Abstract
Background & Aims
Altered levels and functions of microRNAs (miRs) have been associated with inflammatory bowel diseases (IBDs), although little is known about their roles in pediatric IBD. We investigated whether colonic mucosal miRs are altered in children with ulcerative colitis (UC).
Methods
We used a library of 316 miRs to identify those that regulate phosphorylation of STAT3 in NCM460 human colonocytes incubated with interleukin-6. Levels of miR-124 were measured by real-time PCR analysis of colon biopsies from pediatric and adult patients with UC and patients without IBD (controls), and of HCT-116 colonocytes incubated with 5-aza-2’-deoxycytidine. Methylation of the MIR124 promoter was measured by quantitative methylation-specific PCR.
Results
Levels of phosphorylated STAT3 and the genes it regulates (encoding VEGF, BCL2, BCLXL, and MMP9) were increased in pediatric patients with UC, compared to control tissues. Overexpression of miR-124, let-7, miR-125, miR-26, or miR-101 reduced STAT3 phosphorylation by ≥75% in NCM460 cells; miR-124 had the greatest effect. miR-124 was downregulated specifically in colon tissues from pediatric patients with UC and directly targeted STAT3 mRNA. Levels of miR-124 were decreased whereas levels of STAT3 phosphorylation increased in colon tissues from pediatric patients with active UC, compared to those with inactive disease. Furthermore, levels of miR-124 and STAT3 were inversely correlated in mice with experimental colitis. Downregulation of miR-124 in tissues from children with UC was attributed to hypermethylation of its promoter region. Incubation of HCT-116 colonocytes with 5-aza-2’ deoxycytidine upregulated miR-124 and reduced levels of STAT3 mRNA.
Conclusions
MiR-124 appears to regulate the expression of STAT3. Reduced levels of miR-124 in colon tissues of children with active UC appear to increase expression and activity of STAT3, which could promote inflammation and pathogenesis of UC in children.
Keywords: IL6, BCLXL, Let-7, gene regulation
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory bowel diseases (IBDs) that affect both children and adults. The complex nature of these diseases both in severity, frequency as well as interactions between the intestinal mucosa, the innate and adaptive immune systems, and the microbiome have not resulted, as yet, in identifying the etiology of these conditions.1 Westernized lifestyle is linked to the appearance of IBD, but genetic factors are also important though hard to define due to the large number of implicated genes and the small additive effect of each one.2, 3 A recent meta-analysis of genome-wide association studies (GWAS) of IBD cases and controls suggests that host mucosal immune system interaction with luminal and adherent microbes are critical for triggering IBD.4 Out of the more than 1 million individuals affected with IBD in the US, there are ~100,000 children. Because children have fewer environmental confounders than adults, this population provides advantages in studying the genetic contributions to IBD pathogenesis. Mechanisms relevant to the pediatric population may also apply to adult patients and such studies have only recently started.5
Previous studies have shown the importance of the IL6-STAT3 signaling pathway in IBD and specifically STAT3 is up-regulated in adult patients with UC.6, 7 This pathway is involved not only in the development of UC but has also been implicated in the progression of UC to colon cancer.8 However, the involvement of the IL6-STAT3 pathway in pediatric-UC has not been completely delineated.9 For all these reasons, we chose to investigate the IL6-STAT3 pathway in pediatric-UC pathogenesis.
MicroRNAs (miRs) are small non-coding RNA oligonucleotides that can regulate the expression of a large number of genes and have been involved in different human diseases.10 MiRs are centrally involved in the pathogenesis of different human inflammatory diseases.11–14 Recently, we identified that miRs are essential regulators of Toll-like receptor signaling. Specifically, microRNA let-7 targets directly the Toll-like receptor 4 (TLR4) and miR-155 regulates the suppressor of cytokine signaling 1 (SOCS1), two critical proteins in LPS-driven TLR signaling.15 Furthermore, signal transducer and activator of transcription 3 (STAT3) activity, a major factor in inflammatory response, depends on miR-21 and miR-181b.16
Certain miRs are deregulated in IBD.17 MiR-192, which inhibits chemokine production, is decreased in tissue samples from UC patients.18 Several miRs are associated with adult ileal and colonic CD,19 while miRs in peripheral blood can distinguish UC and CD,20, 21 however, their role and function in both adult and pediatric IBD remains to be elucidated.
Here, we identify miRs that are potent regulators of STAT3 activity in human colonocytes. Among those, miR-124 is deregulated specifically in pediatric patients with active UC, leading to increased levels of STAT3 expression and the transcriptional activation of its downstream targets. Moreover, in active pediatric-UC the miR-124/STAT3 pathway is epigenetically regulated, suggesting the involvement of epigenetic-transcription regulatory circuits in the pathogenesis of pediatric-UC.
Materials and Methods
RNA isolation from patient samples and mouse tissues
RNA was extracted from colonic biopsies and mouse colonic tissues, after homogenization using Trizol® (Invitrogen), according to the manufacturer’s instructions, with some modifications. Specifically, two extra washing steps of the RNA pellet with 70% ethanol were added, in order to ensure minimal phenol contamination of the sample. The purity of the samples was evaluated by estimating the ratios of A260nm/A230nm and A260nm/A280nm for phenol and protein contamination, respectively, using a microplate spectrophotometer (Synergy HT, BioTek); only samples with ratios ≥2.0 were used in our analyses. Furthermore, the integrity of the RNA was evaluated by the Bioanalyzer 2100 (Agilent) and samples with RNA integrity number (RIN) higher than 7.5 were included to the study.
Study Population/Patient Characteristics
Pediatric tissue samples were obtained from subjects enrolled in the MassGeneral Hospital for Children Pediatric inflammatory bowel disease biorepository with informed consent/assent from subjects or their legal guardians and approval by the Partners Health Care Institutional Review Board (Protocol #2009P001287). Sigmoid colon mucosal biopsies were collected at the time of colonoscopy, snap frozen in liquid nitrogen and stored at −80 °C. Matched sigmoid biopsies were also obtained and sent to pathology for routine histologic evaluation.
Sigmoid biopsies from a total of 45 subjects diagnosed with IBD before the age of 18 (n = 33) or non-IBD (controls, n = 12) were analyzed. The IBD group included 18 pediatric subjects with active-UC, 9 with inactive-UC and 6 with ileocolonic Crohn’s disease. Patient characteristics including demographics, type of IBD, medications, disease duration and disease activity are listed in Supplementary Table 1 and Supplementary Data.
