Abstract
“Sighs, tears, grief, distress” expresses Johann Sebastian Bach in a musical example for the relationship between sighs and deep emotions. This review explores the neurobiological basis of the sigh and its relationship with psychology, physiology, and pathology. Sighs monitor changes in brain states, induce arousal, and reset breathing variability. These behavioral roles homeostatically regulate breathing stability under physiological and pathological conditions. Sighs evoked in hypoxia evoke arousal and thereby become critical for survival. Hypoarousal and failure to sigh have been associated with sudden infant death syndrome. Increased breathing irregularity may provoke excessive sighing and hyperarousal, a behavioral sequence that may play a role in panic disorders. Essential for generating sighs and breathing is the pre-Bötzinger complex. Modulatory and synaptic interactions within this local network and between networks located in the brainstem, cerebellum, cortex, hypothalamus, amygdala, and the periaqueductal gray may govern the relationships between physiology, psychology, and pathology. Unraveling these circuits will lead to a better understanding of how we balance emotions and how emotions become pathological.
Keywords: anxiety, panic, arousal, cardiorespiratory, PAG, rhythm generation, SIDS, breathing, pre-Bötzinger complex
1 INTRODUCTION
Breathing is an essential component of life, and must be maintained from the first to the last breath. Disturbances in the neuronal control of breathing have devastating consequences and may ultimately be fatal. Various neurological conditions are associated with severe breathing disturbances. These disorders include multiple system atrophy (Schwarzacher et al., 2011), Rett syndrome (Ramirez et al., 2013b; Weese-Mayer et al., 2006, 2008b), Familial Dysautonomia (Carroll et al., 2012; Weese-Mayer et al., 2008a), sudden infant death syndrome (Garcia et al., 2013; Kinney et al., 2009; Paterson, 2013), congenital central hypoventilation syndrome (Ramanantsoa and Gallego, 2013), sleep apnea (Gozal and Kheirandish-Gozal, 2008; Ramirez et al., 2013a), Pitt Hopkins Syndrome (Gallego, 2012), and sudden death of epilepsy (Kalume, 2013; Sowers et al., 2013). Thus, understanding how breathing is generated within the nervous system and how the CNS controls ventilatory functions is of great clinical interest.
But breathing does not have an important role only in controlling ventilatory functions. This behavior has been implicated in the control of a variety of central nervous system functions that are not obviously associated with the control of lung ventilation. Indeed, breathing is perhaps one of the most centrally integrated motor behaviors with functional roles that reach well beyond a “simple control of lung ventilation.” Most respiratory physiologists are aware of the fact that respiratory activity changes dramatically during wakefulness and sleep, and that modulatory and sensory control of breathing is state dependent. But, surprisingly, many scientists have no appreciation, or only very little knowledge, that breathing also has “nonventilatory” functions. Breathing behavior is, for example, highly influenced by emotional states. This behavior is greatly affected by negative (panic, anxiety, and pain) and positive emotions (pleasure, love, and relief), and one of the purposes of this review is to discuss the possibility that breathing is modulated by various circadian, cognitive, and emotional brain states, and at the same time itself plays a major role in centrally affecting emotions, arousal, and other brain states. Breathing disturbances, such as hyperventilation and an increased sigh frequency, are characteristic of panic disorders. And we will argue that hyperventilation is possibly causal for the initiation of panic. Hyperventilation-induced seizures are another example of a brain state that may be centrally caused by the respiratory system (Tsiptsios et al., 2010; Yang et al., 2011). Although hyperventilation-induced seizure activation is a commonly used approach in electroconvulsive therapy (Datto et al., 2002; Haeck et al., 2011; Loo et al., 2010) or in the assessment of epilepsy (Abubakr et al., 2010; Jonas et al., 2011; Tsiptsios et al., 2010), we have very little mechanistic insight into how hyperventilation causes the seizures. It is generally believed that the seizure induction and duration relate to the degree of hypocapnia (Bergsholm et al., 1984), but when it comes to the existing data, the relationship between the level of hypocapnia and seizure activity is not always straightforward (Wirrell et al., 1996). Thus, the induction of epilepsy may in part be neurogenic. Indeed, it has been suggested that hyperventilation-induced mesiotemporal epilepsy and panic disorders may share common neurobiological mechanisms that involve maladaptive amygdalar activation (Gerez et al., 2011; Yang et al., 2011).
