Summary
Apolipoprotein A5 (apoA5) is a potent regulator of triglyceride (TG) metabolism and therefore may contribute to the pathogenesis of non-alcoholic fatty liver disease (NAFLD), a disease characterised by excessive TG-rich lipid droplets in hepatocytes. To test this hypothesis, we examined the mRNA expression of apoA5 in paediatric NAFLD livers in comparison to healthy controls. According to microarray and quantitative real-time PCR, human NAFLD livers exhibited elevated apoA5 expression compared to healthy controls. The apoA5 expression levels were positively correlated with hepatic TG storage and a marker for lipid droplets (perilipin), but were not correlated with plasma TG levels. These observations were confirmed with a NAFLD rat model. Interestingly, apoA5 expression was not altered in cultured fat-laden HepG2 cells, demonstrating that fat storage does not induce apoA5 in NAFLD livers. Therefore, the correlation between apoA5 and intracellular fat storage is likely explained by the potent effect of apoA5 in promoting intracellular fat storage. Our NAFLD patients and rats had elevated insulin resistance, which may have a role in elevating apoA5 expression in NAFLD livers. Our data support the hypothesis that apoA5 promotes hepatic TG storage and therefore contributes to the pathogenesis of NAFLD, and may represent a potential target for therapeutic intervention.
Keywords: apoA5, NAFLD, NASH, perilipin, triglyceride
INTRODUCTION
Apolipoprotein A5 (apoA5) is a low abundance plasma apolipoprotein (apo)1 involved in the regulation of plasma triglyceride (TG) levels.2 Transgenic mice overexpressing human apoA5 exhibited a 60% decrease in plasma TG concentrations, while the apoA5 knockout mice exhibited four times greater plasma TG compared to controls. These animals had normal plasma cholesterol concentrations.2
It has been proposed that the ability of apoA5 to regulate plasma TG is related to its ability to promote efficient TG hydrolysis by lipoprotein lipase (LPL).3,4 This mechanism also involves glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1 (GPIHBP1). Evidence for the latter is suggested by studies wherein intravenous injection of apoA5 into knockout mice reduced plasma TG levels by ~60%; however, no reduction in TG occurred in gpihbp1 knockout mice. Efficient TG hydrolysis requires coordinate interaction among LPL, GPIHBP1 and apoA5 on the endothelial cell surface.
ApoA5 has an intracellular role in TG homeostasis wherein apoA5 promotes intracellular TG storage. Initial studies demonstrated that increased apoA5 lowers VLDL TG secretion without affecting apoB secretion resulting in the formation of smaller VLDL particles in mouse liver.5 In line with this study, apoA5 transgenic mice exhibit elevated liver TG content compared to wild type or apoA5 knockout animals.6 Similarly, increased expression of apoA5 in cultured rat hepatoma cells leads to increased cellular TG and decreased TG secretion.7 Additional support for the intracellular role of apoA5 in TG metabolism comes from the observation that apoA5 binds to intracellular lipid droplets via its carboxyl terminus6,8,9 and that, following partial hepatectomy, rat livers exhibited elevated apoA5 expression10 and increased intracellular lipid accumulation.11,12
A role for apoA5 in TG regulation in humans is supported by the discovery of several single nucleotide polymorphisms SNPs) that correlate with elevated plasma TG levels.13–16 Additional evidence came from a rare case of homozygous apoA5 truncation exhibiting severe hypertriglyceridaemia.17 Recently, an interesting case of homozygous partial deletion of apoA5 signal peptide was reported. The patient exhibited an altered intracellular apoA5 distribution and hypertriglyceridemia.18
Because of the link between apoA5 and storage of TG in lipid droplets, it is expected that apoA5 may play a role in the pathogenesis of non-alcoholic fatty liver diseases (NAFLD), a disease commonly associated with obesity. In parallel with the lingering high prevalence of obesity in adult and paediatric populations,19 the prevalence of NAFLD has been high and increasing steadily among adult and paediatric populations, with a current prevalence of <46% for adults and <11% for adolescents in the US.20,21 NAFLD comprises a spectrum of liver diseases ranging from simple steatosis, steatohepatitis, fibrosis, cirrhosis, liver failure and hepatocellular carcinoma.22 A hallmark feature of NAFLD is the accumulation of TG-rich lipid droplets in hepatocytes. NAFLD liver with evidence of inflammation and variable degree of fibrosis is termed nonalcoholic steatohepatitis (NASH). Examination of the apoA5 mRNA expression in the livers of the NAFLD patients undergoing bariatric surgery revealed that hepatic apoA5 mRNA expression decreases after weight loss together with improvements in hepatic steatosis.23 However, these authors found that apoA5 expression did not correlate with the grade of steatosis. A limitation in this study was that no healthy control subjects were included. To address this issue, we studied apoA5 expression in human NAFLD livers in comparison with normal livers from healthy donors. Furthermore, studies with a rat NAFLD model and cultured cells were performed to test the hypothesis that apoA5 plays a role in intracellular TG accumulation in the pathogenesis of NAFLD.
