Abstract
Epidemiologic data on phocomelia are scarce. This study presents an epidemiologic analysis of the largest series of phocomelia cases known to date. Data were provided by 19 birth defect surveillance programs, all members of the International Clearinghouse for Birth Defects Surveillance and Research. Depending on the program, data corresponded to a period from 1968 through 2006. A total of 22,740,933 live births, stillbirths and, for some programs, elective terminations of pregnancy for fetal anomaly (ETOPFA) were monitored. After a detailed review of clinical data, only true phocomelia cases were included. Descriptive data are presented and additional analyses compared isolated cases with those with multiple congenital anomalies (MCA), excluding syndromes. We also briefly compared congenital anomalies associated with nonsyndromic phocomelia with those presented with amelia, another rare severe congenital limb defect. A total of 141 phocomelia cases registered gave an overall total prevalence of 0.62 per 100,000 births (95% confidence interval: 0.52–0.73). Three programs (Australia Victoria, South America ECLAMC, Italy North East) had significantly different prevalence estimates. Most cases (53.2%) had isolated phocomelia, while 9.9% had syndromes. Most nonsyndromic cases were monomelic (55.9%), with an excess of left (64.9%) and upper limb (64.9%) involvement. Most nonsyndromic cases (66.9%) were live births; most isolated cases (57.9%) weighed more than 2,499 g; most MCA (60.7%) weighed less than 2,500 g, and were more likely stillbirths (30.8%) or ETOPFA (15.4%) than isolated cases. The most common associated defects were musculoskeletal, cardiac, and intestinal. Epidemiological differences between phocomelia and amelia highlighted possible differences in their causes.
Keywords: phocomelia, epidemiology, prevalence, frequency, ICBDSR
INTRODUCTION
Phocomelia is a rare congenital anomaly in which the proximal part of the limb (humerus or femur, radius or tibia, ulna or fibula) is absent or markedly hypoplastic, with normal or nearly normal hand or foot. True phocomelia is characterized by the total absence of the intermediate segments of the limb, with the hand or foot directly attached to the trunk. Etymologically, the term phocomelia comes from the Greek: φώκη— fóke—“seal,” plus µέλoς—melos— “limb,” and it refers to the similarity of the patient’s limb shape to the flipper on a seal.
Little is known about the epidemiology of phocomelia. Although phocomelia is one of the most characteristic defects known to be produced by thalidomide, the causes of most cases of phocomelia today are still to be determined. Despite the occurrence of thousands of infants born with phocomelia and other defects as a consequence of the prenatal exposure to thalidomide, recent new cases of thalidomide embryopathy have been reported in South America, especially in Brazil. Castilla et al. [1996] reported 34 children with malformations due to thalidomide exposure, born in endemic areas of leprosy after the remarketing of the drug. Schuler-Faccini et al. [2007] reported three additional thalidomide-associated cases of phocomelia. Because all of these cases are in principle preventable, the use of thalidomide by pregnant women remains a significant problem, especially in underdeveloped countries due to poorly regulated or uncontrolled use of the drug. In developed countries, although a wide range of new indications for thalidomide use continues, there are local strict regulations enforced to prevent their use during pregnancy. For instance, the Food and Drug Administration, in its website [U.S. Food and Drug Administration, 2011], provides a summary of warnings and information for safe use on thalidomide. Apart from this important issue on thalidomide exposures, an overview of the literature on several key aspects of phocomelia is provided in the following paragraphs.
Historical Aspects
It is said that Étienne Geoffroy Saint-Hilaire coined the term “phocomelia” in the first half of the 19th century. However, much earlier, in the middle of the first century BC, Lucretius, in his poem “De rerum natura” already described beings produced by the earth, like creatures disabled by the adhesion of their limbs to the trunk, so that they could neither do anything nor go anywhere nor keep out of harm nor take what they needed. This could be one of the first conserved historical descriptions of patientswith phocomelia. Much later, in 1642, Aldrovandus [1642] reported a patient with three-finger phocomelia of right arm and amelia of left arm. In 1681, Bouchard [1681] described a child born in France in 1671 with tetraphocomelia, cleft lip, and abnormal ears, possibly a case of Roberts syndrome. In 1800, Isenflamm and Rosenmüller [1800] described a patient with a foot with four toes attached to the hip on the left side, one toe in place of foot also directly attached to the hip on the right side, and amelia of arms. A century later, in 1907, Slingenberg [1907] presented a child born in 1904 in the Netherlands with tetraphocomelia, hands with the thumb and two fingers, and each foot having a big toe and three toes, also possibly a Roberts syndrome [Czeizel et al., 1994].
EMBRYOLOGYOF THE LIMBS
In another article of this issue devoted to the study of amelia in the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) (Bermejo-Sánchez et al., 2011], the processes of human limb development are described in detail. Briefly, the human limb development initiates in the 26th day after fertilization for the upper limb, and day 28 for the lower limb, and extends until day 56 both for the upper and the lower limbs. The appendicular skeleton develops from the lateral (paraxial and somatic) plate mesoderm. Each tissue (cartilage, bone and muscle) goes through many specific mechanisms of differentiation. In the limb bud, at 33 days, mesenchyme covered by a layer of cuboidal ectoderm forms the apical ectodermal ridge (AER), which has an inductive influence on the underlying mesenchyme. By the 6th week after fertilization the hand and footplates are observable. Fingers and toes are formed when programmed cell death (apoptosis) in the AER separates the ridge into five parts. The hand and foot plates become separated from the proximal segment of the limb by a circular constriction, which becomes the wrist and ankle, and later a second constriction (at the level of the elbow and knee) divides the proximal portion into two segments, so that the main segments of the limb (proximal stylopod, middle zeugopod, and distal autopod) can be recognized. By the 6th week of development the first hyaline cartilage can be recognized. Primary ossification centers are present in all long bones of the limbs by the 12th week of development.
MOLECULAR EMBRYOLOGY
This subject is also detailed in the article on amelia in this issue [Bermejo-Sánchez et al., 2011]. The genetic processes that control limb development are complicated and still not fully understood, but several gene families are known to be involved in the spatially and temporally coordinated growth and differentiation of the developing limb. Some of these genes are involved in the initiation and patterning of both the upper and the lower limbs, and others are differentially expressed in the developing forelimb and hindlimb. The most prominent among these genes or families of genes are detailed in Bermejo-Sánchez et al. [2011]. Regarding phocomelia, retinoic acid (RA) signaling may be important since it affects the expression of Meis1/2, which expands distally on RA treatment [Mercader et al., 2000]. On the other hand, the distal expression of Hox genes is reduced, revealing that exogenous RA proximalizes the limb-bud mesenchyme [Mercader et al., 2000]. RA is synthesized in the proximal mesenchyme and spreads into the distal limb bud, in which it is actively degraded [Yashiro et al., 2004], so that high levels of RA would specify proximal cell fates and inhibit distal ones. In fact, the genetic inactivation of CYP26B1, an enzyme involved in the degradation of RA [Yashiro et al., 2004] may play a role. Genes encoding many other secreted signaling molecules are expressed in the limb, for example, insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), etc., and diffusible signaling molecules, such as retinoic acid, have also been shown to contribute to generating the pattern of the limb buds [Tickle et al., 1982]. Genes that encode molecules involved in direct cell–cell signaling such as the Notch/Delta system [Vargesson et al., 1998], and Ephrins/Ephrin receptors [Araujo et al., 1998] are expressed in the developing limb and these interactions may fine-tune the limb bud pattern and/or govern local cell behaviour. Several genes encoding transcription factors have been identified that are expressed in specific domains in the developing limb in response to signaling along antero-posterior, proximodistal, and dorso-ventral axes [Towers and Tickle, 2009]. These include the 5’ genes of the Hox A and D clusters, LIM, Tbx, Sall, and Shox genes. Functional inactivation of these genes in mice and/or their mutations, such as in SHOX [Blaschke and Rappold, 2006], in human patients, lead to limb defects indicating that these genes play a role in the generation of limb bud pattern [Towers and Tickle, 2009]. However, little is known about the gene targets of these transcription factors, and it is often unclear what cellular activities are primarily affected and lead to limb defects [Towers and Tickle, 2009].
CLINICAL GENETICS
Phocomelia is part of a variety of known syndromes or phenotypes. Using the term “phocomelia” as a search criterion in the Winter-Baraitser Dysmorphology Database [Winter and Baraitser, 2010] combined with the same search in the OMIM (Online Mendelian Inheritance in Man) database [2011] generated a list of at least 25 syndromes or recognized clinical entities presenting with phocomelia (Table I). For those with a known chromosome location of a responsible gene, this information is also provided in Table I.
TABLE I.