This review discusses the neuronal and ventilatory functions controlled by breathing and we evaluate to what extent brain states interact in a reciprocal manner with the neuronal networks that control breathing. For obvious reasons, it is impossible to cover the entire field of neuronal and ventilatory control of breathing in one review. Thus, at the outset of this review, we would like to emphasize that we do not attempt to provide a complete overview of the various neuronal mechanisms associated with breathing. Instead, we focus on some specific, yet fascinating, examples with a special emphasis on the generation of the sigh.
The sigh is a deep augmented breath with distinct neurobiological, physiological, and psychological properties that distinguish it from a normal eupneic breath. Sighs are typically triggered by a normal eupneic breath and are followed by a respiratory pause, which is referred to as “postsigh apnea.” Figure 1 shows the respiratory trace of a typical sigh with its two distinct components (Fig. 1): a normal eupneic breath, triggering the large-amplitude sigh, followed by the postsigh apnea (Fig. 1). Under certain conditions, the sigh can become uncoupled and independent from eupneic activity (Lieske et al., 2000). Sighs have important ventilatory functions as they lead to a maximal expansion of the lungs, which prevents the progressive collapse of alveoli (atelectasis) (Bendixen et al., 1964; Cammarota et al., 2011; Hess and Bigatello, 2002; Hoch et al., 1998; Koch et al., 2012).Sighs also restore lung compliance (Caro et al., 1960; Ferris and Pollard, 1960) and maintain normal lung function (Cherniack et al., 1981). Genetically engineered mice that are unable to sigh eventually die of major lung problems (Koch et al., 2012), suggesting that sighs are essential for survival. Because of the large tidal volume associated with sighs and the ability to maximally expand the lung, sighs are often referred to as “augmented breaths.” But in this review, we want to emphasize that the sigh is not just a breath that is augmented. Sighs have distinct