MATERIALS AND METHODS
Study design
Based on our microarray data, a power analysis was conducted using G*Power version 3.1.9.2 to estimate the sample size appropriate for the quantitative real-time PCR analysis of ApoA5 mRNA expression in NAFLD livers and controls. The effect size for the differential ApoA5 expression was 1.86 (Cohen’s d) according to the microarray study, which is considered large according to Cohen’s criteria. With an alpha of 0.05, a power of 0.80, and an allocation ratio of N2/N1 = 2 (for limited availability of control samples), the projected sample sizes are N1 = 5 and N2 = 9 for a two-tailed Student t test. The actual sample sizes are slightly larger than the calculated numbers. This effort is worthwhile as it greatly increased the power from 0.80 to 0.95. For the animal study, with an effect size of 1.86, an alpha of 0.05, a power of 0.80, an allocation ratio of 1, the projected number of animals is N1 = N2 = 6 for a two-tailed Student t test.
Patients
This study was approved by Children and Youth Institutional Review Board of the State University of New York at Buffalo. Patients included in this study were biopsy-proven NAFLD patients fulfilling Kleiner’s criteria.22 In general, our NAFLD patients were obese, hyperglycaemic, hypertriglyceridaemic, exhibited elevated blood transaminases, and more importantly, the histopathology slides showed fatty change of various grades and inflammation of various stages. No other liver disease was diagnosed for any of the NAFLD patients. Liver biopsy samples were collected between July 2010 through September 2013. NAFLD patients were randomly enrolled for this study. The age, gender and ethnicity of the NAFLD patients in this study are typical of the entire NAFLD patients in our hospital. Prior to sample collection, written consent from parents of patients and assent from children were obtained. Patients signed a consent form for use of tissue in research. For normal controls, total liver RNA was purchased from Admet Technologies (USA). These samples were derived from healthy donor livers intended for liver transplantation. Patient characteristics for the quantitative real-time PCR are summarised in Table 1.
Table 1.
Characteristics of the human subjects
| Control (n = 6)* | NAFLD(n = 17) | |
|---|---|---|
| Sex (F/M) | 3/3† | 3/14 |
| Age (y) | 7.8 ± 7.4‡ | 13.4 ± 2.9 |
| BMI (kg/m2) | 17.7 ± 3.4§ | 35 ± 9.4‖ |
| Steatosis (S1/S2/S3) | NA | 6/6/5 |
| Inflammation (G0/G1/G2/G3) | NA | 3/11/3/0 |
| ALT (U/L) | Normal | 64.4 ± 31.7 |
| AST(U/L) | Normal | 44.6 ± 15.9 |
| TG (mmol/L) | NA | 1.84 ± 0.79 |
| VLDL-C (mmol/L) | NA | 0.80 ± 0.40 |
| LDL-C (mmol/L) | NA | 2.46 ± 0.61 |
| HDL-C (mmol/L) | NA | 0.75 ± 0.17 |
| Cholesterol (mmol/L) | NA | 4.07 ± 0.83 |
| Fasting glucose (mmol/L) | NA | 5.90 ± 1.95 |
| Fasting insulin (U/L) | NA | 15.9 ± 13.3 |
| IR-HOMA | NA | 4.4 ± 3.9 |
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; F, female; HDL-C, high density lipoprotein-cholesterol; IR-HOMA, insulin resistance-homeostasis model assessment; LDL-C, low density lipoprotein-cholesterol; M, male; NA, not available; TG, triglyceride; VLDL-C, very low density lipoprotein-cholesterol.
Normal healthy liver intended for transplantation, no liver disease reported.
Sex is not matched between control and NAFLD groups due to the limited availability of normal livers. This is justified as our data showed that there was no difference in APOA5 and PLIN expression between female and male patients.
Mean ± standard deviation.
Normal controls are below 80 percentile of the population.
All patients are above 95 percentile of the population.