Syndromes or Defined Phenotypes Presenting With Phocomelia [Winter and Baraitser, 2010; OMIM, 2011]
| Syndrome or defined phenotype | OMIM number, or reference | Location | Gene/locus |
|---|---|---|---|
| Acrofacial dysostosis-type Rodríguez | 201170 | — | — |
| Alveolar capillary dysplasia with misalignment of pulmonary veins |
265380 | 2q35; 16q24 | CPS1; FOXF1 |
| Baraitser-brachyphalangia-polydactyly | 609945 | — | — |
| Cornelia de Lange syndrome 1 (Brachmann-de Lange syndrome) |
122470 | 5p13.2 | NIPBL |
| DK-phocomelia (with encephalocele and thrombocytopenia) |
223340 | — | — |
| Ectrodactyly-distal phocomelia | Delrue and Lacombe [2002] | — | — |
| Femur-Fibula-Ulna complex (FFU syndrome) | 228200 | — | — |
| Fetal thalidomide | Lenz [1961, 1962, McCredie and Willert 1999] | — | — |
| Fetal valproate syndrome | Verloes et al. [1990] | — | — |
| Fuhrman syndrome | 228930 | 3p25.1 | WNT7A |
| Gollop-monodactylous ectrodactyly, split femur | 228250 | — | — |
| Holt-Oram syndrome | 142900 | 12q24.1 | TBX5 |
| Hydrocephaly-features of VACTERL | 276950 | 10q23.3 | PTEN |
| Meinecke-Peper- Frontonasal dysplasia, phocomelia, absent thumbs |
Meinecke and Peper [1992] | — | — |
| Microgastria-limb reduction defects association | 156810 | — | — |
| Murray-peromelia/phocomelia | Murray et al. [2002] | — | — |
| Phocomelia-ectrodactyly, ear malformation, deafness and sinus arrhythmia |
171480 | — | — |
| Renal dysplasia-Limb defects syndrome | 266910 | — | — |
| Roberts (pseudothalidomide) syndrome/SC Phocomelia | 268300, 269000 | 8p21.1 | ESCO2 |
| Schinzel-Phocomelia and additional anomalies | 276820 | 3p25.1 | WNT7A |
| Steinfeld syndrome | 184705 | — | — |
| Stiles-Dougan-malformed upper extremities | 107900 | — | — |
| Tetra-amelia autosomal recessive | 273395 | 17q21 | WNT3 |
| Thrombocytopenia-absent radius (TAR) | 274000 | 1q21.1 | — |
| Waardenburg syndrome-tetraphocomelia | Wu et al. [2009] | — | — |
VACTERL, vertebral, anal, cardiac, tracheo-esophageal, renal, and limb defects.
PATHOGENESIS
In 1971, Van der Horst and Gotsman [1971] described phocomelia found in a patient with an anomalous origin of the right subclavian artery, suggesting that phocomelia could be a result of a locally reduced blood supply due to the abnormal anatomical route taken by the artery. More recently, Weaver [1998] suggested that the failure of formation of the intermediate limb segments could be influenced by disruptions of the developing arterial supply.
Phocomelia has been interpreted as a patterning defect in the context of the progress zone model, which states that a cell’s proximo-distal identity is determined by the length of time spent in such progress zone in the distal limb region [Summerbell et al., 1973]. If proximal cells remain within range of the AER-produced fibroblast growth factor (FGF) signal for a longer time than normal, those cells will ultimately be specified to distal fates so that the limb develops with distal structures in proximal positions, as it occurs in phocomelia. However, according to more recent experiments [Galloway et al., 2009], phocomelia would not be a patterning defect, but rather results from a time-dependent loss of skeletal progenitors. Because skeletal condensation proceeds from the shoulder to fingers, the proximal elements are differentially affected in limb buds exposed to radiation at early stages. This occurs, not by producing a smaller limb bud in the context of a progress zone but by eliminating chondrogenic precursors during a time window when proximal condensation is compromised but distal differentiation has not yet commenced. This suggests a defect in progenitor cell survival and differentiation. Increased cell death has been thought to underlie thalidomide-induced limb truncations in chick embryos, but whether this is a result of direct activation of caspase pathways, or an indirect result of angiogenic inhibition, it still remains unclear [Galloway et al., 2009]. Cell death was also linked to phocomelia in experiments with whole embryo exposure to nitrogen mustard [Salzgeber, 1969, 1975]. Other authors also suggest that thalidomide in humans may cause apoptosis predominantly in the progress zone, and to a lesser extent in the AER, thus, causing phocomelia [Knobloch and Rüther, 2008].
In spite of some uncertainties, one of the best-studied mechanisms of action is that of the thalidomide-induced limb defects. Phocomelia is one of the most frequent types of limb deficiency associated with the prenatal exposure to the drug. Thalidomide has a complex chemistry and multiple actions. It exists as two isomeric forms that have different biological properties. The S(–) isomer is thought to be responsible for the teratogenic actions, but due to the ability of the isomers to interchange under physiological conditions, it is not possible to isolate one form from the other for clinical applications [Vargesson, 2009]. Thalidomide exerts anti-inflammatory, immunomodulatory, and anti-angiogenic actions. Specifically, it has been shown that thalidomide [Vargesson, 2009] (a) blocks angiogenesis in the chick limb; (b) can induce cell death and formation of reactive oxygen species in limb tissue; (c) antagonizes integrin expression in marmoset embryos and can bind to N-cadherin, and inhibits specific vascular integrins; (d) could cause distalization of the limb bud by blocking or reducing growth factor signaling during limb development, causing loss of proximal tissue, but allowing remaining tissue to be distalized, thus producing phocomelia. It seems that only the anti-angiogenic analogue of thalidomide CPS49 causes limb reduction defects, whereas the anti-inflammatory metabolites and other hydrolysis products do not [Therapontos et al., 2009]. Thus, the changes in gene expression, including the loss of Fgf8 and Fgf10 signaling, and increased cell death would all be secondary to the effect on the vessels. During the defined critical period, the limb vasculature is highly angiogenic, and the limb outgrowth is very rapid, in contrast to the rest of the embryo, which has more mature blood vessels at that time period. Earlier in embryogenesis, when all vessels are angiogenic, the drug is lethal or has a polytopic effect. The exact mechanism underlying phocomelia remains unclear and a challenge. Nevertheless, it could be hypothesized that blocking angiogenesis could produce an almost complete loss of mesenchyme, whereas if some signaling remains in the AER, FGF signaling could be reestablished in the remaining mesenchymal cells so that the limb outgrowth and specification of distal fate could continue. In fact, it has been demonstrated that irradiating and destroying the proximal limb element precursor cells results in phocomelia [Galloway et al., 2009]. Therefore, once the drug effect has worn off, the remaining cell populations expand in response to recovered FGF signaling from the AER and form distal structures, thus, producing phocomelia [Therapontos et al., 2009].
EPIDEMIOLOGY
In many studies, phocomelia has been evaluated within the larger groups of limb reduction defects such as intercalary defects, or more general groups of limb reduction, rather than a specific category. This is true for studies of descriptive epidemiology [Smith et al., 1977; Källén et al., 1984; Froster-Iskenius and Baird, 1989; Calzolari et al., 1990; Froster and Baird, 1992; Froster and Baird, 1993; Lin et al., 1993; Evans et al., 1994; Castilla et al., 1995], as well as studies of possible risk factors [Smith et al., 1977; Aro et al., 1983; Polednak and Janerich, 1985; Botting, 1994; Wasserman et al., 1996; Källén, 1997].
Regarding the prevalence of phocomelia, Källén et al. [1984] estimated that it occurs in 4.2 per 100,000 births, after studying 1,368,024 births. In other studies, that included phocomelia as part of a more general group of intercalary defects, the global prevalence of intercalary defects varied between 0.3 per 100,000 pregnancy outcomes [Rosano et al., 2000], 1.1 per 100,000 births [Evans et al., 1994], and 4.6 per 100,000 births [Calzolari et al., 1990].
Laterality of phocomelia was studied by Källén et al. [1984] among 48 cases with no other limb reduction defects. In that study, 29.2% had right side involvement, 22.9% had left side, and 47.9% were bilateral. In the same study, 68.8% had the upper limbs involved, 29.2% had lower limb involvement, and 2.1% had both the upper and lower limbs affected. The sex distribution of patients, survival, and number of limbs involved, as well as other variables such as birth weight, were analyzed together with other limb reduction defects and, therefore, that study does not provide specific data on phocomelia.
Risk Factors
There is not a published study specifically focusing on the risk factors for phocomelia yet. The only teratogen that has been explicitly related to phocomelia, is thalidomide. Precisely, the unusually high occurrence of severe limb defects (including phocomelia) was the major clue that led to the discovery of thalidomide as one of the most potent, and now quite well known, human teratogens [Lenz, 1961, 1962, 1980]. From the experience of thalidomide, it was concluded that the sensitive periods for phocomelia were between days 24 and 33 (after fertilization) for the involvement of the upper limb, and between days 28 and 33 for the lower limb involvement [Brent and Holmes, 1988].
Associated Defects
With respect to the associated defects, Evans et al. [1994] found that 50% of intercalary defects (9/18) had multiple congenital anomalies. Rosano et al. [2000] found that intercalary defects were significantly associated with omphalocele (present in 4 cases among 17); a defect for which cases with intercalary defects had a fivefold increased risk.
Taking into account all the previous antecedents in the literature, and the limited information we found on the epidemiology of phocomelia, we conducted a descriptive analysis of prevalence data collected on phocomelia by ICBDSR. Such analysis included the variation in total prevalence by program and by selected maternal and case characteristics. We also took advantage of the rare opportunity of a joint analysis and publishing to compare amelia and phocomelia cases.
METHODS
A total of 19 surveillance programs of congenital anomalies (Table II) from 22 countries, every continent except Africa, provided data for this joint study. All the participating programs are members of ICBDSR [ICBDSR, 2011a,b). The study period was variable for the different programs, with the oldest data corresponding to year 1968, extending up to 2006. The underlying birth cohort included 22,740,933 births surveyed, considering live births (LB), stillbirths (SB) and, for some programs, elective terminations of pregnancy for fetal anomalies (ETOPFA). For each participating program, the maternal age distribution of the births was requested.
TABLE II.