nonventilatory, behavioral functions that go well beyond a simple role in augmenting lung volume. Indeed, sighs have inspired philosophers, musicians, and poets for several centuries. “Sigh no more, Ladies” is a famous poem by William Shakespeare, and Johann Sebastian Bach (1685–1750) expresses in his cantata BWV13: “Meine Seufzer, meine Tränen” (my sighs, my tears) or the chorale BWV 254: “Ach Gott, erhör mein Seufzen und Wehklagen” (Oh god, listen to my sighs and cries of despair).
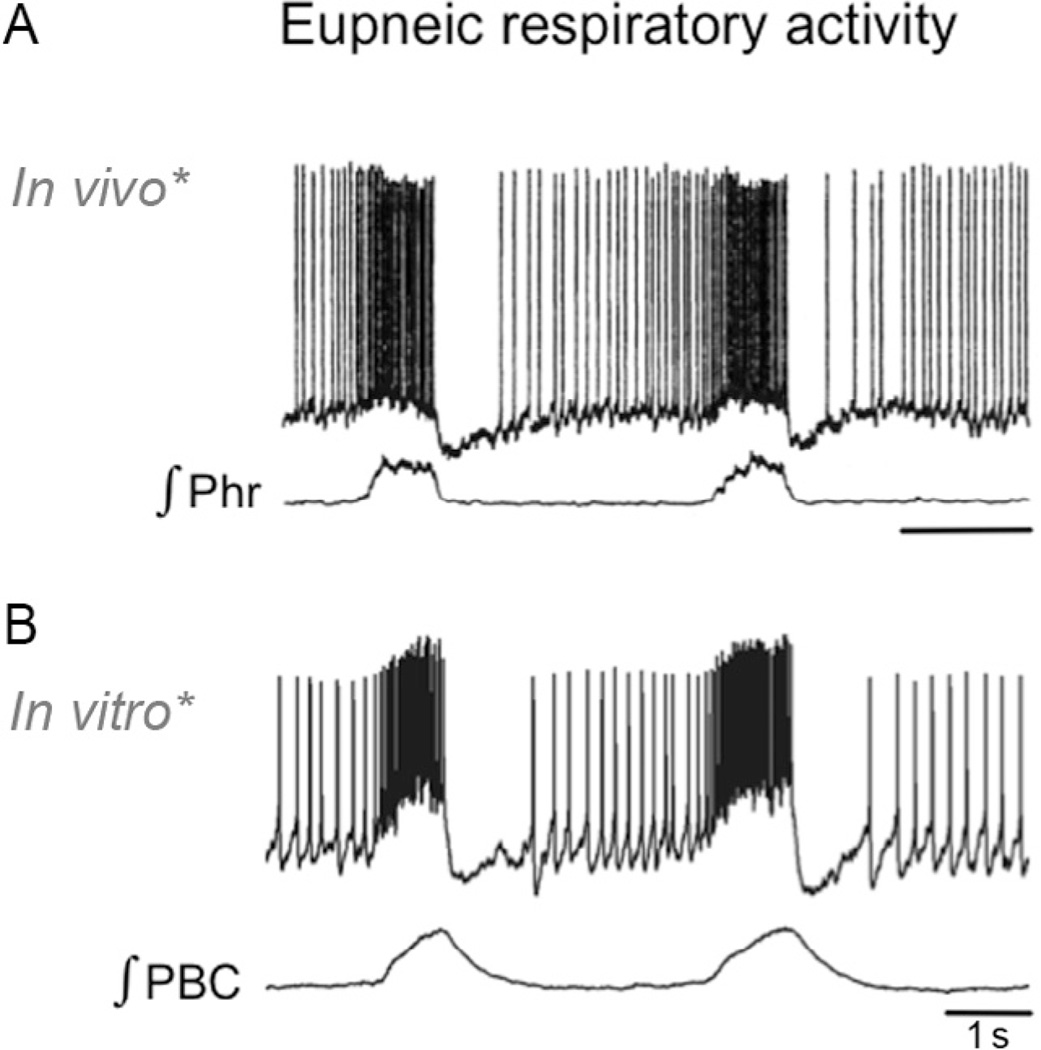
Figure 1.
The neuronal, anatomical, and physiological characteristics of the sigh and the pre-Bötzinger complex in an intact animal, in a human, and in an in vitro preparation. The sigh recorded as integrated phrenic nerve activity from an intact animal (A) and as integrated population activity (D) within the pre-Bötzinger complex (B and C), isolated in a transverse slice from the ventrolateral medulla of a mouse (B). (A) The sigh is characterized by a large inspiratory burst of activity (A1) that is triggered from a normal eupneic breath and that is followed by an apnea (A2) alsoreferred to as “postsigh apnea.” (B) Rhythmically active transverse slice: the right side of the slice depicts an activity map of inspiratory activity. In this activity map, red represents the location of maximal integrated neuronal inspiratory activity generated during the sigh, which overlaps with the site of the pre-Bötzinger complex. (C) The pre-Bötzinger complexin a human. Note the close proximity to the Nucleus ambiguous (NA), which contains cardiac vagal neurons that are responsible for the generation of parasympathetic activity in the heart. The close proximity of NA and the pre-Bötzinger complex presumably plays a role in the cardiorespiratory coupling. (D) Integrated population activity recorded from the pre-Bötzinger complex. Note that the large-amplitude burst is followed by a period of apnea. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
Modified from (B) Lieske et al. (2000), (C) Schwarzacher et al. (2010), and (D) Lieske et al. (2000).
