapy had an impact on preventing subsequent colistin resistance. Existing data do not demonstrate that combination therapy with colistin prevents the emergence of resistance.10 Because heteroresistance to colistin can occur relatively rapidly, the use of a second agent is sometimes advocated so at least 1 antimicrobial agent with some potential activity against the causative pathogen is being administered.18,27–30 In 1 study, in vitro evidence of heteroresistance to colistin was reported to be as high as 93%,27 though the clinical significance of heteroresistance identified in vitro remains unclear. Resistance to colistin emerged in 20.5% of children in our cohort, which is in accord with other studies reporting rates of resistance (range 3–27.9%).31–34 The mechanisms of colistin resistance are poorly defined and have been attributed to alterations in the bacterial outer membranes, electrolyte imbalances or production of efflux pumps.35,36 Existing data suggest that patients with colistin-resistant pathogens have increased mortality.37 All 9 patients who developed colistin resistance in our cohort died during therapy. These findings underscore the need for colistin to be prescribed judiciously taking into account that (1) prolonged use and suboptimal colistin levels may lead to the emergence of resistance and (2) treatment options for colistin-resistant organisms are often nonexistent.
Clinical studies concerning the synergistic activity of colistin with other antimicrobial agents in humans are limited. Similarly, data demonstrating improved clinical outcomes or the prevention of the emergence of resistance with combination regimens is lacking. Although our study did not demonstrate a survival benefit with colistin combination therapy, it did not have adequate power to evaluate this outcome. In vitro studies have demonstrated synergy when colistin is administered in combination with carbapenems and rifampin.38–42 Colistin’s rapid destruction of outer membranes allows enhanced penetration of agents such as carbapenems and rifampin and is especially helpful when carbapenem resistance is a result of porin defects.38 Studies in adults have failed to show improved outcomes when combination therapy is used, but as these studies were observational, they may have been subject to confounding by indication.43–46 Although the role of combination therapy remains uncertain, combination therapy may nonetheless prove to be beneficial. As the distribution of colistin to the lung parenchyma, bones and cerebrospinal fluid is relatively poor, the addition of a second agent may ensure more acceptable antibiotic concentrations in these tissues.47,48
Nephrotoxicity was the most commonly observed adverse event in our cohort, which appeared to increase with age. Acute kidney injury generally became apparent within the first week of colistin initiation and in all cases reversed after discontinuation of colistin. In a retrospective study of 14 children who received colistin in the 1990s, Goverman et al reported a significant rise in creatinine in 2 children (14%).5 This is lower than colistin-associated nephrotoxicity rates reported in the 1960s and 1970s, possibly due to improvements in supportive therapy and the decreased use of additional nephrotoxic agents in recent decades.16,49–51 A systematic review of case series and case reports on colistin use in children without CF by Falagas et al found the incidence of nephrotoxicity to be 2.8%.52 Most children included in this review were hospitalized outside the United States, thus the generalizability to children in the United States should be interpreted with caution as a large portion of included children received doses of colistin lower than recommended by the US package insert.17,52 In recent adult literature, rates of nephrotoxicity range from 3.5 to 58% and appear to be dose dependent.9–16 Although it is unclear why adolescents and adults are predisposed to higher rates of nephrotoxicity than younger children, it may be related to baseline renal dysfunction and multiple minor injuries to the kidneys that have occurred over time. The definition of "nephrotoxicity" varies both within adult studies and between adult and pediatric studies.
Four patients in our cohort developed neurotoxicity, manifesting as paresthesias or severe headaches. These reversed in all patients but it is interesting to note that all patients who reported these symptoms on initial colistin use experienced them again when colistin was prescribed subsequently. Colistin-induced neurotoxicity appears to occur more frequently in children with CF (consistent with our data), although the cause is unclear. In 1 study of 31 patients with CF receiving colistin, 68% experienced reversible paresthesias and/or ataxia.53
This study has several limitations to consider. First, this was a retrospective study where cases were ascertained by voluntary response to a survey of pediatric infectious diseases physicians. As a result, the representativeness of our findings is unknown; however, as there was an 84% response, we believe these findings are still largely generalizable. Second, the findings may be subject to recall bias. It is possible that physicians disproportionately submitted cases with more memorable outcomes. Similarly, they may have been more likely to recall recent cases. However, because colistin is infrequently administered to the pediatric population in the United States, we anticipate that physicians may remember most children to whom they prescribed colistin. Additionally, several of the authors reviewed their institution’s pharmacy databases and submitted all cases of colistin prescription in their respective institutions in the past 7 years (n = 67) so these cases would not be subject to recall bias. Third, as not all patients had long-term follow-up, there may have been long-term sequelae related to colistin use that was not captured by the study. Despite these limitations, these data provide important information to assist physicians with current practices regarding colistin use in children.