Total Prevalence of Phocomelia in 19 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Surveillance program | Period | Births | Total number of cases |
% Of total cases that were SB |
% Of total cases that were ETOPFAa |
Total prevalence per 100,000 births |
95% CI |
|---|---|---|---|---|---|---|---|
| Canada Alberta | 1980–2005 | 1,062,483 | 5 | 0 | 16.7 | 0.47 | 0.15–1.10 |
| USA Utah | 1997–2004 | 380,706 | 1 | 0 | 0 | 0.26 | 0.01–1.46 |
| USA Atlanta | 1968–2004 | 1,283,999 | 11 | 27.3 | 9.1 | 0.86 | 0.43–1.53 |
| USA Texas | 1996–2002 | 2,054,788 | 12 | 8.3 | 0 | 0.58 | 0.30–1.02 |
| Mexico RYVEMCE | 1978–2005 | 1,058,885 | 9 | 11.1 | NP | 0.85 | 0.39–1.61 |
| South America ECLAMC | 1982–2006 | 4,556,173 | 7 | 28.6 | NP | 0.15 | 0.06–0.32 |
| Finland | 1993–2004 | 713,494 | 2 | 0 | 100 | 0.28 | 0.03–1.01 |
| Germany Saxony—Anhalt | 1980–2004 | 355,184 | 4 | 0 | 50.0 | 1.13 | 0.31–2.88 |
| Slovak Republic | 2000–2005 | 318,257 | 4 | 0 | 0 | 1.26 | 0.34–3.22 |
| France Central East | 1979–2004 | 2,500,214 | 19 | 10.5 | 44.8 | 0.76 | 0.46–1.19 |
| Italy North East | 1981–2004 | 1,186,497 | 2 | 0 | 0 | 0.17 | 0.02–0.61 |
| Italy Emilia Romagna | 1982–2004 | 558,176 | 7 | 0 | 0 | 1.25 | 0.50–2.58 |
| Italy Tuscany | 1992–2004 | 336,744 | 3 | 0 | 100 | 0.89 | 0.18–2.60 |
| Italy Campania | 1992–2004 | 643,962 | 8 | 0 | 12.5 | 1.24 | 0.54–2.45 |
| Italy Sicily | 1991–2002 | 216,257 | 3 | 0 | 0 | 1.39 | 0.29–4.05 |
| Spain ECEMC | 1980–2004 | 2,045,751 | 12 | 25.0 | NR | 0.59 | 0.30–1.02 |
| Israel | 1975–2005 | 151,562 | 1 | 0 | 0 | 0.66 | 0.02–3.68 |
| China Beijing | 1992–2005 | 1,927,622 | 11 | 72.7 | NR | 0.57 | 0.28–1.02 |
| Australia Victoria | 1983–2004 | 1,390,179 | 20 | 35.0 | 10.0 | 1.44 | 0.88–2.22 |
| Total | 22,740,933 | 141 | 19.1 | 14.9a | 0.62 | 0.52–0.73 |
ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino-Americano de Malformaciones Congénitas; RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas; SB, Stillbirths; ETOPFA, elective termination of pregnancy for foetal anomaly; CI, confidence interval; NP, not permitted; NR, not reported.
The percentage computed on the 15 surveillance programs registering ETOPFA is 20.6% (n=21/102).
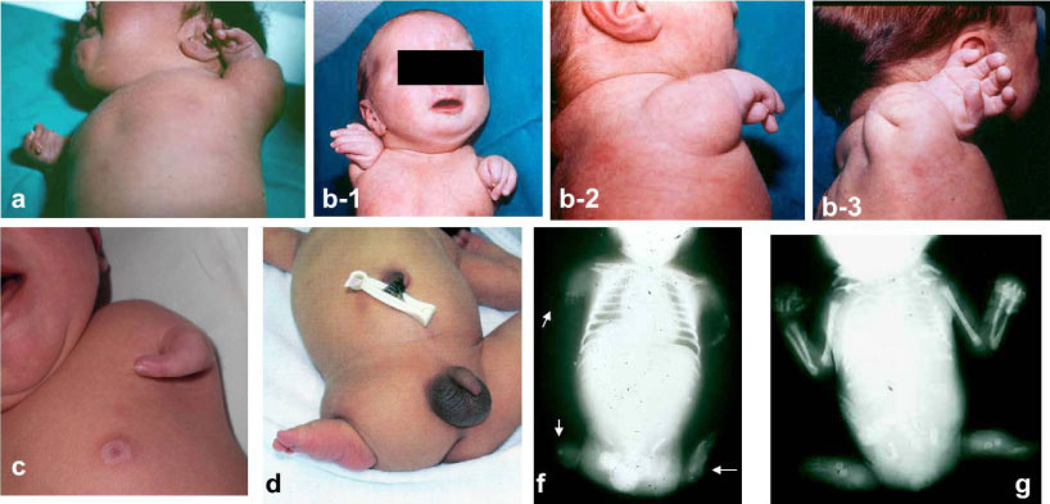
For this study on phocomelia, surveillance programs were asked to provide de-identified information on the cases, following a common protocol, as detailed in the article by Castilla and Mastroiacovo [2011] in this issue of the Journal, with data on phenotype, genetic testing, and selected demographic and prenatal information. The inclusion criterion for this study was to consider only true phocomelia cases. True phocomelia was defined as the total absence of the intermediate segments of the limb, with the hand or foot (normal, almost normal, or malformed) directly attached to the trunk. This strict definition was decided when preparing the study protocol, and the reason to establish it was to limit the cases only to true phocomelia, in order to obtain as much homogeneity as possible. Figure 1 includes several phocomelia cases, showing different expressions of the defect. Local scrutiny of the cases was performed by the most qualified dysmorphologist involved in each surveillance program, using all the available documentation. This means that he/she tried to confirm that the intermediate segments of the limb (humerus/femur, radius/tibia, and ulna/ fibula) were absent. Additionally to the previous local scrutiny of the cases, the collected data were reviewed by three of the authors (E.B.-S., M.-L.M.-F., and P.M.), involving the participating program directors to verify that only true phocomelia cases were to be analyzed for this study. After a detailed clinical assessment of all the case records, in order to identify those with known syndromes, the cases were divided into isolated and those with multiple congenital anomaly (MCA). Cases with recognized syndromes were excluded from subsequent analyses since their cause is either already known or suspected, and one of the aims of this study was to find clues on causes of this congenital anomaly. Therefore, the analyses of variables were restricted to the groups of cases that had isolated phocomelia versus those with MCA.
Figure 1.
Clinical photos of some phocomelia cases showing different expressions of the defect. (a), (b-1), (b-2) and (b-3): Two cases with bilateral phocomelia. (c): Unilateral phocomelia, with just some structures of the hand; (d): unilateral phocomelia of the lower limb; (f): see radiologic detail of a case in which different expressions of phocomelia can be observed in the four limbs; (g): only lower limbs involvement. Courtesy of Dr. Salvador Martínez, Dr. Amparo Sanchis, Dr. Consuelo García, Dr. Jaume Rosal, Dr. Manuel Blanco, and Dr. Ignacio Arroyo.
The total prevalence estimate of phocomelia was computed by surveillance program (LB + SB + ETOPFA cases divided by LB + SB) with its 95% confidence interval (CI) according to the Poisson distribution. More details on the statistical methodology used in this project on phocomelia are provided by Castilla and Mastroiacovo [2011] in this issue of the journal.
Distributions for categorical variables were compared with χ2 tests or Fisher’s exact tests. Prevalence ratios (PR) for maternal age groups relative to the reference age group of mothers younger than 20 years, with corresponding 95% CI were calculated. The odds of developing phocomelia with MCA compared with isolated phocomelia in relation to specific variables was estimated with odds ratios (ORs) and their 95% CI. An adjusted OR (aOR) was obtained after adjustment for participating programs, based on each program’s percentage of MCA cases, by tertile. Those surveillance programs with missing data for more than 20% for each variable were excluded from those analyses. We conducted the logistic regression analyses with Stata (Statistics/ Data Analysis) Special Edition 8.0 program. The P-values lower than 0.05 were considered statistically significant. More detailed information on the variables, data gathered and analyses are provided in the introductory article by Castilla and Mastroiacovo [2011].
Taking advantage of the fact that another study similar to this one on phocomelia was performed on amelia [Bermejo-Sánchez et al., 2011], we gathered data with the same methodology for both defects and with equivalent analyses. The results of the comparison of epidemiological characteristics of phocomelia and amelia are shown in this paper. One of the comparisons performed was that of MCA associated with phocomelia versus amelia, by calculating the PR, as the prevalence of associated defects among nonsyndromic phocomelia cases divided by the prevalence of associated defects among nonsyndromic amelia cases, and establishing the comparison with χ2 tests or Fisher’s exact tests.
RESULTS
There were a total of 141 cases of phocomelia identified among 22,740,933 births (LB, SB and, for some programs, ETOPFA). Therefore, the overall total prevalence was 0.62 per 100,000 (95% CI: 0.52–0.73). Accordingly, there is at least one case with phocomelia in every 136,986–192,308 births.