In contrast to the artists’ early understanding of the deeper nature of the sigh, scientists have largely overlooked the behavioral role of sighing. One of the first behavioral characterizations of the sigh in the scientific literature was made by Haldane et al. (1919). These authors described that the frequency of sighing changes during the transition from sitting to lying. The relative lack of interest in the behavioral role of the sigh in the early scientific literature is somewhat surprising, given that sighs are not a rare breathing behavior. Sighs occur spontaneously, and babies sigh every few minutes (Fig. 2A; Fleming et al., 1984; Hoch et al., 1998), while adults continue to sigh regularly, albeit at a lower frequency (Bell et al., 2011; Vlemincx et al., 2009, 2011, 2013a). Indeed, sighs can occur at surprisingly regular intervals (Fig. 2A). In its extreme, some patients have the irrepressible persistence of sighing. Before the occurrence of irrepressible sighing, a large proportion of such patients seem to experience a traumatic event or anxiety (Sody et al., 2008). However, so far there has been no attempt to mechanistically explain a possible relationship between a traumatic event and the occurrence of a “sigh syndrome.” Indeed, the search for the term “sigh syndrome” yields only this one publication (Sody et al., 2008).
Figure 2.
Sighs occur spontaneously in humans. In addition, the neuronal activity critical for the generation of the sigh can be recorded as a “fictive sigh” when the underlying network is isolated in a brainstem slice preparation from a mouse. (A) Sighs are recorded as large-amplitude breathing movements using inductance plethysmography bands in a young infant. Note the regular occurrence of the large deflections that represent sighs. Inset marked by a red arrow: Each sigh consists of a large-amplitude inspiratory effort that is triggered by a eupneic breath and is followed by a short period of apnea. (B) Fictive sighs occur also spontaneously in the pre-Bötzinger complex isolated in slices obtained from neonatal mice. Note that the recordings in the human infant (A) and isolated slice (B) have the same time scale, illustrating the remarkable regularity of the sigh rhythmic activity, which occurs at a much slower time scale than the eupneic activity. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
Nonetheless, it would be wrong to conclude that nothing is known about sighs. We can trust that Shakespeare already knew that sighs are not just augmented breaths and that it would be misleading to state: “No more augmented breaths, ladies.” In the following sections, we review distinct behavioral functions for the sighs and their behavioral consequences in health and disease.
2 SIGHS MAY SIGNAL CHANGES IN BEHAVIORAL STATE
In a pioneering study by Soltysik and Jelen (2005), the authors demonstrate that sighs have important roles in the body language repertoire of rodents. A safety stimulus signaling to the animal that they will not be exposed to a tail shock leads to a 20-fold increase in sighing. Thus, sighs are specifically expressed during the emotional state of relief, and sighing was even contagious. The authors hypothesize that the sigh could act as a social signal of safety, which is a signal opposite to the alarm cry. It is easy to conclude that sighs may have a similar role in humans. Humans communicate emotional states by smiling, laughing, frowning, and weeping (Teigen, 2008), and the so-called sigh of relief certainly belongs to this family of behaviors (Vlemincx et al., 2009, 2010a). But the behavioral role of sighs goes beyond the sigh of relief. We have all experienced the “sigh of love,” yet sighs are not associated only with positive emotions. Sighs are also generated during negative emotions such as panic (Abelson et al., 2001), distress, sadness, or despair as expressed in the music by Johann Sebastian Bach (“my sigh, my tears,” BWV13, “god listen to my sighs and cries of despair,” BWV254).
In general terms, sighs could signal a behavioral state change: For example, in the “sigh of relief,” sighs signal the change from fear to relaxation. Teigen (2008) also describes situations in which sighs are elicited by a quick drop in physiological arousal, during situations in which humans feel helpless and surrender. The above-mentioned statements by Johann Sebastian Bach could also fall into this category.
The hypothesis that sighs are generated in association with changes in brain states is also consistent with known changes in the sleep/wake cycle. Sighs are frequently generated during the transition from wakefulness to NREM sleep, or from sleep to arousal (Eckert et al., 2007; Orem and Trotter 1993). Based on the tight association with state changes, Orem and Trotter (1993) concluded that the generation of sighs is likely controlled by centrally mediated mechanisms. The concept of a centrally mediated mechanism contrasted the hypothesis that sighs constitute a simple reflex caused by lung hyperinflation, an idea that has prevailed for several decades (Bartlett, 1971; Glogowska et al., 1972; Thach and Taeusch, 1976; Wulbrand et al., 2008). Nevertheless, the concept of a central origin of sighs is consistent with the observation that sighs persist following deafferentation (vagal nerve denervation) in in vivo cats (Cherniack et al., 1981), following lung transplantation in humans (Shea et al., 1988), and following complete deafferentation when isolated in an in vitro preparation as discussed later in the review (Figs. 1B and 2D; Lieske et al., 2000).