Real-time PCR analysis
MiR expression levels were assessed by real-time PCR on a CFX384 detection system (Bio-Rad) using the Exiqon PCR primer sets according to manufacturer’s instructions (Exiqon Inc., Woburn, MA). All primers for the miRs and the reference genes U6 snRNA and 5S rRNA were purchased from Exiqon Inc. Real-time PCR (BioRad) for VEGF, BCL2, BCLXL, MMP9, STAT3 and GAPDH was performed in RNA extracted from biopsies. Primer sequences are provided in Supplementary Data.
STAT3 ELISA assay in human colonic tissue samples
Sandwich ELISA assays (cat. no 171-V22552, Bio-Rad) assessed the phosphorylation status of STAT3 in tyrosine 705 in lysates derived from IL-6-treated NCM460 cells and from pediatric colonic biopsies (9 pediatric-UC and 12 pediatric-control). The data were analyzed in a Bio-Plex FlexMap3D analyzer using the Bio-Plex manager software.
Immunohistochemistry
For the immunohistochemical analysis we used paraffin-embedded sections of biopsies from pediatric patients and controls, according to standard protocols by Cell Signaling Technology, Inc. For antigen unmasking, 1mM EDTA was used. p-STAT3 (Tyr705) (Cell Signaling Technology, Inc.) antibodies were used according to manufacturer’s instructions. DAB-substrate kit and Hematoxylin-QS nuclear counterstain (Vector Laboratories, Inc) were used for staining, according to manufacturer’s instructions.
MicroRNA library screen
A microRNA-library, consisting of 316 microRNA mimics and 2 microRNA negative controls (at a concentration of 75 nM) (Dharmacon Inc) was transfected in human NCM460 colonic epithelial cells in 96-well plates (in three replicates). Thirty six hours post-transfection the cells were treated with 20 ng/ml IL-6 (Peprotech) for 12 hours. STAT3 (Tyr705) phosphorylation status was evaluated by ELISA, as described above. The transfection dose of 75 nM for the microRNA mimics was identified through control experiments performed to identify the maximum dose without any cytotoxic effects. In addition, IL-6 (20ng/ml) was able to induce STAT3 phosphorylation levels, 12 hours post-transfection. MiRs that inhibited STAT3 phosphorylation levels by ≥75% were considered positive hits.
Bioinformatics microRNA analysis
The Targetscan algorithm (www.targetscan.org) was used to identify direct targets for microRNA miR-124.
Cell lines
HT-29 (colorectal adenocarcinoma), RKO and HCT-116 (colorectal carcinoma) were purchased from ATCC and NCM460 (non-transformed coloncytes) human epithelial cells from INCELL Corporation LLC (San Antonio, TX).
Transfection experiments
Colonic epithelial cells were transfected using RNAiMAX reagent with 100 nM of miR-124-3p mirVana® microRNA mimic (miR-124) or the negative control #1 (miR-NC) (Life Technologies). Transfections were repeated at least three times, in triplicates.
3’UTR Luciferase assay
HCT-116 cells (5×105 cells in 100 mm dishes) were transfected using Fugene6 reagent (Roche) with renilla reporter constructs (pLightSwitch) carrying the 3’UTR of STAT3 (SwitchGear Genomics). Cell lysates were prepared 36 hours post transfection and luciferase assays were performed using the Dual-Luciferase Reporter Assay (Promega).
Western blot analysis
Western blot of cell lysates was performed following standard procedures. Frozen tissue biopsies were homogenized using RIPA buffer (Cell Signaling Technology, Inc.), followed by sonication. Antibodies against phospho-Stat3 (Tyr705), STAT3 and GAPDH were purchased from Cell Signaling and antibodies against tubulin from Sigma-Aldrich. Protein bands were quantified using Image Lab software (Bio-Rad).
Mouse models of experimental colitis
Eight to ten week old, C57BL/6 male mice were treated with 5% DSS-water for 5 days, provided ad libitum, as described.22 Mice were then sacrificed and colonic tissues were collected. IL-10 knockout (IL-10 KO) mice on the C57BL/6 background were purchased from Charles River Laboratories. For the experiment, 8 week-old male IL-10 KO mice and their littermates were single housed and treated with piroxicam (Sigma) (80 mg per 250 gr of food) for two weeks. Treatment with piroxicam, a non-steroidal anti-inflammatory drug, can synchronize the induction of colitis in IL-10 KO mice.23 Subsequently, mice were switched to normal chow diet, sacrificed two weeks later and colonic tissues were harvested. RNA was extracted and miR-124 and STAT3 mRNA levels were estimated by real-time PCR analysis. Primer sequences are provided in Supplementary Data.
DNA Methylation Analysis
The DNA-methylation levels of the CpG island in the promoter region of MIR124 was determined by quantitative methylation-specific PCR (qMSP) analysis on sodium bisulfate-treated genomic DNA extracted from 12 control and 9 UC pediatric tissues or from HCT-116 and HT-29 colonic cell lines, as previously described.24 Briefly, genomic DNA was modified using the EZ DNA Methylation kit (Zymo Research, CA) which induces the chemical conversion of unmethylated cytosines into uracils, while methylated cytosines stay protected. Primer sequences designed specifically for the methylated region of the MIR124 promoter are provided in Supplementary Data. As a reference, an unmethylated sequence of β-actin was amplified. Mean values were used for the calculations and the values were normalized to the reference amplicon of β-actin.
Drug treatment of HCT-116 cells
HCT-116 cells (5×105 cells in 100 mm dishes) were plated and after 48 hours they were treated with 1 or 5 µM of the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine (5-AZA) (Sigma-Aldrich). Cells were incubated for 0 to 4 days before RNA extraction.
Statistical analysis
Data were analyzed by unpaired Student’s t-test, Pearson correlation or analysis of variance for multiple comparisons. MicroRNA and mRNA levels are expressed in arbitrary units, normalized to U6 snRNA and 5S rRNA reference RNAs, and GAPDH mRNA, respectively. Results were presented as means ± standard deviation or standard error of the mean, as indicated, or as boxes and whiskers (minimum-to-maximum), using Prism6 (GraphPad Software Inc.). P values of < .05 were considered statistically significant.