Microarray data
A well characterised microarray database24 was used to examine the mRNA expression of apoA5 and perilipin in NASH livers and healthy controls (GEO accession number: GSE24807; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24807). The mRNA expression data from this database has been validated for many genes in our previous studies.25–28
Quantitative real-time PCR
Custom primers were designed for human apoA5 (forward, 5’-GCAGATAATGGCAAGCATGG-3’; reverse, 5’-GCTGGTCTGGCTGAAGTAG-3’), human perilipin (forward, 5’-GCCATGTCCCTATCAGATGC-3’; reverse, 5’-GTTGTCGATGTCCCGGAATT-3’), rat apoA5 (forward, 5’-GAGTACTTCG GCCAGAACAG-3’; reverse, 5’-CAAGGGTCCCAGCTTTTCTAG-3’) and rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward, 5’-CCATCAACGACCCCTTCATT-3’; reverse, 5’-GACCAGCTTCCCATTCTCAG-3’). The primers for human gapdh were described previously.24 These primers were synthesised at Eurofins MWG Operon (USA). Liver biopsies and animal tissues were stored at −80°C after treatment with RNAlater (Qiagen, USA). Total RNA was isolated using the RNeasy kit (Qiagen). From total RNA, complementary DNA (cDNA) was prepared with the i-Script cDNA synthesis kit (Bio-Rad Laboratories, USA). Quantitative RT-PCR was performed on an iCycleriQ real-time detection system (Bio-Rad Laboratories) using SYBR Green (iQ SYBR Green Supermix; Bio-Rad Laboratories) for real-time monitoring. The PCR product was verified by melting curve analysis, confirmed by agarose gel electrophoresis and by DNA sequencing. The expression of each gene was normalised with that of GAPDH and calculated as previously described.24
Animals
All procedures involving rats were reviewed and approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo. Four-week-old male Sprague-Dawley rats were purchased from Harlan Laboratories (USA). After one week of quarantine, these rats were divided into two groups. Group 1 (NAFLD model group) comprised six rats which were fed with a high fat diet (TD.06414; Harlan Laboratories). In addition, dextran sulphate sodium (DSS, 1%) was added to the drinking water for 10 days, followed by a 7 day wash-out period, and then repeat. Group 2 (control group) comprised six rats which were fed with standard rodent chow (2018s; Harlan Laboratories, USA). Similarly, 1% DSS was added to the drinking water to control for the possible influence of DSS induced colitis on the experimental results. Fat contributed 60% of calories in the TD.06414 diet, compared to 18% in the standard rodent chow (2018s). All rats in the study were fed ad lib and had unlimited access to water. After 16 weeks of treatment, rats were sacrificed for tissue collection, blood glucose assays and blood insulin assays following similar procedures described previously.29
Triglyceride analysis
Rat liver and serum triglyceride levels were determined using a colorimetric kit (#10010303; Cayman Chemical Company, USA), according to the manufacturer’s instructions.
Cell culture
HepG2 cell line was obtained from the American Type Culture Collection. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at 37°C, under a humidified atmosphere of 5% carbon dioxide. HepG2 cells were plated on 100 mm dishes at density of 60% confluency. Next day cells were treated with oleic acid (0.5 mM) in combination of palmitic acid (0.25 mM) or with DMSO (solvent for free fatty acids) as a control. After treatment for 24 h, cells were harvested for RNA extraction and western blotting. For Oil Red O staining, cells were plated on cover slips placed in 6-well plates. After treatment for 24 h, cells were stained with Oil Red O and counterstained with haematoxylin.
Western blot
Cultured cells were collected and homogenised into lysates and boiled for 5 min in sodium dodecyl sulfate (SDS) loading buffer. Protein concentrations were determined using RC DC protein assay kit (Bio-Rad). From each sample 60 µg of protein was separated on 10% SDS polyacrylamide electrophoresis gel. Protein bands were transferred onto nitrocellulose membranes using an electrophoresis transfer system. The protein was then probed with a mouse monoclonal anti-human apoA5 antibody (Santa Cruz Biotechnology, USA) at a dilution of 1:1000 and then with the horseradish peroxidase conjugated donkey anti-mouse-IgG at a dilution of 1:5000. The Pierce detection kit was used for chemiluminescent detection and images collected with a ChemiDoc MP imaging system (Bio-Rad). Similar procedures were performed with a β-actin antibody. Results are expressed as a ratio of apoA5 to β-actin.
Statistical methods
Mann–Whitney U tests with a two-tailed distribution were performed to examine possible difference between two experimental groups. Correlations between two variables were examined by Spearman’s rank correlation coefficient tests. A p value of <0.05 was considered to be significant. All statistical analyses were performed with Graphpad Prism 6.0 (Graphpad Software, USA).
RESULTS
The present study evaluated paediatric subjects with known NAFLD; however, since normal control liver samples were not available, use was made of commercially available RNA from paediatric liver donors. The latter samples provided no information on plasma lipid values or glucose; however, liver function was unremarkable. The NAFLD subjects had twice the BMI as the controls and clearly had steatosis (Table 1). Plasma TG levels in NAFLD subjects were only slightly elevated but HDL-cholesterol levels were low. Consistent with the obesity of the NAFLD subjects, their insulin resistance-homeostasis model assessment (IR-HOMA) values were elevated (4.4 versus 2.3 for normal children30).
To study possible roles of apoA5 in the pathogenesis of NAFLD, mRNA expression levels of apoA5 in NASH livers and that of normal controls were examined with our well-characterised microarray database.24 NAFLD livers exhibited highly elevated expression of apoA5 mRNA when compared to healthy donor livers intended for transplantation (Table 2). Perilipin, a lipid-droplet associated protein,31 was also highly elevated in NAFLD livers compared to normal controls (Table 2). These observations were confirmed by qRT-PCR with a different cohort of NAFLD patients and normal controls (Fig. 1).
Table 2.