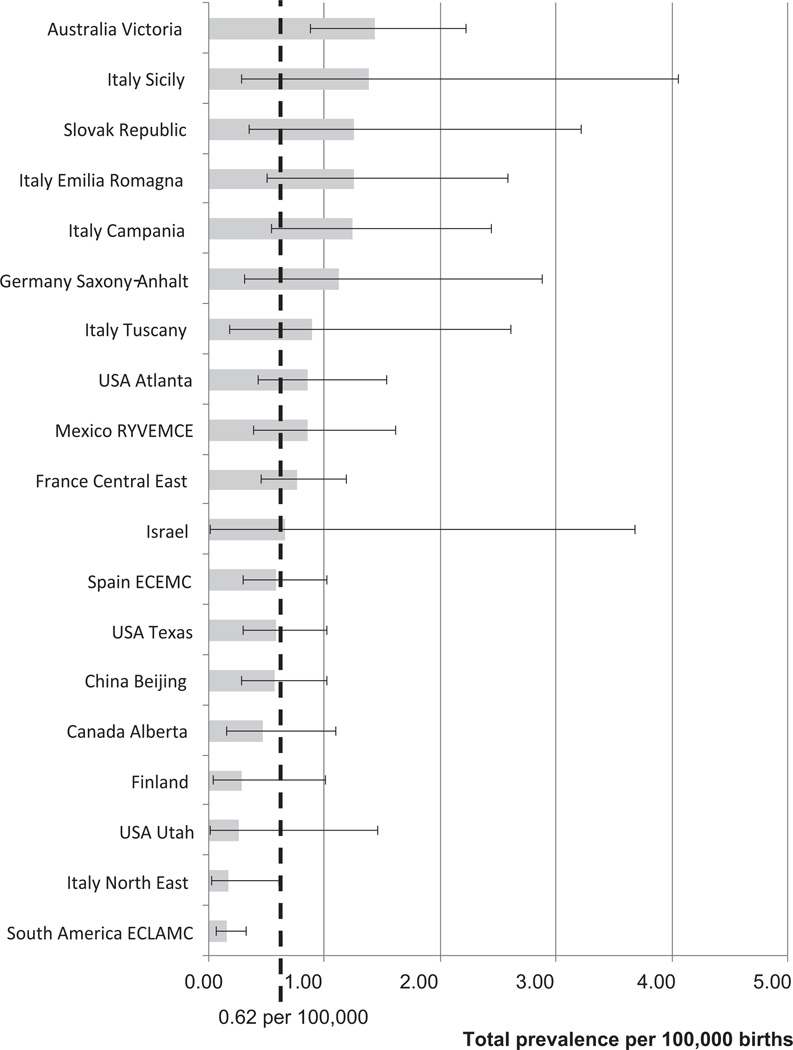
Table II shows the distribution and total prevalence of phocomelia cases by participating program. For each program, the study period, number of births surveyed, number of phocomelia cases, percentage of SB and ETOPFA, total prevalence, and 95% CI are shown. Four programs (France Central East, Australia Victoria, USA Texas, and Spain ECEMC) contributed close to 50% of cases. Figure 2 represents the total prevalence by program (and 95% CI), sorted by decreasing total prevalence, and together with the overall total prevalence represented as a vertical dashed line, for comparison. The total prevalences by program were not significantly different from the overall total prevalence, except for Australia Victoria, where the total prevalence was significantly higher (1.44 per 100,000; CI: 0.88–2.22; P = 0.0006), and for South America ECLAMC (0.15 per 100,000; CI: 0.06–0.32; P < 0.0001), and Italy North East (0.17 per 100,000; CI: 0.02–0.61; P = 0.023) where the total prevalence was significantly lower.
Figure 2.
Total prevalence of phocomelia per 100,000 births (bar) and 95% confidence interval (bracketed line) by surveillance program, and overall total prevalence (dotted line), in 19 surveillance programs of the International Clearinghouse for Birth Defects Surveillance and Research.
With respect to the clinical presentation of phocomelia, 53.2% of cases (75 out of 141) were isolated (only had phocomelia), 36.9% (52/141) had additional major malformations (MCA), and 9.9% (14/141) were associated with different syndromes. Therefore, phocomelia was observed as an isolated defect in about half of the cases. The syndromes registered among phocomelia cases, by decreasing prevalence, were: Roberts syndrome (5 cases), thrombocytopenia with radial aplasia (TAR) (3 cases), the “syndrome of severe limb defects, vertebral hypersegmentation, and mirror polydactyly,” with suggested autosomal recessive inheritance [Urioste et al., 1996; Martínez-Frías et al., 1997] (2 cases), trisomy 18 (2 cases), a derivative chromosome X (1 case), and Nager syndrome (1 case). Cases with recognized syndromes were excluded from further epidemiological analyses.
Table III shows the distribution of the remaining nonsyndromic phocomelia cases by limb involvement. Most cases had only one (monomelic, 55.9%) or two (dimelic, 40.2%) limbs involved. Four cases had phocomelia of the four limbs. Among monomelic cases, the limb involved was more often on the left side (64.9%) and an upper limb (64.9%). Among dimelic cases, the upper limbs were also more often involved (58.5%) than the lower limbs.
TABLE III.
Distribution of Nonsyndromica Phocomelia Cases by Number of Affected Limbs, Upper/Lower Limb Involvement, and Laterality of the Defect, Among 19 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| N | % | % Of total cases | |
|---|---|---|---|
| Monomelic | |||
| Upper right | 15 | 26.3 | |
| Upper left | 22 | 38.6 | |
| Lower right | 5 | 8.8 | |
| Lower left | 15 | 26.3 | |
| Total monomelic | 57 | 100 | 55.9 |
| Dimelic | |||
| Upper/upper | 24 | 58.5 | |
| Lower/lower | 11 | 26.8 | |
| Upper/lower | 6 | 14.6 | |
| Total dimelic | 41 | 100 | 40.2 |
| Trimelic | 0 | — | 0 |
| Tetramelic | 4 | — | 3.9 |
| Total (specified) | 102 | 100 | 100 |
Syndromic cases (n = 14) were excluded from the analysis.
Table IV summarizes some characteristics of the nonsyndromic cases (total, and distributed as isolated or in MCA) with phocomelia. Overall, the male-to-female ratio was 1.23 (65/53). Among isolated cases the male-to-female ratio was 1.11, and among MCA cases it was 1.44. Of the seven cases with sexual ambiguity, only four had specific data on the limb(s) involved, and interestingly all of them had the lower limbs involved, and one also had the upper right limb affected.
TABLE IV.
Characteristics of Nonsyndromica Cases With Phocomelia and by Clinical Phenotype Among 19 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| All casesa (n = 127a) |
Cases with isolated phocomelia (n = 75) |
Cases with phocomelia and MCA (n = 52) |
||||
|---|---|---|---|---|---|---|
| Variables | n | (%) | n | (%) | n | (%) |
| Sex | ||||||
| Male | 65 | 51.2 | 39 | 52.0 | 26 | 50.0 |
| Female | 53 | 41.7 | 35 | 46.7 | 18 | 34.6 |
| Indeterminate | 7 | 5.5 | 0 | 0.0 | 7 | 13.5 |
| Missing data | 2 | 1.6 | 1 | 1.3 | 1 | 1.9 |
| Outcome | ||||||
| Live births | 85 | 66.9 | 57 | 76.0 | 28 | 53.8 |
| Stillbirths | 24 | 18.9 | 8 | 10.7 | 16 | 30.8 |
| ETOPFA | 18 | 14.2 | 10 | 13.3 | 8 | 15.4 |
| Missing data | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Birth weight among live births (g) | ||||||
| <1,500 | 12 | 14.1 | 6 | 10.5 | 6 | 21.4 |
| 1,500–2,499 | 27 | 31.8 | 16 | 28.1 | 11 | 39.3 |
| ≥2,500 | 40 | 47.1 | 33 | 57.9 | 7 | 25.0 |
| Missing data | 6 | 7.1 | 2 | 3.5 | 4 | 14.3 |
| Gestational age among live births (weeks) | ||||||
| <32 | 10 | 11.8 | 5 | 8.8 | 5 | 17.9 |
| 32–36 | 19 | 22.4 | 9 | 15.8 | 10 | 35.7 |
| ≥37 | 52 | 61.2 | 40 | 70.2 | 12 | 42.9 |
| Missing data | 4 | 4.7 | 3 | 5.3 | 1 | 3.6 |
| Parity | ||||||
| 0 | 32 | 25.2 | 18 | 24.0 | 14 | 26.9 |
| 1 | 34 | 26.8 | 23 | 30.7 | 11 | 21.2 |
| ≥2 | 26 | 20.5 | 18 | 24.0 | 8 | 15.4 |
| Missing data | 35 | 27.6 | 16 | 21.3 | 19 | 36.5 |
| Previous spontaneous abortions | ||||||
| 0 | 55 | 43.3 | 38 | 50.7 | 17 | 32.7 |
| ≥1 | 10 | 7.9 | 5 | 6.7 | 5 | 9.6 |
| Missing data | 62 | 48.8 | 32 | 42.7 | 30 | 57.7 |
| Plurality | ||||||
| Singleton | 116 | 91.3 | 68 | 90.7 | 48 | 92.3 |
| Twin | 4 | 3.1 | 2 | 2.7 | 2 | 3.8 |
| Missing data | 7 | 5.5 | 5 | 6.7 | 2 | 3.8 |
| Maternal age | ||||||
| <20 | 12 | 9.4 | 4 | 5.3 | 8 | 15.4 |
| 20–24 | 25 | 19.7 | 14 | 18.7 | 11 | 21.2 |
| 25–29 | 34 | 26.8 | 17 | 22.7 | 17 | 32.7 |
| 30–34 | 29 | 22.8 | 20 | 26.7 | 9 | 17.3 |
| 35–39 | 11 | 8.7 | 7 | 9.3 | 4 | 7.7 |
| ≥40 | 5 | 3.9 | 5 | 6.7 | 0 | 0.0 |
| Missing data | 11 | 8.7 | 8 | 10.7 | 3 | 5.8 |
| Parental age difference | ||||||
| Mother older | 16 | 12.6 | 11 | 14.7 | 5 | 9.6 |
| Mother same age or 2 years younger | 19 | 15.0 | 10 | 13.3 | 9 | 17.3 |
| Mother 3–4 years younger | 7 | 5.5 | 4 | 5.3 | 3 | 5.8 |
| Mother>4 years younger | 12 | 9.4 | 6 | 8.0 | 6 | 11.5 |
| Missing data | 73 | 57.5 | 44 | 58.7 | 29 | 55.8 |
| Maternal education (years) | ||||||
| <9 | 15 | 11.8 | 8 | 10.7 | 7 | 13.5 |
| ≥9 | 50 | 39.4 | 30 | 40.0 | 20 | 38.5 |
| Missing data | 62 | 48.8 | 37 | 49.3 | 25 | 48.1 |
Syndromic cases (n = 14) were excluded from the analysis.