Results
Increased STAT3 activity in pediatric ulcerative colitis patients
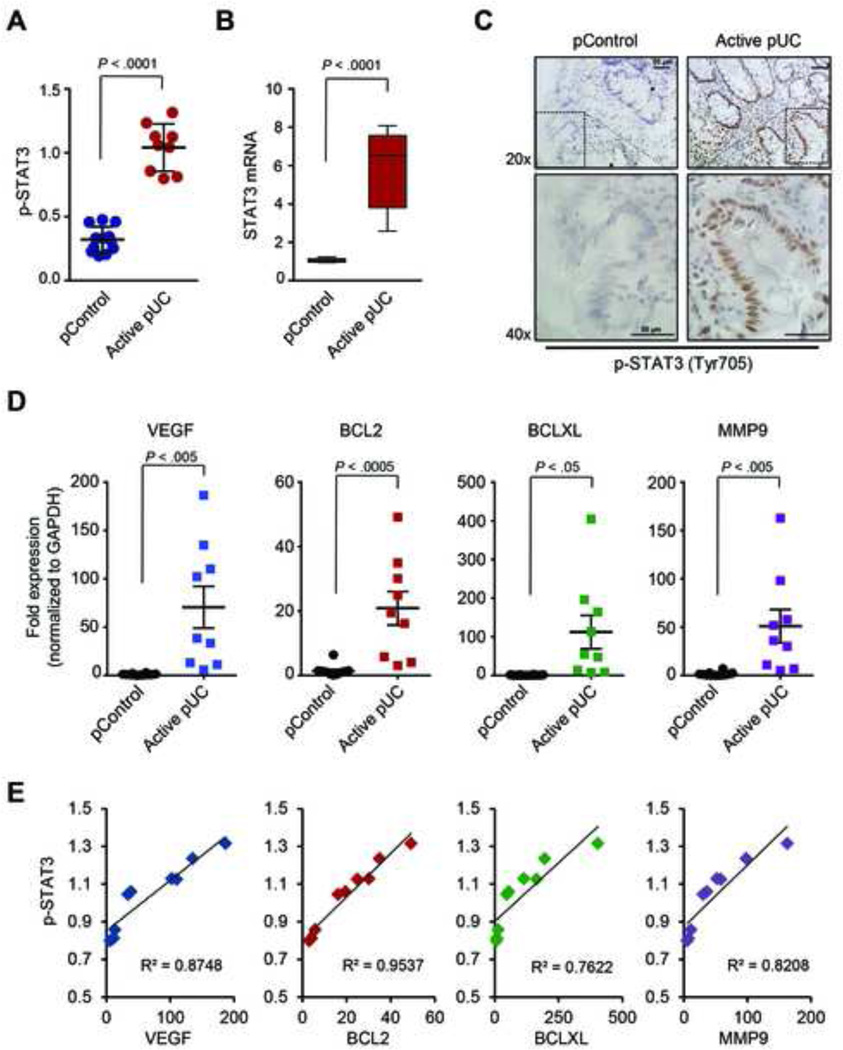
We first examined the levels of phosphorylated STAT3 (p-STAT3) at tyrosine 705 (Tyr705) by ELISA in colonic tissue biopsies derived from pediatric patients with active UC (pUC) and no-IBD pediatric samples (pControl). The phosphorylation levels as well as the mRNA levels of STAT3 are significantly increased in biopsies derived from pediatric-UC patients, relative to controls (Figure 1A–B). Moreover, immunohistochemical analysis of biopsy sections reveals nuclear staining of p-STAT3, specifically in epithelial cells of the pediatric-UC and not of the pediatric-control group (Figure 1C and Supplementary Figure 1). The increased activity of STAT3 in pediatric-UC tissues, was validated by the expression levels of direct target genes of STAT3, such as VEGF, BCL2, BCLXL and MMP9.25 All STAT3 direct target genes are highly up-regulated in biopsies derived from pediatric-UC patients, relative to controls (Figure 1D). Furthermore, there was a strong correlation between the mRNA levels of VEGF, BCL2, BCLXL and MMP9 and p-STAT3 in the pediatric-UC patient samples (Figure 1E). Overall, these data suggest that the STAT3 and its downstream directs are highly active in pediatric-UC patients.
Figure 1.
Increased levels of phosphorylated STAT3 (p-STAT3) correlate with the expression levels of its direct targets in pediatric-UC biopsies. (A) Levels of p-STAT3 in biopsies from pediatric patients with active UC (pUC, n = 9) and non-IBD pediatric samples (pControl, n = 12), as determined by ELISA assays. Values represent mean ±SD. (B) Relative levels of STAT3 mRNA were determined by real-time PCR. Data are presented as boxes with whiskers (minimum-to-maximum). (C) Immunostaining for p-STAT3 (Tyr705) (brown stain) in pControl (left) and pediatric-UC (right) biopsies. Hematoxylin is used as nuclear counterstain (blue). Representative microscopy pictures are shown in original magnification 20× and 40× (dotted area), as indicated. Scale bar represents 50 µm. (D) mRNA levels of VEGF, BCL2, BCLXL and MMP9 in pediatric-UC samples, compared to pControls, based on real-time PCR. (E) Correlation of p-STAT3 with mRNA levels of the indicated target genes. t-test analysis and correlation coefficient were calculated using Prism6 (GraphPad Software, Inc.).