In conclusion, colistin use in children has been associated primarily with nephrotoxicity and, to a lesser extent, neurotoxicity, both of which were reversible in our cohort. The growing emergence of resistance is concerning. Pharmacokinetic studies to determine optimal dosing in children should be prioritized. Given the alarming emergence of MDR Gram-negative pathogens, prospective controlled studies assessing the efficacy and safety of colistin use in children are warranted.
ACKNOWLEDGMENTS
The authors thank the members of the Pediatric Colistin Study Group who participated in this case series including Grace Appiah, Alana Arnold, Rebecca Brady, Sean Elliot, Stephen Eppes, Michele Estabrook, Kelly Flett, Diana Florescu, Bruno Granwehr, Alice Jenh, Kathryn Moffett, David Michalik, Nizar Maraqa, Aaron Milstone, Cecile Punzalan, Priya Prasad, Jana Shaw, Mark Travassos and Kevin Winthrop.
This work was supported by a National Institutes of Health grant (grant number KL2RR025006 to PDT).
Footnotes
The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.Lee CY, Chen PY, Huang FL, et al. Microbiologic spectrum and susceptibility pattern of clinical isolates from the pediatric intensive care unit in a single medical center - 6 years’ experience. J Microbiol Immunol Infect. 2009;42:160–165. [PubMed] [Google Scholar]
- 2.Zhanel GG, DeCorby M, Laing N, et al. Canadian Antimicrobial Resistance Alliance (CARA) Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005–2006. Antimicrob Agents Chemother. 2008;52:1430–1437. doi: 10.1128/AAC.01538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidron AI, Edwards JR, Patel J, et al. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 4.Tamma PD, Lee CK. Use of colistin in children. Pediatr Infect Dis J. 2009;28:534–535. doi: 10.1097/INF.0b013e3181ac4980. [DOI] [PubMed] [Google Scholar]
- 5.Goverman J, Weber JM, Keaney TJ, et al. Intravenous colistin for the treatment of multi-drug resistant, gram-negative infection in the pediatric burn population. J Burn Care Res. 2007;28:421–426. doi: 10.1097/BCR.0B013E318053D346. [DOI] [PubMed] [Google Scholar]
- 6.Rosanova M, Epelbaum C, Noman A, et al. Use of colistin in a pediatric burn unit in Argentina. J Burn Care Res. 2009;30:612–615. doi: 10.1097/BCR.0b013e3181abffb6. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, DeSantis ER, Kuper J. Resurgence of colistin use. Am J Health Syst Pharm. 2007;64:2462–2466. doi: 10.2146/ajhp060501. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Karageorgopoulos DE, Nordmann P. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 2011;6:653–666. doi: 10.2217/fmb.11.49. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CY, Sheng WH, Wang JT, et al. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents. 2010;35:297–300. doi: 10.1016/j.ijantimicag.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53:879–884. doi: 10.1093/cid/cir611. [DOI] [PubMed] [Google Scholar]
- 12.Doshi NM, Mount KL, Murphy CV. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy. 2011;31:1257–1264. doi: 10.1592/phco.31.12.1257. [DOI] [PubMed] [Google Scholar]
- 13.Falagas ME, Fragoulis KN, Kasiakou SK, et al. Nephrotoxicity of intravenous colistin: a prospective evaluation. Int J Antimicrob Agents. 2005;26:504–507. doi: 10.1016/j.ijantimicag.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Levin AS, Barone AA, Penço J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 15.Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48:1724–1728. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 16.Koch-Weser J, Sidel VW, Federman EB, et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72:857–868. doi: 10.7326/0003-4819-72-6-857. [DOI] [PubMed] [Google Scholar]
- 17.X-Gen Pharmaceuticals Inc. Colistimethate for injection, USP: for intramuscular and intravenous use. [Accessed April 9, 2010]; Available at: http://www.x-gen.us/injectables/colistimethate/Colistimethate_files/insert/Colistimethate_pi.pdf. [Google Scholar]
- 18.Bergen PJ, Li J, Nation RL. Dosing of colistin-back to basic PK/PD. Curr Opin Pharmacol. 2011;11:464–469. doi: 10.1016/j.coph.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markou N, Markantonis SL, Dimitrakis E, et al. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther. 2008;30:143–151. doi: 10.1016/j.clinthera.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Daikos GL, Skiada A, Pavleas J, et al. Serum bactericidal activity of three different dosing regimens of colistin with implications for optimum clinical use. J Chemother. 2010;22:175–178. doi: 10.1179/joc.2010.22.3.175. [DOI] [PubMed] [Google Scholar]