Microarray analysis of apoA5 and PLIN mRNA expression in NAFLD livers*
| GenBank accession# | Gene description | NAFLD (n = 12) | Control (n = 5) | NAFLD/Control† | p value‡ |
|---|---|---|---|---|---|
| NM_052968.2 | apolipoprotein A-V (APOA5) | 127.80 ± 16.93 | 44.21 ± 10.87 | 2.89 | 0.008 |
| NM_002666.1 | perilipin (PLIN) | 50.96 ± 6.81 | 9.35 ± 2.11 | 5.45 | 0.002 |
The gene expression results (sample mean ± SEM) shown were median normalised.
Fold difference of gene expression (sample mean) between NAFLD livers and controls.
Two tailed Student t test.
Fig. 1.
Altered gene expression in NASH livers. The mRNA expression of (A) apolipoprotein A5 and (B) perilipin was examined in NASH livers and controls by qRT-PCR. Relative gene expression (mRNA copy numbers of the target gene normalised to that of the GAPDH) was plotted as mean ± SEM with p values indicated. ApoA5, apolipoprotein A5; PLIN, perilipin.
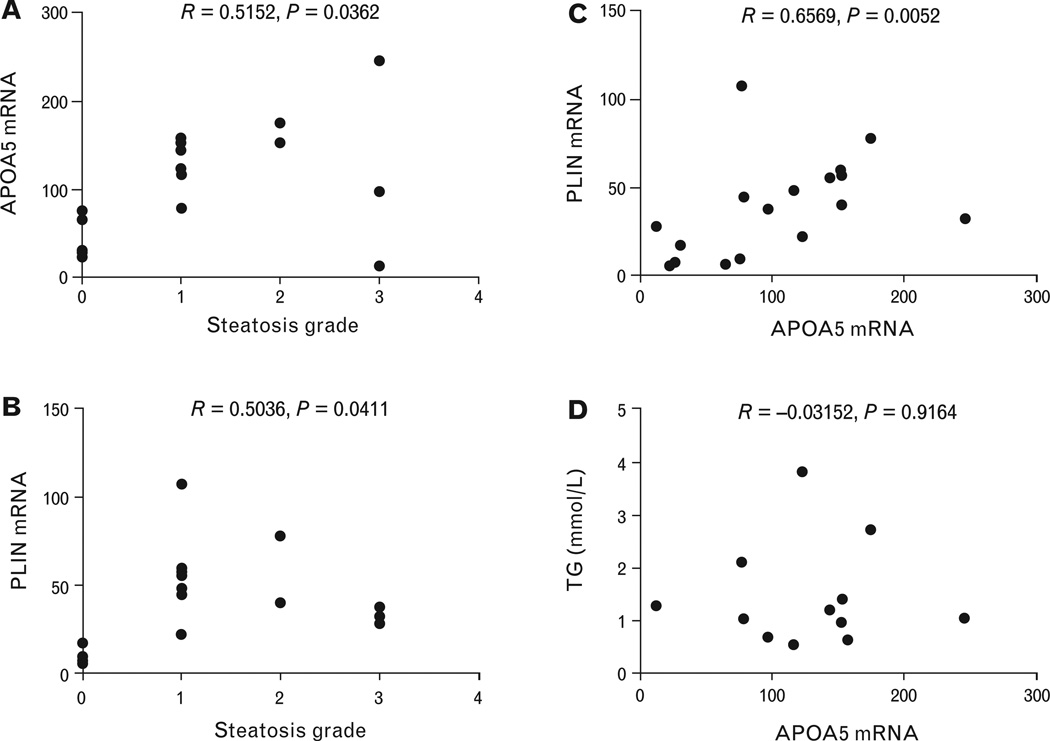
Correlation analysis was performed between apoA5 mRNA levels and the steatosis grades of the NAFLD livers. A positive correlation was observed (Fig. 2A). Notably, although the general trend was that higher steatosis grades are associated with higher levels of apoA5 mRNA, some NAFLD livers with steatosis grade 3 exhibited reduced or moderate levels of apoA5 expression. A similar pattern was observed when examining the correlation between perilipin mRNA levels and the steatosis grades of the NAFLD livers (Fig. 2B). These results predicted a positive correlation between apoA5 and perilipin expression levels, which was observed (Fig. 2C). However, there was no correlation between apoA5 mRNA expression levels and the plasma triglyceride concentrations (Fig. 2D).
Fig. 2.
Correlation analyses in NAFLD patients. (A) Correlation of apolipoprotein A5 expression levels with steatosis grade; (B) correlation of perilipin expression levels withsteatosis grade; (C) correlation of apolipoprotein A5 expression levels with perilipin expression levels; and (D) lack of correlation between apolipoprotein A5 expression levels with serum triglyceride concentrations. Spearman’s correlation coefficient r and p values are indicated. ApoA5, apolipoprotein A5; PLIN, perilipin; TG, triglyceride.