Identification of microRNA regulators of STAT3 activity in colonic epithelial cells by high throughput microRNA screening
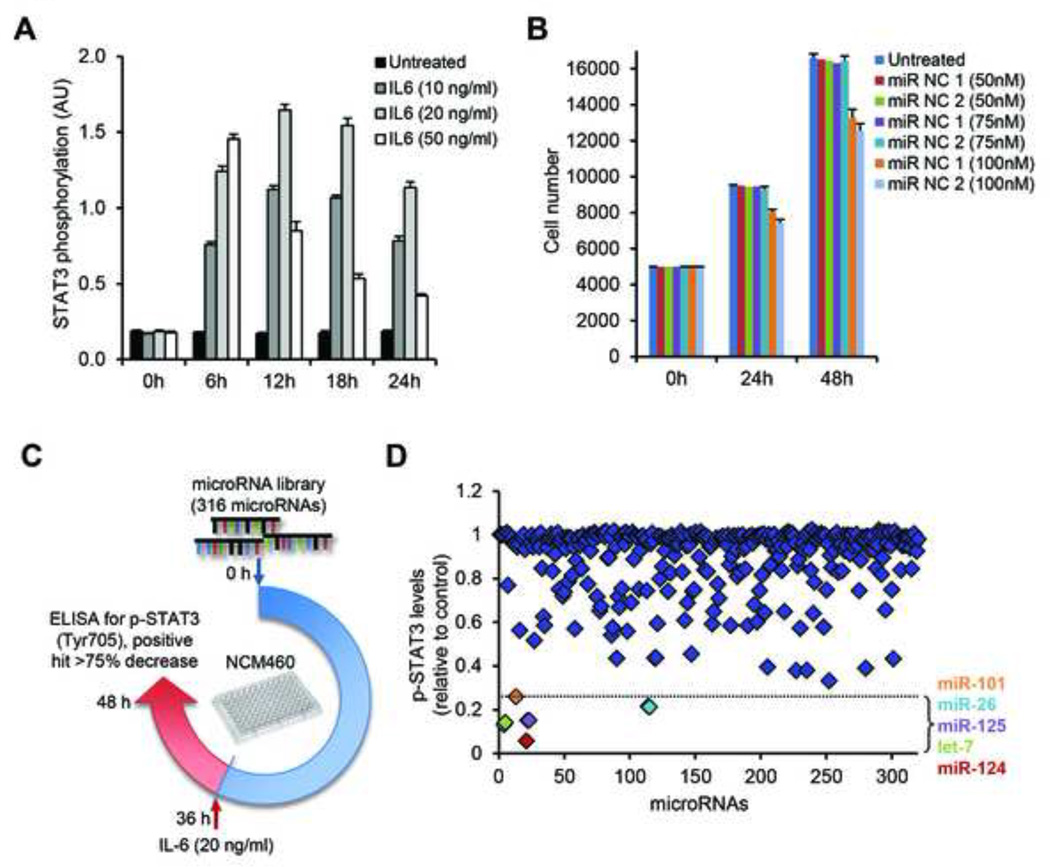
Recent studies support the notion that microRNAs are central regulators of inflammatory responses in mammary epithelial cells and hepatocytes by controlling NF-κB and IL-6/STAT3 signaling pathways.11, 26 Here, we were interested in identifying whether microRNAs are regulators of inflammatory responses and specifically STAT3 activity in human colonocytes. To address this question, we performed a high-throughput microRNA library screen in NCM460 colonic epithelial cells. We initially performed control experiments. IL-6 treatment (20 ng/ml) of NCM460 cells induced STAT3 activation in 12 hours (Figure 2A). Transfection of up to 75 nM of miR mimics did not affect the growth of NCM460 cells (Figure 2B), and the highest levels of miRs were detected 36 hours post-miR transfection (Supplementary Figure 2). Using these experimental conditions we transfected a library of 316 miRs in NCM460 cells and evaluated their ability to inhibit IL-6-dependent activation of STAT3 (Figure 2C). We identified five miRs (let-7, miR-125, miR-101, miR-26 and miR-124) that suppressed STAT3 activation by more than 75%, compared to controls (Figure 2D). Interestingly, miR-124 had the maximum inhibitory effect on STAT3 phosphorylation (greater than 90%) suggesting a potential role as a central regulator of inflammatory response in human colonocytes.
Figure 2.
MicroRNA-library screen in NCM460 colonocytes. (A) Optimization of IL-6 treatment for time and dosage, as assessed for STAT3 phosphorylation, by ELISA assay. NCM460 cells were treated with 10, 20 and 50 ng/ml of IL-6 for 0, 6, 12, 18 and 24 hours. (B) Cell growth assays on NMC460 cells transfected with 50, 75 or 100 nM of microRNA negative controls (miR-NC 1 and miR-NC 2), as compared to untreated cells. (C) Schematic representation of the 316 microRNA-library screen and (D) the effect on p-STAT3 levels, after IL-6 treatment. NCM460 cells were transfected with the microRNA-library and activation with IL-6 (20 ng/ml) followed 36 hours later. Cells were harvested at 48 hours post-transfection and p-STAT3 levels were estimated. Data points correspond to each individual microRNA of the library. The dotted line indicates the 75%-decrease point, set as the cut-off for a positive hit. Positive hits are color-coded corresponding to the microRNAs indicated on the right-hand side of the panel, presented in the order of increasing p-STAT3 inhibition.
The miR-124/STAT3 pathway is deregulated specifically in pediatric ulcerative colitis patients
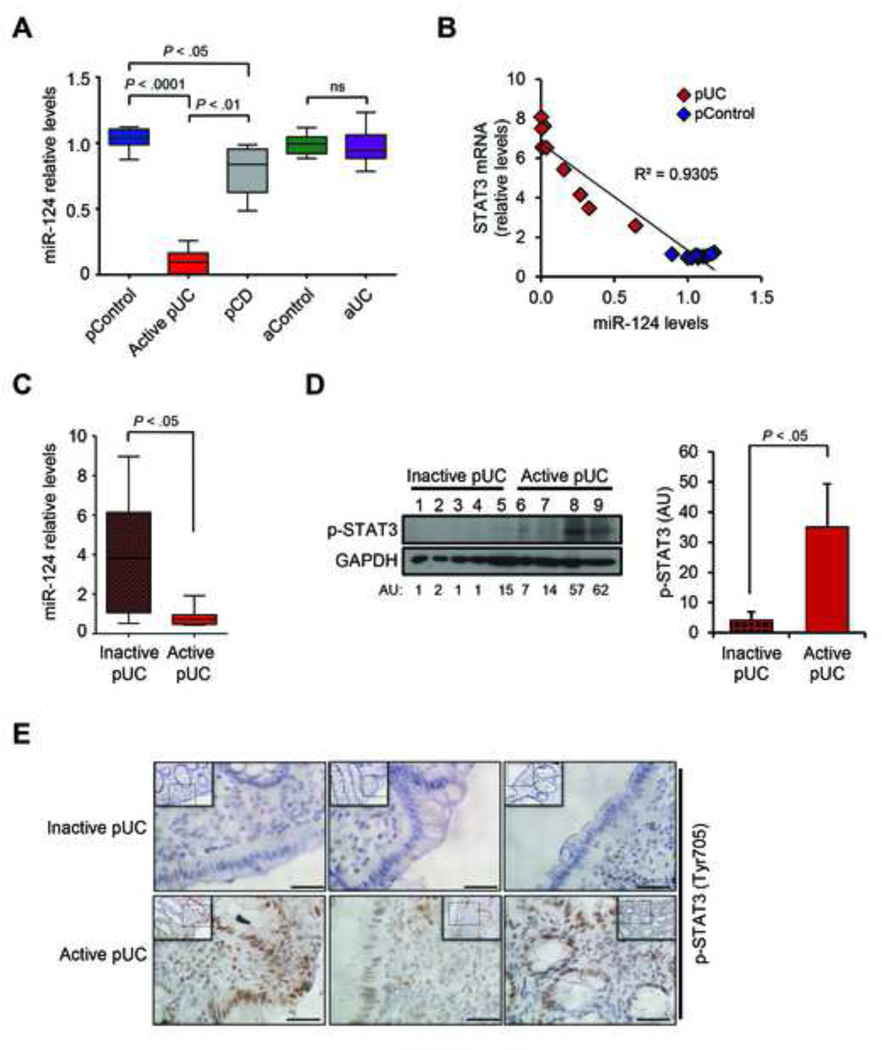
From the five miRs identified from our screen, we investigated whether any of those are specifically deregulated in pediatric-UC patients. We performed real-time PCR for these miRs in colonic biopsies derived from pediatric and adult, control and UC patients. Their clinical information and statistical analysis are provided in the Supplementary Table 1. Our results indicate that let-7 and miR-125 are specifically down-regulated in adult UC tissues, as compared to adult control tissues, while miR-101 and miR-26 are down-regulated in both pediatric and adult UC tissues, as compared to control tissues (Supplementary Figure 3). Interestingly, miR-124 is markedly decreased in pediatric-UC samples but not in adult UC tissues, suggesting a specific role for this miR in pediatric-UC patients (Figure 3A). Parallel analysis in biopsies from pediatric patients with Crohn’s disease (pCD) revealed that miR-124 is decreased in pediatric-CD patients but not as dramatically as in pediatric-UC patients (Figure 3A). Additionally, we identified a strong inverse correlation between miR-124 and STAT3 mRNA levels in pediatric-control and UC tissues (Figure 3B).