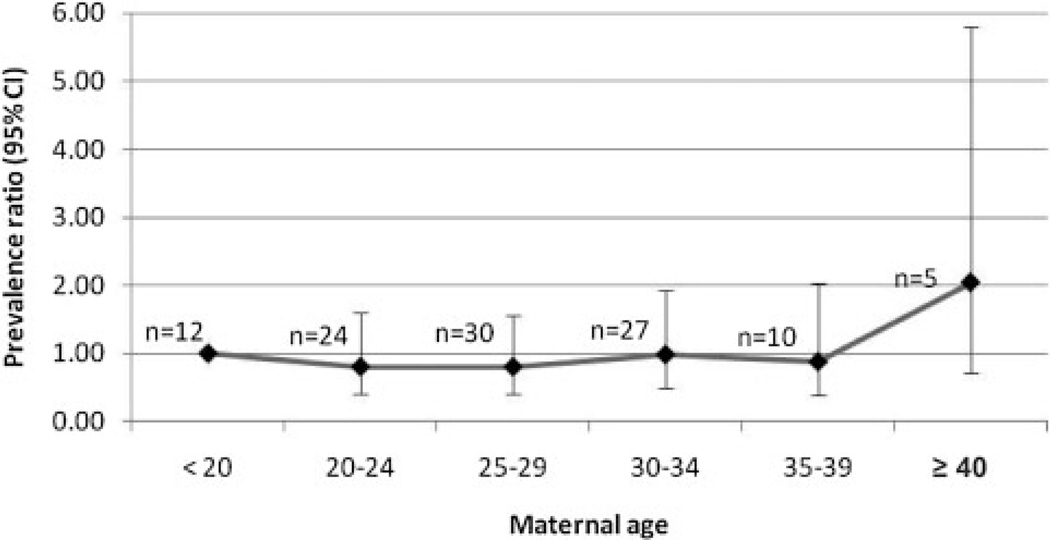
In regards to pregnancy outcomes, 66.9% of 127 phocomelia cases (75 isolated plus 52 with MCA) were LB, 18.9% were SB, and 14.2% were ETOPFA (these percentages are slightly different from those shown in Table II because Table II includes the 14 syndromic cases). Among the isolated cases, 76% were LB, while only 53.8% were LB among those with MCA. The percentage of ETOPFA was similar among isolated (13.3%) and among MCA phocomelia cases (15.4%). Regarding the birth weight of LB cases, most of the isolated cases (57.9%) weighed 2,500 g or more, while the majority of cases with associated malformations weighed less than 2,500 g (60.7%). With respect to the gestational age among LB, most of the isolated cases (70.2%) were born at term (≥37 weeks), and most of the MCA cases were preterm infants (53.6%). For the other characteristics listed in Table IV, except for plurality and maternal age, there were high percentages of missing data. Only 3.1% of the cases (N = 120) were twins. Regarding maternal age, as it can be observed in Figure 3, representing the PR for phocomelia by maternal age group (reference group: <20 years), there was no statistically significant trend or difference among the maternal age groups considered.
Figure 3.
Prevalence ratios for maternal age groups relative to the reference age of <20 years with corresponding 95% CIs for phocomelia in 17 surveillance programs★ of the International Clearinghouse for Birth Defects Surveillance and Research (syndromic cases excluded). ★Cases and births excluded for the following programs because no births by maternal age were available: China Beijing <1997 and >2003, Germany Saxony– Anhalt <1991, Italy Emilia Romagna <1985, Italy North East, Italy Sicily.
Table V depicts the crude and aOR for associations of the various maternal and case characteristics shown in Table IV, for MCA cases with phocomelia compared with isolated phocomelia cases. MCA cases were more commonly SB (aOR = 6.70, CI: 1.40– 32.00) and ETOPFA (aOR = 4.47, CI: 1.21 – 16.53) than the isolated cases, and weighed less than 2,500 g more frequently than the isolated cases. For the other variables included in Table V, no statistically significant difference between isolated and MCA cases was obtained.
TABLE V.
Crude and Adjusted Odds Ratios (OR) With 95% Confidence Intervals (95% CI) for the Association of Various Characteristics Among Multiple Congenital Anomalies Cases (Cases) Versus Isolated Cases (controls) of Phocomelia Reported by 19 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research
| Crude OR | 95% CI | Adjusted OR (aOR)a |
95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 1.00 | Referent | 1.00 | Referent | ||||
| Female | 0.77 | 0.36 | 1.64 | 0.72 | 0.32 | 1.63 | ||
| Outcome | ||||||||
| Live births | 1.00 | Referent | 1.00 | Referent | ||||
| Stillbirths | 4.47 | 1.17 | 17.15 | 6.70 | 1.40 | 32.00 | ||
| ETOPFA | 2.04 | 0.70 | 6.01 | 4.47 | 1.21 | 16.53 | ||
| Birth weight among live births (g) | ||||||||
| <1,500 | 4.71 | 1.17 | 19.02 | 6.60 | 1.20 | 36.31 | ||
| 1,500–2,499 | 3.24 | 1.06 | 9.93 | 4.66 | 1.20 | 18.15 | ||
| ≥2,500 | 1.00 | Referent | 1.00 | Referent | ||||
| Gestational age among live births (weeks) | ||||||||
| <32 | 3.33 | 0.82 | 13.48 | 2.72 | 0.76 | 9.74 | ||
| 32–36 | 3.70 | 1.22 | 11.21 | 4.06 | 0.77 | 21.41 | ||
| ≥37 | 1.00 | Referent | 1.00 | Referent | ||||
| Parity | ||||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||||
| 1 | 0.77 | 0.26 | 2.28 | 0.49 | 0.14 | 1.66 | ||
| ≥2 | 0.76 | 0.24 | 2.37 | 0.42 | 0.11 | 1.58 | ||
| Previous spontaneous abortions | ||||||||
| 0 | 1.00 | Referent | 1.00 | Referent | ||||
| ≥1 | 2.77 | 0.69 | 11.14 | 4.45 | 0.84 | 23.59 | ||
| Plurality | ||||||||
| Single | 1.00 | Referent | 1.00 | Referent | ||||
| Twin | 1.42 | 0.19 | 10.41 | 0.86 | 0.09 | 8.12 | ||
| Maternal age | ||||||||
| <20 | 1.00 | Referent | 1.00 | Referent | ||||
| 20–24 | 0.39 | 0.09 | 1.65 | 0.65 | 0.13 | 3.18 | ||
| 25–29 | 0.50 | 0.13 | 1.98 | 0.86 | 0.19 | 3.94 | ||
| 30–34 | 0.22 | 0.05 | 0.94 | 0.35 | 0.07 | 1.67 | ||
| 35–39 | 0.28 | 0.05 | 1.59 | 0.63 | 0.09 | 4.29 | ||
| ≥40 | — | — | ||||||
| Parental age difference | ||||||||
| Mother older | 0.50 | 0.12 | 2.02 | 0.61 | 0.11 | 3.26 | ||
| Mother same age or 2 years younger |
1.00 | Referent | 1.00 | Referent | ||||
| Mother 3–4 years younger | 0.83 | 0.14 | 4.78 | 1.01 | 0.12 | 3.26 | ||
| Mother >4 years younger | 1.11 | 0.26 | 4.72 | 0.85 | 0.15 | 4.71 | ||
| Maternal education (years) | ||||||||
| <9 | 1.40 | 0.42 | 4.62 | 0.75 | 0.19 | 2.95 | ||
| ≥9 | 1.00 | Referent | 1.00 | Referent | ||||
OR computed only for the 15 programs reporting ETOPFA; Surveillance programs with more than 20% missing data were excluded from the analysis; fourteen cases with syndromes were excluded from the analysis.
ETOPFA, elective termination of pregnancy for fetal anomalies; aOR, adjusted odds ratio
Adjustments were made for tertiles of percentage of MCA cases in each program.
Table VI summarizes the frequency of associated defects (excluding other limb reduction defects) among non-syndromic MCA phocomelia, according to the three-digit level of the International Classification of Diseases, Tenth Revision (ICD-10) classification system. Congenital deformities of feet; spine and bony thorax; and other musculoskeletal malformations were each present in 28.8% of cases; other congenital malformations of the limbs; and defects of cardiac septa in 26.9% of cases; absence, atresia or stenosis of large intestine; and congenital malformations of the face and neck in 17.3%; indeterminate sex and pseudohermaphroditism in 15.4%; and hydrocephalus in 13.5%. Congenital malformations of great arteries, malformations of the lung, cleft palate, renal agenesis and other reduction of kidney, congenital malformations of hips, polydactyly and syndactyly were each present in 11.5% of nonsyndromic phocomelia cases with MCA.
TABLE VI.