3 SIGHS AND THE CONTROL OF AROUSAL
Glogowska and collaborators (1972) were perhaps the first to report that sighs are triggered by stimuli that initiate arousal. Subsequently, McGinty and coworkers (1979) described in detail a stereotypic sequence of events in which the generation of sighs is associated with increased somatic activity, variable heart rate deceleration, and sleep state transition. This observation was confirmed in a series of elegant studies demonstrating that in infants, arousal begins with the occurrence of a sigh (i.e., augmented breath), followed by thrashing, eye opening, and repositioning of the head (Lijowska et al., 1997; McNamara et al., 1998). Interestingly, the same stereotypical sequence of behaviors was observed under different conditions (McNamara et al., 1998; Thach and Lijowska, 1996). Spontaneously occurring sighs can be associated with arousal in the absence of an obvious sensory stimulus (Anderson et al., 1996; Thach and Lijowska 1996), but sighs followed by arousal could also be triggered mechanically (McNamara et al., 1998; Thach and Lijowska, 1996). Perhaps most importantly, sighs can also be triggered by changes in blood gases, as the generation of the sigh is particularly sensitive to hypoxic challenges (Bartlett, 1971; Bell and Haouzi, 2010; Bell et al., 2009; Cherniack et al., 1981; Hill et al., 2011; Lieske et al., 2000; Schwenke and Cragg, 2000). This chemical sensitivity is in part mediated centrally and does not even require peripheral chemoreceptors (Hill et al., 2011; Lieske et al., 2000). The intrinsic chemical sensitivity becomes highly significant in the context of arousal, as it is a protective mechanism that alerts an individual when exposed to potentially dangerous hypoxic and hypercapnic conditions. A specific example is sleeping babies lying with their face down (prone position). The prone position will lead to a buildup of CO2 and a decrease in inspired O2 (Bolton et al., 1993; Chiodini and Thach, 1993; Kemp and Thach, 1991; Kemp et al., 1993). This hypercapnic/hypoxic challenge evokes sighs, followed by arousal and head turning, which ultimately ameliorates the conditions that endanger the sleeping infant (Lijowska et al., 1997; McNamara et al., 1998). Thus, the generation of the sigh is a central nervous system mechanism that is not only temporarily associated with changes in brain states but also seems to be instrumental in initiating both subcortical and cortical arousals. Consequently, one can conclude that the sigh is an essential central nervous system adaptation that responds to changes in blood gases and initiates a series of events that lead to an arousal response, which will refresh the inspired O2 supply of the infant (Ayas et al., 2000; Fewell, 2005; Horne et al., 2005; Lijowska et al., 1997; Masa et al., 2003; McNamara et al., 1998; Parslow et al., 2003; Thach, 2002).
4 SIGHS AND THEIR IMPLICATIONS FOR SIDS AND OTHER PATHOLOGIES
The stereotypical response from respiratory distress associated with the prone position protects a healthy child, but may fail in children that later died of SIDS. Prospective studies have shown that spontaneous and induced arousals during sleep are decreased in SIDS victims (Dunne et al., 1992; Kahn et al., 1992; Kato et al., 2006; McCulloch et al., 1982; Sawaguchi et al., 2005; Schechtman et al., 1992). An important aspect of this behavioral sequence is the coupling between the respiratory behavior and heart rate control. Indeed, one of the hallmarks of sighs is their association with an initial heart rate increase, which is followed by a heart rate decrease (Fig. 3; Haupt et al., 2012; McNamara et al., 1998; Porges et al., 2000; Weese-Mayer et al., 2008a; Wulbrand et al., 2008). This sigh-coupled heart rate change not only seems to be important for coordinating cardiorespiratory function but also correlates with the degree of cortical arousal (Thach, 2002; Thach and Lijowska, 1996). Infants that later died of SIDS had a lower heart rate variability during a sigh (Franco et al., 2003). This is interesting, since lower heart rate variability is typically associated with higher parasympathetic tone, a sign of dysautonomia.