To further test our hypothesis for a role of apoA5 in the pathogenesis of NAFLD, a NAFLD rat model was employed. NAFLD was induced by high-fat diet in addition to dextran sulfate sodium (DSS) treatment. Control animals were DSS treated. Significant difference in body weight was observed between NAFLD animals and the controls (Fig. 3A). Consistent with the increased body weights, significantly elevated IRHOMA value was observed with the NAFLD animals (Fig. 3B). Oil red O staining of the liver cryosections revealed extensive distribution of fat-laden cells in NAFLD livers compared to sporadic staining in the control livers (Fig. 3C). ApoA5 mRNA expression in NAFLD rat livers was elevated compared to the control livers according to qRT-PCR analysis (Fig. 3D). On the other hand, no difference in serum TG was observed between NAFLD rats and control animals (Fig. 3E). Consistent with human studies, no correlation was observed between apoA5 expression and serum TG levels in rats (Fig. 3F).
Fig. 3.
Elevated apolipoprotein A5 expression in NAFLD rats. Comparison of (A) the body weight and (B) insulin resistance between NAFLD model rats and control rats. Plotted are means ± SEM. p values are indicated. (C) Oil red O staining of liver cryosections from NAFLD rats and control animals after whole-body perfusion. Two representative images from each group are shown. (D) Comparison of the mRNA expression of apolipoprotein A5 in NAFLD rat livers and controls. Relative gene expression data from qRT-PCR (mRNA copy numbers of the target gene normalised to that of the GAPDH) was plotted as mean ± SEM with p values indicated. ApoA5, apolipoprotein A5. (E) Comparison of the serum triglyceride in NAFLD rats and controls. Plotted value is mean ± SEM with p value indicated. (F) Lack of correlation between apolipoprotein A5 expression levels with serum triglyceride concentrations in the rats. Spearman’s correlation coefficient r and p values are indicated.
To determine whether cultured HepG2 cells can recapitulate the metabolic events found in human and rat liver tissue, HepG2 cells were treated with free fatty acids to induce elevated presence of intracellular fat, evidenced by oil red O staining (Fig. 4A). As noted in Fig. 4A, fatty acid induced a large increase in intracellular lipid droplets. Consistently, increased expression of perilipin was observed in fat-laden HepG2 cells compared to control cells (Fig. 4B), indicating elevated levels of intracellular lipid droplets. Surprisingly, in contrast to in vivo studies with NAFLD patients and NAFLD rats, apoA5 expression was not altered in cultured fat-laden HepG2 cells (Fig. 4C). Unlike liver specimens from NAFLD subjects, there was no correlation between apoA5 mRNA expression and perilipin mRNA expression (Fig. 4D). There also was no difference in apoA5 protein levels between fat-laden HepG2 cells and control cells (Fig. 4E).
Fig. 4.
Apolipoprotein A5 expression was not altered in cultured fat laden HepG2 cells. (A) Elevated fat storage in HepG2 cells treated with oleic acid (0.5 mM) and palmitic acid (0.25 mM). Cells were stained with Oil Red O and counterstained with haematoxylin. Left, control cells; right, cells treated with free fatty acids (FFA). Two typical samples are shown for each treatment. (B) The mRNA expression of perilipin and (C) apolipoprotein A5 were examined in fat laden HepG2 cells and controls by qRT-PCR. Relative gene expression (mRNA copy numbers of the target gene normalised to that of the GAPDH) was plotted as mean ± SEM with p value indicated. (D) Lack of correlation between apolipoprotein A5 expression levels with perilipin expression levels. Spearman’s correlation coefficient r and p value are indicated. (E) Western blot analysis of apolipoprotein A5 expression in fat laden HepG2 cells and control cells. β-actin blot was used for normalisation purposes. Average densitometry from two experiments was plotted with p value indicated. Apolipoprotein A5 signals were normalised with those of β-actin. ApoA5, apolipoprotein A5; FFA, free fatty acid treatment.
DISCUSSION
Elevated apoA5 may contribute to the pathogenesis of NAFLD
Here we report that the apoA5 expression in the livers of biopsy-diagnosed NAFLD patients was highly elevated compared to healthy donor livers intended for transplantation. We further observed that, apoA5 expression was correlated with the steatosis grade of the NAFLD livers. Similar observations were made with a NAFLD rat model. These observations support the premise that apoA5 may contribute to NAFLD pathogenesis.
Previous studies suggested that apoA5 regulates plasma TG by two mechanisms: one extracellular, the other intracellular.32 Firstly, apoA5 functions extracellularly by increasing the efficiency of LPL activity3,33 and clearance of VLDL remnants.34 Indeed, SNPs that decrease apoA5’s lipolytic function are associated with hypertriglyceridaemia.2,16,33,35–37 Interestingly, in our studies, elevated apoA5 mRNA expression in NAFLD livers was not associated with lower plasma TG levels; in fact, there was very mild hypertriglyceridaemia as noted in Table 1. Our result is consistent with the observations that elevated plasma apoA5 was not associated with lower plasma TG in type-2 diabetes,38,39 patients of higher risk for coronary artery disease,40 and severe hypertriglyceridaemia patients.41 In those studies, as well as ours, the patients are usually obese38 or overweight.40,41 Therefore, in these patients, the plasma TG-lowering effect of apoA5 could be overridden by other factors such as persistent TG supply from diet, from adipose tissue, or from elevated hepatic VLDL secretion.