Prevalence of Associated Defects Among Nonsyndromic Phocomelia Cases, Excluding Other Limb Reduction Defects, Reported by 19 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research, and Comparison With the Prevalence of Associated Defects Among Nonsyndromic Amelia [Bermejo-Sánchez et al., 2011] (the ICD-10 Codes for Which No Case Was Registered Are Not Listed)
| Associated defects | ICD-10 code (3 digits) |
Phocomelia | Amelia | PR | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Anencephaly | Q00 | 2 | 3.8 | 22 | 10.1 | 0.4 |
| Encephalocele | Q01 | 3 | 5.8 | 13 | 6.0 | 1.0 |
| Microcephaly | Q02 | 3 | 5.8 | 2 | 0.9 | 6.3 |
| Congenital hydrocephalus | Q03 | 7 | 13.5 | 18 | 8.3 | 1.6 |
| Other CM of brain | Q04 | 1 | 1.9 | 14 | 6.4 | 0.3 |
| Spina bifida | Q05 | 2 | 3.8 | 9 | 4.1 | 0.9 |
| Other CM of spinal cord | Q06 | 0 | 0.0 | 2 | 0.9 | 0.0 |
| CM of eyelid, lacrimal apparatus and orbit | Q10 | 1 | 1.9 | 2 | 0.9 | 2.1 |
| Anophthalmos, microphtalmos and macrophthalmos | Q11 | 3 | 5.8 | 15 | 6.9 | 0.8 |
| CM of the lens | Q12 | 0 | 0.0 | 1 | 0.5 | 0.0 |
| CM of posterior segment of eye | Q14 | 0 | 0.0 | 1 | 0.5 | 0.0 |
| Other CM of eye | Q15 | 1 | 1.9 | 8 | 3.7 | 0.5 |
| CM of ear causing impairment of hearing | Q16 | 4 | 7.7 | 6 | 2.8 | 2.8 |
| Other CM of ear | Q17 | 5 | 9.6 | 20 | 9.2 | 1.0 |
| Other CM of face and neck | Q18 | 9 | 17.3 | 18 | 8.3 | 2.1 |
| CM of cardiac chambers and connections | Q20 | 2 | 3.8 | 6 | 2.8 | 1.4 |
| CM of cardia septa | Q21 | 14 | 26.9 | 24 | 11.0 | 2.4★ |
| CM of pulmonary and tricuspid valves | Q22 | 2 | 3.8 | 5 | 2.3 | 1.7 |
| CM of aortic and mitral valves | Q23 | 2 | 3.8 | 3 | 1.4 | 2.8 |
| Other CM of heart | Q24 | 3 | 5.8 | 14 | 6.4 | 0.9 |
| CM of great arteries | Q25 | 6 | 11.5 | 7 | 3.2 | 3.6★ |
| CM of great veins | Q26 | 1 | 1.9 | 1 | 0.5 | 4.2 |
| Other CM of peripheral vascular system | Q27 | 0 | 0.0 | 15 | 6.9 | 0.0 |
| Other CM of circulatory system | Q28 | 0 | 0.0 | 1 | 0.5 | 0.0 |
| CM of nose | Q30 | 3 | 5.8 | 8 | 3.7 | 1.6 |
| CM of larynx | Q31 | 1 | 1.9 | 0 | 0.0 | — |
| CM of lung | Q33 | 6 | 11.5 | 17 | 7.8 | 1.5 |
| Other CM of respiratory system | Q34 | 0 | 0.0 | 7 | 3.2 | 0.0 |
| Cleft palate | Q35 | 6 | 11.5 | 6 | 2.8 | 4.2★ |
| Cleft lip | Q36 | 1 | 1.9 | 6 | 2.8 | 0.7 |
| Cleft palate with cleft lip | Q37 | 2 | 3.8 | 24 | 11.0 | 0.3 |
| Other CM of tongue, mouth and pharynx | Q38 | 0 | 0.0 | 6 | 2.8 | 0.0 |
| CM of esophagus | Q39 | 3 | 5.8 | 8 | 3.7 | 1.6 |
| Other CM of upper alimentary tract | Q40 | 1 | 1.9 | 0 | 0.0 | — |
| Absence, atresia and stenosis of small intestine | Q41 | 3 | 5.8 | 4 | 1.8 | 3.1 |
| Absence, atresia and stenosis of large intestine | Q42 | 9 | 17.3 | 41 | 18.8 | 0.9 |
| Other CM of intestine | Q43 | 0 | 0.0 | 13 | 6.0 | 0.0 |
| CM of gallbladder, bile ducts and liver | Q44 | 3 | 5.8 | 4 | 1.8 | 3.1 |
| Other CM of digestive system | Q45 | 1 | 1.9 | 2 | 0.9 | 2.1 |
| CM of ovaries, fallopian tubes and broad ligaments | Q50 | 0 | 0.0 | 12 | 5.5 | 0.0 |
| CM of uterus and cervix | Q51 | 0 | 0.0 | 8 | 3.7 | 0.0 |
| Other CM of female genitalia | Q52 | 0 | 0.0 | 9 | 4.1 | 0.0 |
| Undescended and ectopic testicle | Q53 | 3 | 5.8 | 7 | 3.2 | 1.8 |
| Hypospadias | Q54 | 1 | 1.9 | 4 | 1.8 | 1.0 |
| Other CM of male genital organs | Q55 | 3 | 5.8 | 11 | 5.0 | 1.1 |
| Indeterminate sex and pseudohermaphroditism | Q56 | 8 | 15.4 | 32 | 14.7 | 1.0 |
| Renal agenesis and other reduction defects of kidney | Q60 | 6 | 11.5 | 36 | 16.5 | 0.7 |
| Cystic kidney disease | Q61 | 3 | 5.8 | 7 | 3.2 | 1.8 |
| Cong. obstructive defects of renal pelvis and CM of ureter | Q62 | 1 | 1.9 | 17 | 7.8 | 0.2 |
| Other CM of kidney | Q63 | 1 | 1.9 | 8 | 3.7 | 0.5 |
| Other CM of urinary system | Q64 | 2 | 3.8 | 11 | 5.0 | 0.8 |
| Congenital deformities of hips | Q65 | 6 | 11.5 | 3 | 1.4 | 8.4★★ |
| Congenital deformities of feet | Q66 | 15 | 28.8 | 30 | 13.8 | 2.1★★ |
| Musculoskeletal deformities of head, face, spine and chest | Q67 | 5 | 9.6 | 30 | 13.8 | 0.7 |
| Other congenital musculoskeletal deformities | Q68 | 4 | 7.7 | 9 | 4.1 | 1.9 |
| Polydactyly | Q69 | 6 | 11.5 | 6 | 2.8 | 4.2★ |
| Syndactyly | Q70 | 6 | 11.5 | 14 | 6.4 | 1.8 |
| Other CM of limb(s) | Q74 | 14 | 26.9 | 46 | 21.1 | 1.3 |
| Other CM of skull and face bones | Q75 | 1 | 1.9 | 11 | 5.0 | 0.4 |
| CM of spine and bony thorax | Q76 | 15 | 28.8 | 49 | 22.5 | 1.3 |
| Other musculoskeletal CM, not elsewhere classified | Q79 | 15 | 28.8 | 87 | 39.9 | 0.7 |
| Other CM of skin | Q82 | 2 | 3.8 | 12 | 5.5 | 0.7 |
| CM of breast | Q83 | 3 | 5.8 | 3 | 1.4 | 4.2 |
| Other CM of integument | Q84 | 0 | 0.0 | 3 | 1.4 | 0.0 |
| Phacomatosis, not elsewhere classified | Q85 | 1 | 1.9 | 0 | 0.0 | — |
| Other specified syndromes affecting multiple systems | Q87 | 0 | 0.0 | 17 | 7.8 | 0.0 |
| Other CM, not elsewhere classified | Q89 | 5 | 9.6 | 18 | 8.3 | 1.2 |
| Total | 52 | 100.0 | 218 | 100.0 | ||
CM, congenital malformations; PR, prevalence ratio, prevalence of associated defects among nonsyndromic phocomelia cases, divided by prevalence of associated defects among nonsyndromic amelia cases.
P<0.05.
P<0.01.
COMPARISON OF CHARACTERISTICS OF PHOCOMELIA AND AMELIA
Another collaborative study of the ICBDSR was performed for amelia with an identical methodology [Bermejo-Sánchez et al., 2011] and studying the same variables, and this provides a unique opportunity to compare the results obtained for these two rare severe defects affecting the limbs. In the last column of Table VI, the PR is presented to estimate how many times a defect is more or less frequent among nonsyndromic MCA phocomelia cases than among those with amelia. Some defects were significantly more frequent among phocomelia than among amelia cases: congenital deformities of the hips or feet (P < 0.01); cleft palate only (without cleft lip); polydactyly; and congenital malformations of the great arteries or cardiac septa (P < 0.05). No defect was significantly more frequent among amelia than among phocomelia cases.
With respect to the other aspects studied for both defects, we observed (data not shown in a joint table, although the data for both defects are shown in this article for phocomelia and in Bermejo-Sánchez et al. [2011] for amelia) that the proportion of LB cases was significantly lower for amelia than for phocomelia cases (P = 0.01). Regarding the clinical presentation of both defects (in the groups of isolated, MCA, and syndromes), phocomelia presented as an isolated defect more frequently than amelia, and was observed in more cases with syndromes (P < 0.0000001). There was not any statistically significant difference between both defects in the number of involved limbs among nonsyndromic cases, although amelia seems to be monomelic more frequently (64.1%) than phocomelia (55.9%). With respect to laterality of the defect, phocomelia seems to affect the left side (64.9%) more frequently than amelia (50.0%) among nonsyndromic monomelic cases, although again no statistically significant difference was observed. While for amelia an increased risk was found among young mothers, there was no relationship with any maternal age strata for phocomelia. We did not find any statistically significant difference between phocomelia and amelia regarding the male-to-female sex ratio, birth weight, and gestational age of LB cases, and twinning, after having compared both defects separately for isolated, MCA, and total nonsyndromic cases. The comparison between phocomelia and amelia for the aOR of the association of those characteristics to MCA cases, did not reveal any statistically significant difference.
DISCUSSION
After a thorough review of the literature, we were not able to identify even a single published study specifically focused on the epidemiology of phocomelia. Phocomelia has generally been studied jointly in the context of other intercalary defects, together with other severe limb reduction defects like amelia, or as part of the general group of limb reduction defects. Therefore, to our knowledge, this epidemiological study is the first one known to date specifically performed on phocomelia separately from other intercalary limb defects. Furthermore, our case definition established the inclusion of only true phocomelia cases for our analyses, what is also exceptional.
This is also the first time a comparison is performed between the epidemiological characteristics of phocomelia and amelia, another severe and very rare defect involving the limbs.
One of the main challenges we had to face was the critical review of the cases to include only true phocomelia, according to the study protocol, that is, cases with total absence of intercalary structures, with hand/foot present. Based on our experience, for the evaluation of cases with intercalary defects, it is important to clearly define the bones affected, and for these purposes it is essential to have a good radiological examination of the limb, which also helps clearly distinguish true phocomelia cases. Also, in cases of ETOPFA, a complete pathological study (including radiological examination) of the fetus is mandatory in order to precisely define not only phocomelia but all the defects present in the fetus (what is essential to provide an accurate counseling to the parents regarding recurrence risks and the possibilities of early detection in future pregnancies).