Figure 3.
Cardiorespiratory coupling of a sigh recorded in a healthy human subject. The sigh recorded with an inductance plethysmography brand from the abdomen (upper trace) is characterized by a heart rate increase followed by a heart rate decrease shown here in the simultaneously recorded electrocardiogram (lower trace). (For the color version of this figure, the reader is referred to the online version of this chapter.)
Although not all studies report a difference in sigh generation (Franco et al., 1998; Kahn et al., 1992; Kato et al., 2001), there is some evidence suggesting that SIDS victims exhibit significantly fewer sighs (Kahn et al., 1988). Interestingly, one report suggests that infants sigh more often in prone than in supine position. Yet, the children that later died of SIDS had less arousal even though prone position was more frequent, suggesting that the coupling between the sigh and arousal may have been disturbed (Groswasser et al., 2001; Kahn et al., 1992). Thus, a genetic predisposition or any condition that blunts the initiation or effectiveness of this arousal sequence may convey an increased risk for SIDS (Franco et al., 2010; Garcia et al., 2013).
An abnormal cardiovascular–respiratory coupling associated with the sigh may also have detrimental consequences in a variety of other human disease states. An attenuated response to endogenous sympathetic stimulation following a sigh has been reported in congenital hypoventilation syndrome (CCHS) (O’Brien et al., 2005), and an increased sympathetic activation has been reported in children with sleep-disordered breathing (O’Brien and Gozal, 2005). Disturbances in cardiorespiratory coupling are also characteristic of familial dysautonomia and sickle-cell anemia (Sangkatumvong et al., 2011; Weese-Mayer et al., 2008a). In sickle-cell anemia, the frequency of sighs is not different from controls, but sighs are much more likely to induce pronounced perfusion drops that are presumably associated with an exaggerated sympathetic and suppressed parasympathetic response. Hypoperfusion in sickle-cell anemia is a particularly dangerous situation, because it could lead to red blood cell polymerization followed by a vaso-occlusive crisis (Platt et al., 1994; Sangkatumvong et al., 2010, 2011). Indeed, the sigh-triggered hypoperfusion may be responsible for the sudden death that occurs frequently in this patient population (Sangkatumvong et al., 2011).
The sigh may also have an important role in obstructive sleep apnea, specifically during the recovery from an airway obstruction (Alvarez et al., 1993; Ramirez et al., 2013a; Wulbrand et al., 1998). The termination of airway occlusion is typically abrupt and associated with a sudden burst of genioglossus activity (Berry and Gleeson, 1997; Rees et al., 1995; Remmers et al., 1978; Wulbrand et al., 1998, 2008) and the recruitment of phasic inspiratory motor units (Wilkinson et al., 2010). Reflex recruitment of pharyngeal dilator muscles seems to be insufficient for this abrupt response, and central mechanisms that stage the initiation of arousal become important. As similarly described above in the context of SIDS, arousal is stimulated by the increasingly hypoxic and hypercapnic conditions associated with the airway occlusion (Berry and Gleeson, 1997; Gleeson et al., 1990; Kimoff et al., 1994). Although cortical arousal is not always observed following airway occlusion, sighs consistently coincide with subcortical arousal involving a sudden rise in limb motor activity and a distinct neck extension, an adaptive response that can contribute to the termination of an airway occlusion (Perez-Padilla et al., 1983; Wulbrand et al., 2008).