Figure 3.
Inverse correlation of miR-124 and STAT3 levels is specific to pediatric biopsies of active UC. (A) Expression levels of miR-124 in pediatric non-IBD (pControl, n = 8), pediatric active UC (pUC, n = 7), pediatric Crohn’s disease (pCD, n = 6), adult non-IBD (aControl, n = 6) and adult UC (aUC, n = 11) patient biopsies, determined by real-time PCR. Results are presented as boxes with whiskers (minimum-to-maximum), relative to pediatric-control samples (pControl). ns: not statistically significant (One-way ANOVA, Prism6, GraphPad Software Inc.). (B) Correlation of miR-124 levels to STAT3 mRNA levels in pediatric-UC and pediatric-Control biopsies. Correlation coefficient was calculated using Prism6 (GraphPad Software, Inc.). (C) Expression levels of miR-124 in biopsies from pediatric patients with inactive UC (Inactive-pUC, n = 6), compared to active pediatric-UC biopsies (Active-pUC, n = 6). (D) Western blot analysis of lysates from frozen biopsies from inactive UC (lanes 1 to 5) and active UC (lanes 6 to 9) pediatric patients for p-STAT3 and quantitation of the intensity of the detected bands, normalized to GAPDH levels and expressed in arbitrary units (AU). Quantitation for each group (right panel); data are presented as mean ±SE of the mean. t-test analysis was performed using Prism6 (GraphPad Software, Inc.). (E) Immunostaining for p-STAT3 in biopsies from inactive and active pediatric-UC patients. Representative microscopy pictures are shown in original magnification 20× (insert) and 40× (magnified dotted area), as indicated. Scale bars represent 50 µm.
We next sought to investigate the miR-124/STAT3 pathway, identified in pediatric-UC patients, in relation to the activity of the disease. For this reason, we compared the levels of miR-124 and the levels of p-STAT3 in biopsies from pediatric patients with inactive UC or active UC. MiR-124 levels are significantly decreased (Figure 3C) and p-STAT3 levels are significantly increased (Figure 3D) in pediatric-UC patients with active disease, relative to those with inactive disease. Immunohistochemical analysis of sections from additional biopsies revealed increased staining of phosphorylated STAT3 in the nucleus of epithelial cells mainly in the active UC samples (Figure 3E and Supplementary Figure 1), indicating the functional relevance of this pathway to the disease activity.
MiR-124 regulates STAT3 mRNA levels through direct binding in its 3’UTR
Based on the information above, we investigated the potential direct interaction between miR-124 and STAT3. Bioinformatic analysis identified sequence complementarity of miR-124 with the 3’UTR of STAT3 (Figure 4A). To verify the direct interaction between miR-124 and STAT3, we employed luciferase assays. Delivery of miR-124 suppresses STAT3-3’UTR luciferase activity by more than 60% (Figure 4B). Furthermore, the endogenous STAT3 mRNA levels in HCT-116 colonic epithelial cells, after miR-124 overexpression, decrease by more than 70% (Figure 4C). To further validate the correlation between STAT3 expression and phosphorylation status, we performed western blot analysis for total and phosphorylated STAT3 in three different human colonic cell lines HT-29, RKO and HCT-116, after miR-124 transfection. MiR-124 delivery suppresses both total and phosphorylated STAT3 at similar levels, suggesting that miR-124 regulates STAT3 activity through its transcript (Figure 4D). Overall, we identified a direct regulation of STAT3 expression by miR-124 in human colonocytes.
Figure 4.
MiR-124 targets STAT3 mRNA by direct binding to its 3’UTR. (A) Bioinformatic analysis identified complementarity of STAT3 mRNA and the “seed sequence” of miR-124. (B) Luciferase reporter assays using the 3’UTR of STAT3 and (C) real-time PCR analysis for endogenous mRNA levels of STAT3 in mock-transfected (Control) HCT-116 cells, or transfected with negative control microRNA (miR-NC) (100 nM) or miR-124 (100 nM), for 36 hours. Experiments were performed in triplicates; values represent mean ±SE, t-test analysis, Prism6 (GraphPad Software Inc.). (D) Western blot analysis for p-STAT3 and total-STAT3 (STAT3) in three colonic cell lines (HT-29, RKO and HCT-116) after mock transfection (Control) or transfection with negative-control microRNA (miR-NC) or miR-124 (100 nM), as indicated, for 36 hours. Tubulin was used as loading control.