- 22.Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couet W, Grégoire N, Gobin P, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89:875–879. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Coulthard K, Milne R, et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003;52:987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 25.Markou N, Apostolakos H, Koumoudiou C, et al. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit Care. 2003;7:R78–R83. doi: 10.1186/cc2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen RJ, Li J, Nation RL, et al. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 29.Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother. 2008;52:351–352. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh JY, Son JS, Chung DR, et al. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob Agents Chemother. 2010;54:560–562. doi: 10.1128/AAC.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko KS, Suh JY, Kwon KT, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60:1163–1167. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 33.Pitt TL, Sparrow M, Warner M, et al. Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax. 2003;58:794–796. doi: 10.1136/thorax.58.9.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schülin T. In vitro activity of the aerosolized agents colistin and tobramycin and five intravenous agents against Pseudomonas aeruginosa isolated from cystic fibrosis patients in southwestern Germany. J Antimicrob Chemother. 2002;49:403–406. doi: 10.1093/jac/49.2.403. [DOI] [PubMed] [Google Scholar]
- 35.Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young ML, Bains M, Bell A, et al. Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob Agents Chemother. 1992;36:2566–2568. doi: 10.1128/aac.36.11.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarkotou O, Pournaras S, Voulgari E, et al. Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J Clin Microbiol. 2010;48:2271–2274. doi: 10.1128/JCM.02301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon J, Urban C, Terzian C, et al. In vitro double and triple synergistic activities of Polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2004;48:753–757. doi: 10.1128/AAC.48.3.753-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CH, Tang YF, Su LH, et al. Antimicrobial effects of varied combinations of meropenem, sulbactam, and colistin on a multidrug-resistant Acinetobacter baumannii isolate that caused meningitis and bacteremia. Microb Drug Resist. 2008;14:233–237. doi: 10.1089/mdr.2008.0840. [DOI] [PubMed] [Google Scholar]
- 40.Rynn C, Wootton M, Bowker KE, et al. In vitro assessment of colistin’s antipseudomonal antimicrobial interactions with other antibiotics. Clin Microbiol Infect. 1999;5:32–36. doi: 10.1111/j.1469-0691.1999.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 41.Giamarellos-Bourboulis EJ, Xirouchaki E, Giamarellou H. Interactions of colistin and rifampin on multidrug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2001;40:117–120. doi: 10.1016/s0732-8893(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 42.Ostenson RC, Fields BT, Nolan CM. Polymyxin B and rifampin: new regimen for multiresistant Serratia marcescens infections. Antimicrob Agents Chemother. 1977;12:655–659. doi: 10.1128/aac.12.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tascini C, Gemignani G, Palumbo F, et al. Clinical and microbiological efficacy of colistin therapy alone or in combination as treatment for multidrug resistant Pseudomonas aeruginosa diabetic foot infections with or without osteomyelitis. J Chemother. 2006;18:648–651. doi: 10.1179/joc.2006.18.6.648. [DOI] [PubMed] [Google Scholar]
- 44.Falagas ME, Rafailidis PI, Kasiakou SK, et al. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect. 2006;12:1227–1230. doi: 10.1111/j.1469-0691.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 45.Conway SP, Pond MN, Watson A, et al. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax. 1997;52:987–993. doi: 10.1136/thx.52.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linden PK, Kusne S, Coley K, et al. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2003;37:e154–e160. doi: 10.1086/379611. [DOI] [PubMed] [Google Scholar]
- 47.Jiménez-Mejías ME, Pichardo-Guerrero C, Márquez-Rivas FJ, et al. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur J Clin Microbiol Infect Dis. 2002;21:212–214. doi: 10.1007/s10096-001-0680-2. [DOI] [PubMed] [Google Scholar]
- 48.Stein A, Raoult D. Colistin: an antimicrobial for the 21st century? Clin Infect Dis. 2002;35:901–902. doi: 10.1086/342570. [DOI] [PubMed] [Google Scholar]
- 49.Wolinsky E, Hines JD. Neurotoxic and nephrotoxic effects of colistin patients with renal disease. N Engl J Med. 1962;266:759–762. doi: 10.1056/NEJM196204122661505. [DOI] [PubMed] [Google Scholar]
- 50.Brown JM, Dorman DC, Roy LP. Acute renal failure due to overdosage of colistin. Med J Aust. 1970;2:923–924. doi: 10.5694/j.1326-5377.1970.tb63262.x. [DOI] [PubMed] [Google Scholar]
- 51.Price DJ, Graham DI. Effects of large doses of colistin sulphomethate sodium on renal function. Br Med J. 1970;4:525–527. doi: 10.1136/bmj.4.5734.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falagas ME, Vouloumanou EK, Rafailidis PI. Systemic colistin use in children without cystic fibrosis: a systematic review of the literature. Int J Antimicrob Agents. 2009;33:503.e1–503.e13. doi: 10.1016/j.ijantimicag.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Reed MD, Stern RC, O’Riordan MA, et al. The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol. 2001;41:645–654. doi: 10.1177/00912700122010537. [DOI] [PubMed] [Google Scholar]