5 SIGHS HOMEOSTATICALLY RESET BREATHING VARIABILITY
The previous sections suggest that (a) sighs signal state changes and (b) sighs can trigger arousal. But there may be a third, equally important function for sighs. Based on a series of fascinating human psychophysiological studies, Vlemincx and coworkers (Vlemincx et al., 2010b, 2013a; Wuyts et al., 2011) proposed the intriguing hypothesis that the sigh acts as a psychophysiological mechanism that resets eupneic breathing variability associated with different psychological states. Indeed, a high degree of breathing variability is normal (Donaldson, 1992; Mangin et al., 2011; Small et al., 1999; Tobin et al., 1995; Wuyts et al., 2011; Wysocki et al., 2006). This variability is adaptive as it allows the respiratory network to sensitively react to changes in environmental and behavioral demands (Wuyts et al., 2011). Speech, for example, requires extensive variability in respiratory control (Ghazanfar and Rendall, 2008; Loucks et al., 2007). This variability, however, is not random. Long sentences follow large-amplitude breaths, and the timing of individual breaths is associated with the syntax of spoken sentences. Disturbances in respiratory control can lead to dysarthria, which summarizes different forms of speech disorders (Enderby, 2013; Liegeois and Morgan, 2012). Patients suffering, for example, from Parkinson’s disease take a greater percentage of breaths at locations that are unrelated to a syntactic boundary, suggesting that the interaction between cognition and respiratory timing is disturbed in this patient population (Brown and Marsden, 1990; Huber and Darling, 2011; Huber et al., 2012). Thus, respiratory variability, which is highly controlled in healthy speech, can become random in a variety of disorders.
Under healthy conditions, increased respiratory variability characterizes, for example, laughter, when abrupt decreases in inspiratory timing are typical (Boiten, 1998; Boiten et al., 1994). But respiratory variability can also decrease, for example, during sustained attention tasks (Vlemincx et al., 2011). Upon completion of the attention task, breathing variability increases again and the sigh rate increases (Vlemincx et al., 2011). According to the hypothesis by Vlemincx and coworkers (2013a), sighs reinstate normal, healthy variability during increased or after decreased variability (Vlemincx et al., 2011). Indeed, the authors can demonstrate that random unstructured variability increases before a sigh and decreases following a sigh (Vlemincx et al., 2010a,b). Thus, this concept attributes a critical homeostatic role to the sigh.
Consistent with this homeostatic hypothesis is the observation that sighs typically terminate periods of irregular breathing in an animal model (Orem and Trotter, 1993). In these experiments, sighs were also associated with changes in body position, which is consistent with their role in monitoring changes in behavioral state and arousal (Orem and Trotter, 1993). We conclude that sighs function as (a) a homeostatic resetting mechanism, (b) a monitor for brain state changes, and (c) a mechanism that induces arousal.
6 WHEN SIGHS ENHANCE BREATHING VARIABILITY AND INDUCE HYPERAROUSAL
While the combination of the above-mentioned properties is adaptive and essential in healthy subjects, sighs could also turn into major drivers of certain human disease states. For example, patients with panic disorder suffer from significantly increased respiratory variability and hyperventilation (Abelson et al., 1996, 2001, 2008; Martinez et al., 2001; Meuret et al., 2001; Roth et al., 2002; Wilhelm et al., 2001a,b). Their breathing irregularity persists even when panic is pharmacologically controlled, indicating that respiratory dysregulation is not secondarily induced by the panic attack but may convey panic attack vulnerability (Abelson et al., 1996, 2001, 2008). Klein suggested that hypercapnia causes a dysregulation of brainstem circuits that drive suffocation fears, which in turn will evoke panic attacks (Gorman et al., 1994; Klein, 1993, 1994; Martinez et al., 2001). He specifically hypothesizes that an episodic dysfunction in endogenous opioidergic regulation involving the same brainstem areas that also regulate breathing is responsible for panic disorder (Klein, 1993; Preter and Klein, 2008). Consistent with brainstem dysfunction is the observation that patients suffering from CCHS are not only unable to sense CO2 but also significantly less anxious than controls (Pine et al., 1994).