The miR-124/STAT3 pathway in two different models of mouse experimental colitis
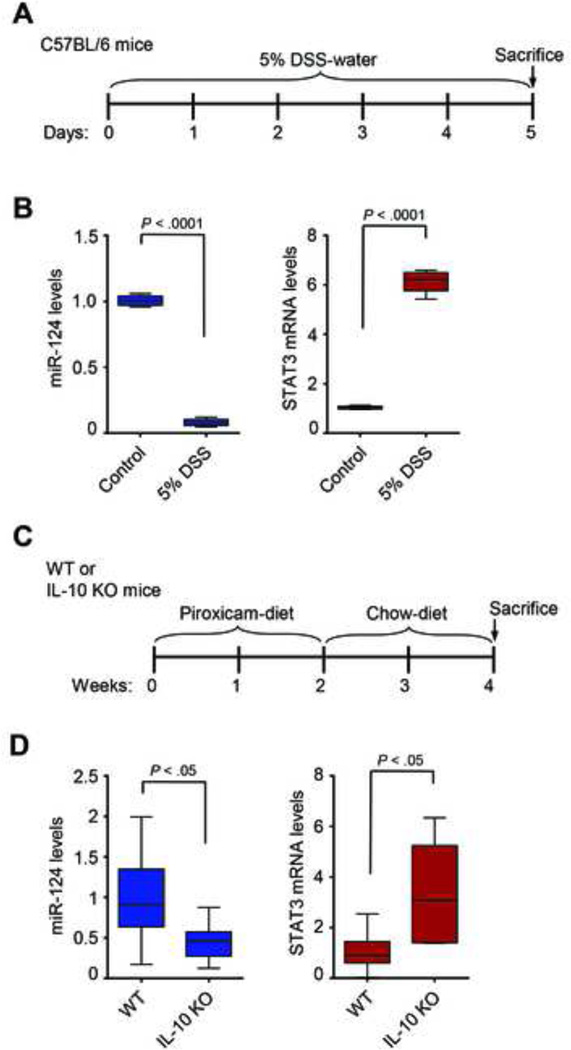
To study further the role of miR-124/STAT3 pathway in colitis, we used the dextran sulfate sodium (DSS) and the IL-10 deficient (IL-10 KO) mouse models of experimental colitis. The DSS-mouse model protocol is outlined in Figure 5A. MiR-124 levels are decreased, while STAT3 mRNA levels are increased in the colonic tissues of DSS-treated mice (n = 5), compared to control mice treated with regular water (n = 5) (Figure 5B).
Figure 5.
MiR-124 and STAT3 expression levels in mouse models of experimental colitis. (A) Schematic representation of the protocol for the DSS-model in C57BL/6 mice. (B) The levels of miR-124 and STAT3 mRNA expression in colonic tissues of control (Control, n = 5) and DSS-treated mice (5% DSS, n = 5) were determined by real-time PCR. (C) Schematic representation of the experimental design for the IL-10 KO-colitis mouse model. (D) miR-124 and STAT3 expression levels in colonic tissues from WT and IL-10 KO mice (n = 7 per group) were determined by real-time PCR analysis. Data are presented as boxes with whiskers (minimum-to-maximum). (t-test, Prism6, GraphPad Software, Inc.).
A more relevant animal model for UC is the IL-10 KO mouse model. Specifically, the protocol that was followed is outlined in Figure 5C. Real-time PCR analysis in colonic tissues from control (n = 7) and IL-10 KO (n = 7) mice reveals a significant decrease of miR-124 and a significant increase of STAT3 mRNA levels in IL-10 KO mice, relative to WT (Figure 5D). Our analyses suggest that the miR-124/STAT3 pathway is deregulated in both DSS and IL10-KO mouse models of colitis, consistent with our findings in human and in vitro studies.
Increased DNA-methylation of MIR124 promoter in pediatric ulcerative colitis
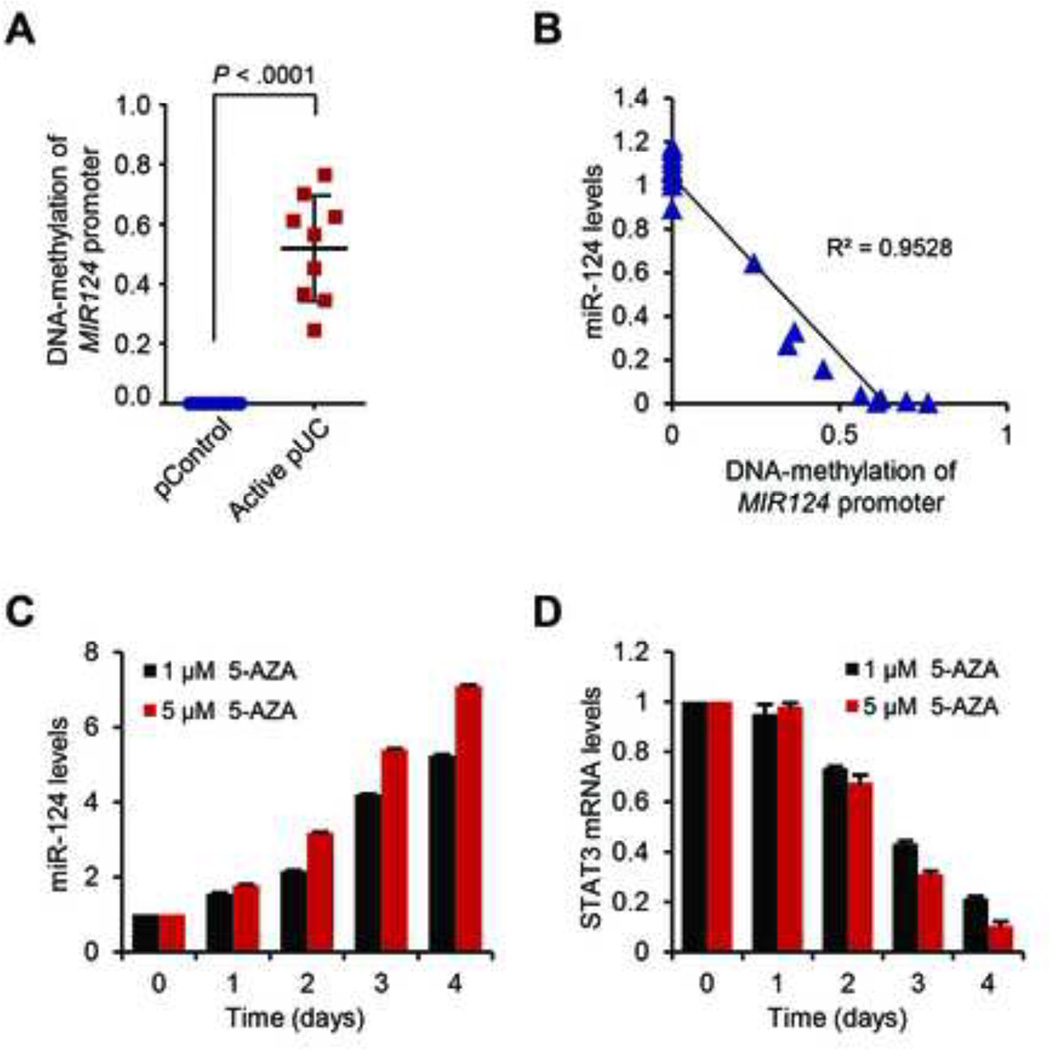
To determine whether the lower levels of miR-124, observed in the pediatric-UC samples were due to hypermethylation, we analyzed the methylation status of MIR124 promoter in these biopsies, using quantitative methylation-specific PCR (qMSP) analysis. This analysis revealed that MIR124 promoter area is hypermethylated in pediatric-UC patient samples, compared to control samples (Figure 6A). Additionally, the DNA-methylation levels of MIR124-promoter region follow a strong inverse correlation to miR-124 levels of pediatric-UC patient samples (R2 = 0.9528), supporting the hypothesis that DNA-methylation regulates miR-124 levels in these patients (Figure 6B).