Secondly, apoA5 promotes intracellular TG storage.5–7 It has also been shown in transgenic mice overexpressing apoA5 that apoA5 associated with lipid droplets increased along with cellular TG levels while wild type mice had low apoA5 associated with lipid droplets and low cellular TG.6 There is evidence that apoA5 promotes intracellular TG storage at the expense of decreased TG secretion, thus increasing the burden of stored TG.7 In this case, apoA5 may reduce hypertriglyceridaemia, but at the same time, contribute to the development of steatosis and lipotoxicity. Our observations, in NAFLD patients and NAFLD rat model, that elevated hepatic apoA5 expression correlated with liver fat accumulation, support the role of apoA5 in hepatic TG storage. Similar observation was observed with adult NAFLD livers from patients who underwent bariatric surgery.23 Ress and colleagues reported that hepatic apoA5 mRNA expression decreases after weight loss together with reduced liver fat. The difference is that apoA5 mRNA and steatosis grade are significantly correlated with our paediatric NAFLD patients, but not with the adult NAFLD patients in the study by Ress et al. We speculate that this difference is mainly because the adult NAFLD patients were likely in more severe disease stages than those of our paediatric NAFLD patients.
Another possible mechanism for the correlation between elevated apoA5 expression and liver fat is that intracellular lipid droplets may induce apoA5 expression. To test this hypothesis, we induced lipid droplets with fatty acids in cultured HepG2 cells and found that apoA5 expression was not induced in fat-laden HepG2 cells at both mRNA and protein levels. This result is in contrast to the observation that increased apoA5 expression leads to increased fat storage in cultured rat hepatoma cells.7 Our results suggest that apoA5 is not required in the formation of lipid droplets. More importantly, together with previous observations, our results suggest that the correlation between apoA5 expression and hepatic lipid storage is likely explained by a uni-directional causal relationship or the modulating role of apoA5 on hepatic lipid storage.
Possible mechanism for elevated apoA5 expression in NAFLD livers
Our data suggest that apoA5 cannot be induced by intracellular fat storage per se. What caused the increased expression of apoA5 in NAFLD livers? Currently, the only known triggers for elevated apoA5 expression in NAFLD livers are from the circulation. In cultured cells and animal models, apoA5 expression is known to be regulated at the transcription level by several different signalling pathways. Thyroid hormone42 and the retinoic acid receptor-related orphan receptor (ROR) alpha43 increase apoA5 promoter transcription activity. A thyroid receptor response element was identified in the human apoA5 promoter.42 On the other hand, insulin44 and LXR-SREBP-1c signalling45 down-regulate apoA5 mRNA expression. A major characteristic of NAFLD patients is insulin resistance.46,47 One limit of our study was the lack of blood biochemical indices for the healthy controls. However, it is very likely that our NAFLD patients were highly insulin resistant because their average IR-HOMA value of 4.4 (Table 1) is much higher than 2.3, the average of normal weight children.30 Similarly, our NAFLD rats also exhibited significantly elevated insulin resistance compared to the controls rats. Studies on the mechanism of insulin resistance in a NAFLD/NASH animal model revealed that a critical step in insulin signalling, the tyrosine phosphorylation of insulin receptor substrate 1 and insulin receptor substrate 2, is impaired in NAFLD/NASH livers.48 Impaired insulin signalling in NAFLD livers could lead to elevated apoA5 expression44 and subsequently lead to elevated hepatic TG storage.
We speculate that in NAFLD/NASH livers, impaired insulin signalling is the major pathway contributing to the up-regulation of apoA5 expression. Other pathways known to regulate apoA5 expression, including thyroid hormone signalling, ROR alpha signalling and LXR signalling, may not contribute to elevated apoA5 expression in NAFLD/NASH livers. (1) There are conflicting data regarding thyroid hormone signalling in NAFLD/NASH.49 (2) In line with its positive effect on apoA5 expression, ROR alpha deficiency protects mice from hepatic steatosis, adipose-associated inflammation, and insulin resistance.50 However, there is no supportive evidence from NAFLD patients regarding the ROR alpha signalling. (3) LXR signalling is elevated in NAFLD livers;51 therefore, this signalling pathway would not contribute to increased apoA5 expression. Rather, elevated LXR signalling may down-regulate apoA5 expression in NAFLD livers.45
In summary, we demonstrated that apoA5 expression positively correlates with liver fat storage in NAFLD patients and rats. Since intracellular fat storage could not induce apoA5 expression, this correlation is likely explained by the potent effect of apoA5 in promoting intracellular fat storage. Additionally, we observed that our NAFLD patients and rats had elevated insulin resistance, which may have a role in elevating apoA5 expression in NAFLD livers. Our data support the hypothesis that apoA5 promotes hepatic TG storage and therefore contributes to the pathogenesis of NAFLD, and may represent a potential target for therapeutic intervention.