Another common problem is classification. The general definition of phocomelia includes the codes Q71.1 (congenital absence of upper arm and forearm with hand present), Q72.1 (congenital absence of thigh and lower limb with foot present), and Q73.1 (phocomelia, unspecified limb(s)) of the ICD-10-CM classification system. The pediatric adaptation of ICD-10 codes made by the Royal College of Paediatrics and Child Health Classification (ICD-BPA), is used by many programs [Castilla and Mastroiacovo, 2011], but totally fits in the ICD for phocomelia. However, it is not uncommon that some cases with severe or even less severe hypoplasia of the intercalary long bones could also be included in those codes, used as the best approximation to the defect observed. Misclassification of phocomelia cases may be a common problem. For instance, Goldfarb et al. [2005] reviewed 41 patients previously classified as phocomelia, and none of them had a true intercalary deficiency. To solve this problem, some ICBDSR programs have created their own additional codes to separate true phocomelia cases from those having other intercalary defects. This approach could be recommended for anybody planning to study phocomelia in the future. Historic difficulties will remain because ICD codes do not differentiate between true phocomelia and other types of severe intercalary defects.
Regarding the total prevalence of phocomelia, only three out of the 19 programs had rates significantly different from the group average; it was higher in Australia Victoria, and lower in South America ECLAMC, and Italy North East. South America ECLAMC has a strict working definition, and is able to differentiate cases of true phocomelia from those having even a minimal bony structure between the trunk and the terminal part of the limb, which are classified as incomplete or atypical phocomelia. This strict definition applies to other programs like Spain-ECEMC and others. Although real differences in total prevalence cannot be ruled out, in spite of a critical review of all the cases, some misclassification could have played a role in the results shown in Table II, as the information available for some cases was less documented. As we have commented, based on our experience, it is crucial to have a good radiological examination of the limb, and the complete necropsy of ETOPFA cases. Problems of misclassification could be present also in the scarce data on the prevalence of phocomelia in the literature. For instance, Källén et al. [1984] estimated it occurring in 4.2 per 100,000 births, after studying 1,368,024 births. This figure seems high for true phocomelia and it is unclear whether only true phocomelia or other intercalary defects were included as well.
With respect to the clinical presentation of phocomelia, half (53.2%) of the cases in our study had an isolated defect, similar to the 50% reported by Evans et al. [1994]. Because half of the cases have associated defects, this has implications in prenatal and postnatal diagnosis: for example, when phocomelia is identified in a fetus or a baby, a thorough search for other associated anomalies is warranted to identify promptly less apparent structural malformations and manage accordingly, because it is likely that other defects are also present in one out of two affected infants or fetuses. Of course, that search should be as complete as possible, but, based on our data, it should especially focus on the musculo-skeletal system, the heart, and large intestine, which were among the organ systems most frequently affected with associated defects. In this sense, we stress that the percentage of ETOPFA was similar among isolated (13.3%) and among MCA phocomelia cases (15.4%), which could indicate that in general phocomelia is the defect that caused the interruption of pregnancy in ETOPFA cases.
We found an excess of upper limb involvement (64.9% among monomelic cases). This is concordant with the results of Källén et al. [1984], who observed that 68.8% of the cases of phocomelia had involvement of the upper limbs. However, in contrast to that study, in which the right side was involved in 29.2% of cases (22.9% had the left side involved, and 47.9% were bilateral), we found that the left side was more commonly involved (64.9%) in an almost threefold larger sample. However, differences in the working definition of true phocomelia could account for the lower proportion of left side involvement in the study by Källén et al. [1984].
Compared with isolated phocomelia cases, we found that those with MCA had low birth weights much more frequently, and we consider that they may be affected by some additional factors causing intrauterine growth retardation as their gestational ages did not differ significantly from those of the isolated cases.
It is true that there could be some clinical and etiological heterogeneity in the groups considered in this study, as in others. Such heterogeneity could affect not only the MCA cases group, but also the isolated ones. Of course, it is not expected that all the MCA cases or all the isolated cases have a common unique cause. However, from this kind of epidemiological studies, which are descriptive and exploratory (also given the scarce data in the literature), we try to obtain clues on the etiology(ies). Such clues can open new avenues to conduct causal studies (epidemiological or genetic including microarray tests performed on the whole genome of phocomelia patients), on specific (groups of) factors, and on specific groups of phocomelia cases (with selected phenotypes).
We observed some epidemiological differences between phocomelia and amelia cases, consistent with possible differences in causes and pathogenesis of these two defects, as has been observed in multiple experimental studies. We would stress that the proportion of phocomelia LB is quite high (66.9% of our 127 cases with the defect in either isolated or MCA), and it is also relatively high among amelia cases (53.9%), both defects representing severe limb affectations, and determining considerable disabilities and dependence. This warrants more research on their possible causes.
Finally, just a note on the terminology, which is also important to properly classify cases, and to strictly select those fitting into the definition for specific studies. The term “phocomelia” is descriptive, and it alludes to the shape of the limb resembling that of a flipper on the seal. However, because of its potentially pejorative implications, we suggest its replacement by other equally descriptive but more neutral, specific and academic terminology: “Defect of intercalary structures of the limbs.” This alludes to the defect, which can be either an absence (in true phocomelia) or hypoplasia (in the other forms of intercalary limb defects) of those intercalary segments of the limb that should be clearly defined for each case.
ACKNOWLEDGMENTS
The authors are grateful to each monitoring system’s staff and members for their work in collecting case data and submission to the ICBDSR Centre. We also thank Dr. Adolfo Correa, from the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA (USA), for his helpful comments in the preparation of the manuscript. This work was in part supported by: Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain, and the Fundación 1000 sobre Defectos Congénitos, of Spain. CIBERER is an initiative of ISCIII. Components of ECEMC’s Peripheral Group are gratefully acknowledged. The work conducted at the ICBDSR Centre was supported by the Center for Disease Control and Prevention (1U50DD000524-02).
Grant sponsor for South America ECLAMC: MCT/CNPq, Brazil; Grant numbers: 573993/2008-4, 476978/ 2008-4, 554755/2009-2; 306750/2009-0; 402045/2010-6. The Tuscany Registry of Birth Defects is funded by the “Direzione Generale Diritti di cittadinanza e Coesione sociale—Regione Toscana”.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- Aldrovandus U. Monstrorum historia cum paralipomenis historiae omnium animalium. Bologna: N. Tebaldini; 1642. (Citeb by Czeizel et al. [1994]). [Google Scholar]
- Araujo M, Piedra ME, Herrera MT, Ros MA, Nieto MA. The expression and regulation of chick EphA7 suggests roles in limb patterning and innervation. Development. 1998;125:4195–4204. doi: 10.1242/dev.125.21.4195. [DOI] [PubMed] [Google Scholar]
- Aro T, Heinonen OP, Saxén L. Risk indicators of reduction limb defects. J Epidemiol Comm Health. 1983;37:50–56. doi: 10.1136/jech.37.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Sánchez E, Cuevas L, Amar E, Bakker MK, Bianca S, Bianchi F, Canfield MA, Castilla EE, Clementi M, Cocchi G, Feldkamp ML, Landau D, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Mutchinick OM, Rissmann A, Ritvanen A, Scarano G, Siffel C, Szabova E, Martínez-Frías ML. Amelia: A multi-center descriptive epidemiologic study in a large dataset from the International Clearinghouse for Birth Defects Surveillance and Research, and overview of the literature. Am J Med Genet C Semin Med Genet. 2011 doi: 10.1002/ajmg.c.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke RJ, Rappold G. The pseudoautosomal regions, SHOX and disease. Curr Opin Genet Dev. 2006;16:233–239. doi: 10.1016/j.gde.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Botting BJ. Limb reduction defects and coastal areas. Lancet. 1994;343:1033–1034. doi: 10.1016/s0140-6736(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Bouchard F. De infante monstroso Lugduni in viam publicam die V. Martii A. MDCLXXI exposito. Ephemeridem. 1681;3:14–17. Obs. 13. [Google Scholar]
- Brent RL, Holmes LB. Clinical and basic science lessons from the thalidomide tragedy: What have we learned about the causes of limb defects? Teratology. 1988;38:241–251. doi: 10.1002/tera.1420380308. [DOI] [PubMed] [Google Scholar]
- Calzolari E, Maservigi GP, Garani GP, Cocchi G, Magnani C, Milan M. Limb reduction defects in Emilia Romagna, Italy: Epidemiologic and genetic study in 173,109 consecutive births. J Med Genet. 1990;27:353–357. doi: 10.1136/jmg.27.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo P. Very rare defects: What can we learn? Am J Med Genet C Semin Med Genet. 2011 doi: 10.1002/ajmg.c.30315. (in press) [DOI] [PubMed] [Google Scholar]
- Castilla EE, Cavalcanti DP, Dutra MG, López-Camelo JS, Paz JE, Gadow EC. Limb reduction defects in South America. Br J Obstet Gynecol. 1995;102:393–400. doi: 10.1111/j.1471-0528.1995.tb11292.x. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Ashton-Prolla P, Barreda-Mejía E, Brunoni D, Cavalcanti DP, Correa-Neto J, Delgadillo JL, Dutra MG, Félix T, Giraldo A, Juárez N, López-Camelo JS, Nazer J, Orioli IM, Paz JE, Pessoto MA, Pino-Neto JM, Quadrelli R, Rittler M, Rueda S, Saltos M, Sánchez O, Schuler L. Thalido-mide, a current teratogen in South America. Teratology. 1996;54:273–277. doi: 10.1002/(SICI)1096-9926(199612)54:6<273::AID-TERA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Evans JA, Kodaj I, Lenz W. Congenital limb deficiencies in Hungary. Genetic and teratologic epidemiological studies. Budapest: Akadémiai Kiadó; 1994. [Google Scholar]
- Delrue MA, Lacombe D. Association of ectrodactyly and distal phocomelia. Genet Couns. 2002;13:319–325. [PubMed] [Google Scholar]
- Evans JA, Vitez M, Czeizel AE. Congenital abnormalities associated with limb deficiency defects: A population study based on cases from the Hungarian Congenital Malformation Registry (1975–1984) Am J Med Genet. 1994;49:52–66. doi: 10.1002/ajmg.1320490111. [DOI] [PubMed] [Google Scholar]
- Froster U, Baird P. Upper limb deficiencies and associated malformations: A population based study. Am J Med Genet. 1992;44:767–781. doi: 10.1002/ajmg.1320440611. [DOI] [PubMed] [Google Scholar]
- Froster U, Baird P. Congenital defects of lower limbs and associated malformations: A population based study. Am J Med Genet. 1993;45:60–64. doi: 10.1002/ajmg.1320450116. [DOI] [PubMed] [Google Scholar]
- Froster-Iskenius UG, Baird PA. Limb reduction defects in over one million consecutive live births. Teratology. 1989;39:127–135. doi: 10.1002/tera.1420390205. [DOI] [PubMed] [Google Scholar]
- Galloway JL, Delgado I, Ros MA, Tabin CJ. A reevaluation of X-irradiation-induced phocomelia and proximodistal limb patterning. Nature. 2009;460:400–404. doi: 10.1038/nature08117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb CA, Manske PR, Busa R, Mills J, Carter P, Ezaki M. Upper-extremity phocomelia reexamined: A longitudinal dysplasia. J Bone Joint Surg Am. 2005;87(12):2639–2648. doi: 10.2106/JBJS.D.02011. [DOI] [PubMed] [Google Scholar]
- ICBDSR (International Clearinghouse for Birth Defects Surveillance and Research) [Accessed July 29, 2011];2011a http://www.icbdsr.org.