Figure 6.
DNA-methylation regulates miR-124 expression in active pediatric-UC. (A) Levels of DNA-methylation of MIR124 promoter region in active pediatric-UC biopsies (pUC), compared to controls (pControl) were determined by bisulfate sequencing. Values represent mean ±SD; t-test, Prism6 (GraphPad Software, Inc.). (B) Correlation of miR-124 expression levels with the DNA-methylation status of the MIR124 promoter. Correlation coefficient was calculated using Prism6 (GraphPad Software, Inc.). (C) Real-time PCR analysis for the expression levels of miR-124 and (D) STAT3, after treatment of HCT-116 colonic cells with two different concentrations (1 and 5 µM) of the DNA-methyltransferase inhibitor 5-aza-2’-deoxycytidine (5-AZA), for 0 to 4 days. The experiment was performed in triplicates.
DNA-methylation regulates miR-124 expression
HCT-116 cells, a transformed colonic cell line with decreased levels of miR-124,27 were treated with the DNA-methyltransferase inhibitor, 5-AZA. Real-time PCR revealed a dose- and time-dependent increase in miR-124 levels in HCT-116 cells (Figure 6C). On the contrary, STAT3 mRNA levels decrease following 5-AZA treatment (Figure 6D), suggesting that the miR-124/STAT3 pathway is epigenetically regulated. Treatment with 5-AZA decreases the methylation levels in the promoter region of MIR124 in HCT-116 cells, as verified by qMSP analysis (Supplementary figure 4A). Similarly, the miR-124 levels are regulated by DNA-methylation of the MIR124 promoter region in the HT-29 colonic adenocarcinoma cell line (Supplementary figure 4B–C). All the above, indicate that DNA-methylation is a regulatory mechanism for MIR124.
Discussion
Our study reveals that an epigenetically regulated microRNA, miR-124, controls the STAT3 signaling pathway specifically in pediatric-UC patients with active disease. Initially, we were interested in identifying the role of STAT3 in pediatric-UC, since it has been involved in adult-UC. Bio-Plex and real-time PCR analyses in pediatric patient samples identified a significant increase in p-STAT3 and STAT3 mRNA levels in UC patients, compared to controls. Additionally, we identified a strong correlation of p-STAT3 with its targets, VEGF, BCL2, BCLXL and MMP9. These factors are implicated in the pathogenesis of IBD and their expression is elevated in IBD patients.28–31
We then focused on miRs that can regulate the activity of STAT3 in the context of inflammation in human colonocytes. Screening of 316 miRs identified the ones that can regulate the activation of STAT3 in NCM460 colonic epithelial cells. MiRs are negative regulators of gene expression thus our screen reveals, by overexpressing them, which miRs can suppress STAT3 activation. This suppression can be due to reduced STAT3 mRNA levels or reduction in the mRNA levels of another factor that regulates STAT3 phosphorylation. The miRs with the greatest effect on STAT3 activation are miR-124, let-7, miR-125, miR-26 and miR-101. Bioinformatic and in vitro analyses identified that only miR-124 has a binding site in the 3’UTR of STAT3 mRNA, thus miR-124 overexpression can suppress STAT3 at the mRNA level, resulting to decreased levels of phosphorylated STAT3. The other 4 miRs identified in our screen, may regulate STAT3 through indirect mechanisms, since they have all been implicated in inflammation. Specifically, the negative NF-κB regulator TNFAIP3 is a direct target of miR-125a and miR-125b in diffuse large B-cell lymphoma,32 while miR-125b directly targets STAT3, as demonstrated by luciferase-reporter assays.33 MicroRNA let-7 has been reported by others34 and us to be involved in inflammatory circuits leading to cell transformation through STAT3 activation.16, 26 MiR-26 plays a role in carcinogenesis by inhibiting EZH235, 36 but is also implicated in gastrointestinal diseases37, 38 indicating the importance of epigenetic regulation in these diseases, as well as in pediatric-UC. Notably, according to our data, miR-101 is significantly down-regulated in both pediatric and adult UC patients. This is of particular importance since miR-101 is a known negative regulator of EZH2 expression.39 EZH2 is a member of the polycomb repressor complex 2 (PRC2), a global suppressor of gene expression. This suggests that miR-101 can regulate chromatin modifications that can affect multiple loci important in the pathogenesis of both pediatric and adult IBD.
Apart from the fact that miR-124 had the strongest effect on STAT3 activation in our high-throughput screen and that it targets STAT3 by direct binding to its mRNA, our data indicate an important specific deregulation of miR-124 to the pediatric-UC and not the adult-UC patients. Taken together, the above findings make miR-124 an attractive and promising target to further study its role and regulation in the context of pediatric-UC.
The decreased miR-124 levels found in pediatric-UC patients may be attributed to DNA hypermethylation of its promoter region, according to our data. On the other hand, recent studies point to global alterations in DNA-methylation in IBD patients.40–42 Although there is deregulation of the DNA methylation machinery in multiple human diseases, the genes that are affected are not always the same. Thus, identifying novel factors that are uniquely deregulated in pediatric-UC patients, compared to adult UC, is valuable in distinguishing the different stages of this disease.
Conversely, different molecular mechanisms may be responsible for the regulation of the expression levels of the same gene in different human diseases. Recent studies from our group revealed that miR-124 is highly down-regulated in liver cancer patients. Thought DNA-methylation was a possible mechanism of miR-124 down-regulation in these patients, instead, we identified that miR-124 was transcriptionally regulated by the HNF4A transcription factor.11
The data presented here suggest that miR-124 is down-regulated in pediatric UC patients due to DNA-methylation in the MIR124 promoter region. This finding is supported by our in vitro data indicating that treatment of cells with 5-AZA results in demethylation of the MIR124 promoter region and up-regulation of miR-124 levels. This type of epigenetic regulation for miR124 has been identified previously in other human diseases. Specifically, in 19 liver cancer cell lines and 41 primary hepatocellular carcinoma tissues, miR124 was reduced and frequent tumor-specific methylation was reported,43 similarly to HCT-116 cells.27 Interestingly, the methylation levels at MIR124 loci in the gastric mucosa of volunteers infected by H.pylori are elevated compared to healthy non-infected individuals44, while DNA-methylation silencing of MIR124 is functionally involved in cervical carcinogenesis.24
The epigenetic regulation of the miR-124/STAT3 signaling pathway in active pediatric-UC presented in this study (Figure 7), serves as the first evidence for an epigenetic regulatory mechanism in pediatric IBD. The specificity and the notable decrease of miR-124 identified in this study, make it a potential candidate as a biomarker for active pediatric-UC. Apart from the insights into the pathology of the disease, our findings provide a new pathway to target specifically pediatric-UC. Perturbations of the epigenetically regulated miR-124/STAT3 pathway may lead to novel therapeutic approaches with great impact in pediatric-UC and other inflammatory diseases where this regulatory mechanism holds an important role.