Acknowledgments
sources of funding: This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U01 DK061728, to SSB), the Peter and Tommy Fund, Inc., Buffalo, NY (to SSB and LZ), the National Natural Science Foundation of China (No. 81374031, to QF), the National Natural Science Foundation of China (No. 81173404, to YH) and a departmental start-up fund (to LZ).
Footnotes
Conflicts of interest: The authors state that there are no conflicts of interest to disclose.
References
- 1.O’Brien PJ, Alborn WE, Sloan JH, et al. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 2.Pennacchio LA, Olivier M, Hubacek JA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 3.Lookene A, Beckstead JA, Nilsson S, et al. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J Biol Chem. 2005;280:25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
- 4.Shu X, Nelbach L, Weinstein MM, et al. Intravenous injection of apolipoprotein A-V reconstituted high-density lipoprotein decreases hypertriglyceridemia in apoav−/− mice and requires glycosylphosphatidylinositolanchored high-density lipoprotein-binding protein 1. Arterioscler Thromb Vasc Biol. 2010;30:2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaap FG, Rensen PC, Voshol PJ, et al. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Biol Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 6.Shu X, Nelbach L, Ryan RO, et al. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim Biophys Acta. 1801;2010:605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blade AM, Fabritius MA, Hou L, et al. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J Lipid Res. 2011;52:237–244. doi: 10.1194/jlr.M010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu X, Chan J, Ryan RO, et al. Apolipoprotein A-V association with intracellular lipid droplets. J Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Shu X, Ryan RO, Forte TM. Intracellular lipid droplet targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J Lipid Res. 2008;49:1670–1676. doi: 10.1194/jlr.M800111-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Vliet HN, Sammels MG, Leegwater AC, et al. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 11.Tijburg LB, Nyathi CB, Meijer GW, et al. Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. J Biol Chem. 1991;277:723–728. doi: 10.1042/bj2770723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell GC. Probing Prometheus: fat fueling the fire? Hepatology. 2004;40:1252–1255. doi: 10.1002/hep.20522. [DOI] [PubMed] [Google Scholar]
- 13.Pennacchio LA, Olivier M, Hubacek JA, et al. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet. 2002;11:3031–3038. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 14.Palmen J, Smith AJ, Dorfmeister B, et al. The functional interaction on in vitro gene expression of APOA5 SNPs, defining haplotype APOA52, and their paradoxical association with plasma triglyceride but not plasma apoAV levels. Biochim Biophys Acta. 1782;2008:447–452. doi: 10.1016/j.bbadis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Talmud PJ, Palmen J, Putt W, et al. Determination of the functionality of common APOA5 polymorphisms. J Biol Chem. 2005;280:28215–28220. doi: 10.1074/jbc.M502144200. [DOI] [PubMed] [Google Scholar]
- 16.Pullinger CR, Aouizerat BE, Movsesyan I, et al. An apolipoprotein A-V gene SNP is associated with marked hypertriglyceridemia among Asian-American patients. J Lipid Res. 2008;49:1846–1854. doi: 10.1194/jlr.P800011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priore Oliva C, Pisciotta L, Li Volti G, et al. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2005;25:411–417. doi: 10.1161/01.ATV.0000153087.36428.dd. [DOI] [PubMed] [Google Scholar]
- 18.Albers K, Schlein C, Wenner K, et al. Homozygosity for a partial deletion of apoprotein A-V signal peptide results in intracellular missorting of the protein and chylomicronemia in a breast-fed infant. Atherosclerosis. 2014;233:97–103. doi: 10.1016/j.atherosclerosis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:2007–2010. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 23.Ress C, Moschen AR, Sausgruber N, et al. The role of apolipoprotein A5 in non-alcoholic fatty liver disease. Gut. 2011;60:985–991. doi: 10.1136/gut.2010.222224. [DOI] [PubMed] [Google Scholar]
- 24.Baker SS, Baker RD, Liu W, et al. Role of alcohol metabolism in nonalcoholic steatohepatitis. PLoS One. 2010;5:e9570. doi: 10.1371/journal.pone.0009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Baker SS, Baker RD, et al. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS One. 2011;6:e24363. doi: 10.1371/journal.pone.0024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Baker SS, Liu W, et al. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2011;60:1001–1011. doi: 10.1016/j.metabol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Moya D, Baker SS, Liu W, et al. Novel pathway for iron deficiency in pediatric non-alcoholic steatohepatitis. Clin Nutr. 2014 doi: 10.1016/j.clnu.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Desai S, Baker SS, Liu W, et al. Paraoxonase 1 and oxidative stress in paediatric non-alcoholic steatohepatitis. Liver Int. 2014;34:110–117. doi: 10.1111/liv.12308. [DOI] [PubMed] [Google Scholar]