- ICBDSR (International Clearinghouse for Birth Defects Surveillance and Research) Annual Report 2009 with data for 2007. Rome: ICBD; 2011b. [Accessed July 29, 2011]. http://www.icbdsr.org/file-bank/documents/ar2005/AR%202009_web.pdf. [Google Scholar]
- Isenflamm HF, Rosenmüller JC. Beiträge Für Die Zergliederungskunst. Leipzig: bey Karl Tauchnitz; 1800. p. 268. [Google Scholar]
- Källén K. Maternal smoking during pregnancy and limb reduction malformations in Sweden. Am J Public Health. 1997;87:29–32. doi: 10.2105/ajph.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B, Rahmani TMZ, Winberg J. Infants with congenital limb reduction registered in the Swedish register of congenital malformations. Teratology. 1984;29:73–85. doi: 10.1002/tera.1420290109. [DOI] [PubMed] [Google Scholar]
- Knobloch J, Rüther U. Shedding light on an old mystery: Thalidomide suppresses survival pathways to induce limb defects. Cell Cycle. 2008;7(9):1121–1127. doi: 10.4161/cc.7.9.5793. [DOI] [PubMed] [Google Scholar]
- Lenz W. Kindliche Mibildungen nach Medicament-Einnahme während der Grav-idität? Dtsch Med Wschr. 1961;86:2555–2556. [Google Scholar]
- Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:271. doi: 10.1016/s0140-6736(62)91943-8. [DOI] [PubMed] [Google Scholar]
- Lenz W. Genetics and limb deficiencies. Clin Orthop. 1980;148:9–17. [PubMed] [Google Scholar]
- Lin S, Marshall EG, Davidson GK, Roth GB, Druschel CM. Evaluation of congenital limb reduction defects in upstate New York. Teratology. 1993;47:127–135. doi: 10.1002/tera.1420470205. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Arroyo I, Bermejo E, Espinosa J, García MJ. Severe limb deficiencies, vertebral hypersegmentation, and mirror polydactyly: Two additional cases that expand the phenotype to a more generalized effect on blastogenesis. Am J Med Genet. 1997;73:205–209. doi: 10.1002/(sici)1096-8628(19971212)73:2<205::aid-ajmg18>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- McCredie J, Willert HG. Longitudinal limb deficiencies and the sclerotomes: An analysis of 378 dysmelic malformations induced by thalidomide. J Bone Joint Surg Br. 1999;81:9–23. doi: 10.1302/0301-620x.81b1.8448. [DOI] [PubMed] [Google Scholar]
- Meinecke P, Peper M. Intrauterine growth retardation, mild frontonasal dysplasia, phocomelic upper limbs with absent thumbs and a variety of internal malformations including choanal atresia, congenital heart defects, polysplenia, absent gall bladder as well as genitourinary anomalies. A possibly “new” MCA syndrome? Genet Couns. 1992;3:53–56. [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Murray RS, Keeling JW, Ellis PM, FitzPatrick DR. Symmetrical upper limb peromelia and lower limb phocomelia associated with a de novo apparently balanced reciprocal translocation. Clin Dysmorphol. 2002;11:87–90. doi: 10.1097/00019605-200204000-00002. [DOI] [PubMed] [Google Scholar]
- OMIM database: Online Mendelian Inheritance in Man, OMIM (TM) [Accessed July 29, 2011];McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD) http://www.ncbi.nlm.nih.gov/omim/
- Polednak AP, Janerich DT. Maternal factors in congenital limb-reduction defects. Teratology. 1985;32:41–50. doi: 10.1002/tera.1420320107. [DOI] [PubMed] [Google Scholar]
- Rosano A, Botto LD, Olney RS, Khoury MJ, Ritvanen A, Goujard J, Stoll C, Cocchi G, Merlob P, Mutchinick O, Cornel MC, Castilla EE, Martínez-Frías ML, Zampino G, Erickson JD, Mastroiacovo P. Limb defects associated with major congenital anomalies: Clinical and epidemiological study from the International Clearinghouse for Birth Defects Monitoring Systems. Am J Med Genet. 2000;93:110–116. doi: 10.1002/1096-8628(20000717)93:2<110::aid-ajmg6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Salzgeber B. Comparative study of the effects of nitrogen mustard on mesodermal and ectodermal limb bud components of chick embryos. J Embryol Exp Morphol. 1969;22:373–394. [PubMed] [Google Scholar]
- Salzgeber B. Ectodermal-mesodermal interactions in chick embryo limb buds treated with nitrogen mustard. Dev Growth Differ. 1975;17:295–296. doi: 10.1111/j.1440-169X.1975.00295.x. [DOI] [PubMed] [Google Scholar]
- Schuler-Faccini L, Soares RC, de Sousa AC, Maximino C, Luna E, Schwartz IV, Waldman C, Castilla EE. New cases of thalidomide embryopathy in Brazil. Birth Defects Res A Clin Mol Teratol. 2007;79:671–672. doi: 10.1002/bdra.20384. [DOI] [PubMed] [Google Scholar]
- Slingenberg B. In: Misvormingen van extremiteiten. De Erven F, Haarlem Bohn, editors. 1907. (Cited by Czeizel et al., 1994) [Google Scholar]
- Smith ESO, Charlotte SD, Miller JR, Banister P. An epidemiological study of congenital reduction deformities of the limbs. Br J Prev Soc Med. 1977;31:39–41. doi: 10.1136/jech.31.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell D, Lewis JH, Wolpert L. Positional information in chick limb morphogenesis. Nature. 1973;244:492–496. doi: 10.1038/244492a0. [DOI] [PubMed] [Google Scholar]
- Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA. 2009;106:8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle C, Alberts B, Wolpert L, Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982;296:564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Towers M, Tickle C. Generation of pattern and form in the developing limb. Int J Dev Biol. 2009;53:805–812. doi: 10.1387/ijdb.072499mt. [DOI] [PubMed] [Google Scholar]
- Urioste M, Lorda-Sánchez I, Blanco M, Burón E, Aparicio P, Martínez-Frías ML. Severe congenital limb deficiencies, vertebral hypersegmentation, absent thymus and mirror polydactyly: A defect expression of a developmental control gene? Hum Genet. 1996;97:214–217. doi: 10.1007/BF02265268. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. [Accessed July 29, 2011];2011 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107296.htm.
- Van der Horst RL, Gotsman MS. Anomalous origin of the subclavian artery associated with phocomelia. S Afr Med J. 1971;45:1397–1399. [PubMed] [Google Scholar]
- Vargesson N. Thalidomide-induced limb defects: Resolving a 50-year-old puzzle. Bioessays. 2009;31:1327–1336. doi: 10.1002/bies.200900103. [DOI] [PubMed] [Google Scholar]
- Vargesson N, Patel K, Lewis J, Tickle C. Expression patterns of Notch1, Serrate1, Serrate2 and Delta1 in tissues of the developing chick limb. Mech Dev. 1998;77:197–199. doi: 10.1016/s0925-4773(98)00138-5. [DOI] [PubMed] [Google Scholar]
- Verloes A, Frikiche A, Gremillet C, Paquay T, Decortis T, Rigo J, Senterre J. Proximal phocomelia and radial ray aplasia in fetal valproic syndrome. Eur J Pediatr. 1990;149:266–267. doi: 10.1007/BF02106290. [DOI] [PubMed] [Google Scholar]
- Wasserman CR, Shaw GM, O’Malley CD, Tolarova MM, Lammer E. Parental cigarette smoking and risk for congenital anomalies of the heart, neural tube, or limb. Teratology. 1996;53:261–267. doi: 10.1002/(SICI)1096-9926(199604)53:4<261::AID-TERA9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Weaver DD. Vascular etiology of limb defects: The subclavian artery supply disruption sequence. In: Herring JA, Birch JG, editors. The child with a limb deficiency. Chicago: American Academy of Orthopaedic Surgeons; 1998. pp. 25–37. [Google Scholar]
- Winter R, Baraitser M. Winter–Baraitser Dysmorphology Database. 2010 http://www.lmdatabases.com. Version 1.0.22.
- Wu H-T, Wainrwright H, Beighton P. Tetraphocomelia with the Waardenburg syndrome and multiple malformations. Clin Dysmorphol. 2009;18:112–115. doi: 10.1097/MCD.0b013e32832443f7. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]