Figure 7.
Schematic representation of the identified epigenetic regulatory mechanism involved in the miR-124/STAT3 pathway in pediatric-UC. DNA-methylation in the promoter of MIR124 decreases the expression levels of the microRNA relieving its inhibitory effect on STAT3 levels, thus leading to increased STAT3 phosphorylation and consequently to higher levels of its direct target genes VEGF, BCL2, BCLXL and MMP9.
Supplementary Material
Acknowledgments
Grant support: This study was supported by the Charles H. Hood Foundation (DI), NIH DK60729 (CP), a grant from the Pediatric IBD Foundation (HSW), and philanthropic support from Martin Schlaff (HSW).
Abbreviations
- IBD
inflammatory bowel disease
- STAT3
signal transducer and activator of transcription 3
- 5-AZA
5-aza-2’-deoxycytidine
- DSS
dextran sulfate sodium salt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have nothing to disclose. No conflicts of interest exist.
Author Contributions: GK, CP, CP, HW, DI contributed to the design of the study, GK, CP, JK, AMF, BG-M, EK, HW, DI contributed to the generation, analysis and interpretation of the data, GK and DI contributed to the preparation of the manuscript.
References
- 1.Scholmerich J. New developments in aetiological mechanisms of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2003;15:585–586. doi: 10.1097/00042737-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 4.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousvaros A, Sylvester F, Kugathasan S, et al. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. doi: 10.1097/01.mib.0000228358.25364.8b. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto K. Role of STAT3 in inflammatory bowel disease. World J Gastroenterol. 2008;14:5110–5114. doi: 10.3748/wjg.14.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso A, Dentelli P, Carlino A, et al. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91–98. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 9.Willson TA, Kuhn BR, Jurickova I, et al. STAT3 genotypic variation and cellular STAT3 activation and colon leukocyte recruitment in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:32–43. doi: 10.1097/MPG.0b013e318246be78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatziapostolou M, Polytarchou C, Aggelidou E, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147:1233–1247. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagakis E, Bertsias G, Verginis P, et al. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann Rheum Dis. 2011;70:1496–1506. doi: 10.1136/ard.2010.139857. [DOI] [PubMed] [Google Scholar]
- 13.Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 14.Smith KM, Guerau-de-Arellano M, Costinean S, et al. miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. J Immunol. 2012;189:1567–1576. doi: 10.4049/jimmunol.1103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Androulidaki A, Iliopoulos D, Arranz A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliopoulos D, Jaeger SA, Hirsch HA, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalal SR, Kwon JH. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2010;6:714–722. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Zhang S, Dassopoulos T, et al. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F, Guo NJ, Tian H, et al. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2011;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paraskevi A, Theodoropoulos G, Papaconstantinou I, et al. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Koon HW, Zhao D, Zhan Y, et al. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci U S A. 2007;104:2013–2018. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 24.Wilting SM, van Boerdonk RA, Henken FE, et al. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer. 2010;9:167. doi: 10.1186/1476-4598-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez JV, Frank DA. Genome-wide analysis of STAT target genes: elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol Ther. 2004;3:1045–1050. doi: 10.4161/cbt.3.11.1172. [DOI] [PubMed] [Google Scholar]
- 26.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lujambio A, Ropero S, Ballestar E, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 28.Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Karamanolis DG, Kyrlagkitsis I, Konstantinou K, et al. The Bcl-2/Bax system and apoptosis in ulcerative colitis. Hepatogastroenterology. 2007;54:1085–1088. [PubMed] [Google Scholar]
- 30.Manfredi MA, Zurakowski D, Rufo PA, et al. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1091–1096. doi: 10.1002/ibd.20419. [DOI] [PubMed] [Google Scholar]
- 31.Meijer MJ, Mieremet-Ooms MA, van der Zon AM, et al. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn's disease phenotype. Dig Liver Dis. 2007;39:733–739. doi: 10.1016/j.dld.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Ramasamy K, Bouamar H, et al. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) Proc Natl Acad Sci U S A. 2012;109:7865–7870. doi: 10.1073/pnas.1200081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surdziel E, Cabanski M, Dallmann I, et al. Enforced expression of miR-125b affects myelopoiesis by targeting multiple signaling pathways. Blood. 2011;117:4338–4348. doi: 10.1182/blood-2010-06-289058. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi Y, Tsujii M, Wang J, et al. CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori-related carcinogenesis. Gut. 2012 doi: 10.1136/gutjnl-2011-301625. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, He ML, Wang L, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Liu QG. The role of miR-26 in tumors and normal tissues (Review) Oncol Lett. 2011;2:1019–1023. doi: 10.3892/ol.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padgett KA, Lan RY, Leung PC, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun. 2009;32:246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating beta-catenin. Gastroenterology. 2012;143:246–256. e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao P, Deng Z, Wan M, et al. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Z, Hegarty JP, Yu W, et al. Identification of disease-associated DNA methylation in B cells from Crohn's disease and ulcerative colitis patients. Dig Dis Sci. 2012;57:3145–3153. doi: 10.1007/s10620-012-2288-z. [DOI] [PubMed] [Google Scholar]
- 41.Lin Z, Hegarty JP, Cappel JA, et al. Identification of disease-associated DNA methylation in intestinal tissues from patients with inflammatory bowel disease. Clin Genet. 2011;80:59–67. doi: 10.1111/j.1399-0004.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 42.Kellermayer R. Epigenetics and the developmental origins of inflammatory bowel diseases. Can J Gastroenterol. 2012;26:909–915. doi: 10.1155/2012/526408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furuta M, Kozaki KI, Tanaka S, et al. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- 44.Ando T, Yoshida T, Enomoto S, et al. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer. 2009;124:2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.