- 29.Patel R, Baker SS, Liu W, et al. Effect of dietary advanced glycation end products on mouse liver. PLoS One. 2012;7:e35143. doi: 10.1371/journal.pone.0035143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JM, Okumura MJ, Davis MM, et al. Prevalence and determinants of insulin resistance among US adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 31.Straub BK, Stoeffel P, Heid H, et al. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 32.Forte TM, Shu X, Ryan RO. The ins (cell) and outs (plasma) of apolipoprotein A-V. J Lipid Res. 2009;50(Suppl):S150–S155. doi: 10.1194/jlr.R800050-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkel M, Loeffler B, Kluger M, et al. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280:21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson SK, Lookene A, Beckstead JA, et al. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 2007;46:3896–3904. doi: 10.1021/bi7000533. [DOI] [PubMed] [Google Scholar]
- 35.van der Vliet HN, Schaap FG, Levels JH, et al. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem Biophys Res Commun. 2002;295:1156–1159. doi: 10.1016/s0006-291x(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 36.Fruchart-Najib J, Bauge E, Niculescu LS, et al. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun. 2004;319:397–404. doi: 10.1016/j.bbrc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Baroukh N, Bauge E, Akiyama J, et al. Analysis of apolipoprotein A5, c3, and plasma triglyceride concentrations in genetically engineered mice. Arterioscler Thromb Vasc Biol. 2004;24:1297–1302. doi: 10.1161/01.ATV.0000130463.68272.1d. [DOI] [PubMed] [Google Scholar]
- 38.Dallinga-Thie GM, van Tol A, Hattori H, et al. Plasma apolipoprotein A5 and triglycerides in type 2 diabetes. Diabetologia. 2006;49:1505–1511. doi: 10.1007/s00125-006-0261-0. [DOI] [PubMed] [Google Scholar]
- 39.Talmud PJ, Cooper JA, Hattori H, et al. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 2006;49:2337–2340. doi: 10.1007/s00125-006-0387-0. [DOI] [PubMed] [Google Scholar]
- 40.Vaessen SF, Schaap FG, Kuivenhoven JA, et al. Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J Lipid Res. 2006;47:2064–2070. doi: 10.1194/jlr.M600233-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Henneman P, Schaap FG, Havekes LM, et al. Plasma apoAV levels are markedly elevated in severe hypertriglyceridemia and positively correlated with the APOA5 S19W polymorphism. Atherosclerosis. 2007;193:129–134. doi: 10.1016/j.atherosclerosis.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 42.Prieur X, Huby T, Coste H, et al. Thyroid hormone regulates the hypotriglyceridemic gene APOA5. J Biol Chem. 2005;280:27533–27534. doi: 10.1074/jbc.M503139200. [DOI] [PubMed] [Google Scholar]
- 43.Genoux A, Dehondt H, Helleboid-Chapman A, et al. Transcriptional regulation of apolipoprotein A5 gene expression by the nuclear receptor RORalpha. Arterioscler Thromb Vasc Biol. 2005;25:1186–1192. doi: 10.1161/01.ATV.0000163841.85333.83. [DOI] [PubMed] [Google Scholar]
- 44.Nowak M, Helleboid-Chapman A, Jakel H, et al. Insulin-mediated down-regulation of apolipoprotein A5 gene expression through the phosphatidylinositol 3-kinase pathway: role of upstream stimulatory factor. Mol Cell Biol. 2005;25:1537–1548. doi: 10.1128/MCB.25.4.1537-1548.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakel H, Nowak M, Moitrot E, et al. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J Biol Chem. 2004;279:45462–45469. doi: 10.1074/jbc.M404744200. [DOI] [PubMed] [Google Scholar]
- 46.Tankurt E, Biberoglu S, Ellidokuz E, et al. Hyperinsulinemia and insulin resistance in non-alcoholic steatohepatitis. J Hepatol. 1999;31:963. doi: 10.1016/s0168-8278(99)80301-8. [DOI] [PubMed] [Google Scholar]
- 47.Choudhury J, Sanyal AJ. Insulin resistance in NASH. Frontiers Biosci. 2005;10:1520–1533. doi: 10.2741/1636. [DOI] [PubMed] [Google Scholar]
- 48.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 49.Eshraghian A, Hamidian Jahromi A. Non-alcoholic fatty liver disease and thyroid dysfunction: A systematic review. World J Gastroenterol. 2014;20:8102–8109. doi: 10.3748/wjg.v20.i25.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang HS, Okamoto K, Takeda Y, et al. Transcriptional profiling reveals a role for RORalpha in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiol Genomics. 2011;43:818–828. doi: 10.1152/physiolgenomics.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima-Cabello E, Garcia-Mediavilla MV, Miquilena-Colina ME, et al. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci. 2011;120:239–250. doi: 10.1042/CS20100387. [DOI] [PubMed] [Google Scholar]