Alternatively, excessive random breathing variability in panic disorder patients may centrally activate sighs possibly in a physiological attempt to reduce and reset this pathological variability. Indeed, patients with panic disorders typically show increased sigh frequency (Abelson et al., 2001; Schwartz et al., 1996; Vlemincx et al., 2013a). However, instead of regularizing variability as is the case in healthy subjects, the increased generation of the sighs may exaggerate the already existing breathing variability by further increasing tidal volume variability, which is a characteristic feature of patients with panic disorder (Abelson et al., 2001; Schwartz et al., 1996; Wilhelm et al., 2001a,c). Sighs could further increase irregularity through the so-called postsigh apnea, which can create conditions of intermittent hypoxia and oxidative stress if sighs occur frequently (Garcia et al., 2013; Ramirez et al., 2013a,b). Consistent with the notion that sighs are centrally generated is a report suggesting that sighs in panic disorder patients are not triggered by a pCO2-dependent chemical stimulus (Abelson et al., 2001). Interestingly, rats bred for high anxiety showed a significantly different breathing behavior with an increased number of sighs when compared to rats bred for low anxiety (Carnevali et al., 2013) further supporting the notion that anxiety and the generation of sighs are centrally linked. Assuming that sighs have also arousal-promoting functions, what follows is that excessive sighing may generate a hyperarousal state, which could exaggerate panic.
Hyperarousal is a characteristic feature of several forms of panic disorders (Ottaviani et al., 2012) and disorders associated with anxiety. Examples are posttraumatic stress disorder (Edmondson and Cohen, 2013; Perez et al., 2012), attention deficit hyperactivity disorder (Antshel et al., 2013; Hanson et al., 2012), general anxiety disorder (Brunet et al., 2013; Hoge et al., 2013), insomnia (Hantsoo et al., 2013; Killgore et al., 2013; Vincent and Walsh, 2013; Wallhausser-Franke et al., 2013), and Fragile X syndrome (Berry-Kravis et al., 2012; Heilman et al., 2011). Autonomic dysregulation is an aspect of some of these disorders, but sighs have not been specifically characterized in these disorders, (Beauchaine et al., 2007; Heilman et al., 2011; Kalk et al., 2011; Musser et al., 2013).
The pathway to an anxiety disorder may begin in a healthy human: homeostatic mechanisms continuously establish a dynamic balance between breathing stability and instability (Vlemincx et al., 2013a,b). In an arithmetic task, an increased sigh frequency may be adaptive in decreasing variability (Vlemincx et al., 2011), but it might also temporarily induce a hyperarousal state. A heightened arousal state could initially be adaptive as it stages the healthy flight-and-fight response. In the example of an infant sleeping in a prone position, this response is essential for survival. However, a heightened arousal state will eventually become maladaptive in chronic worriers. As a result of the chronic nature, these worries may become pathological leading to exaggerated cardiac defense responses, reduced sinus-arrhythmia (Vila et al., 2007; Vlemincx et al., 2013a), and reduced parasympathetic activity which is typical for chronic worriers (Thayer and Brosschot, 2005; Thayer et al., 1996; Vlemincx et al., 2013a,b). Constant worrying and chronic anxiety may also lead to insomnia as frequent arousals may contribute to sleep fragmentation. Anxiety and insomnia are closely related clinical entities (Brown et al., 2013; Dong et al., 2013; Lee et al., 2013). This relationship could be associated with an increased propensity to sigh, a possibility that deserves further investigations.
The above considerations suggest that panic disorders emerge through an imbalance between the cardiorespiratory and arousal system. A physiological, homeostatically controlled cardiorespiratory-coupled flight-and-fight response could become maladaptive and homeostatically uncontrolled in a chronic state. This hypothesis may apply not only to panic disorder but also possibly to many disorders associated with heightened anxiety. But unfortunately, in many of these disorders, the roles of the underlying cardiorespiratory characteristics and the sigh have not been explored.
7 THE PRE-BÖTZINGER COMPLEX, AN ESSENTIAL BRAIN REGION FOR THE GENERATION OF THE SIGH AND EUPNEIC ACTIVITY
Neurobiological correlates for many of these considerations can be found at multiple levels of the CNS. For example, “balanced amygdala activation” in response to fearful stimuli may turn into reduced amygdala activation, as is characteristic for panic disorders (Ottaviani et al., 2012), or into a hyperactive amygdala, which would be equally maladaptive. But before discussing these wider network interactions, we would like to focus on the question of how breathing and sighing are generated within the CNS, as this is at the core of this review.
The French physiologist Pierre Flourens (1794–1867) first proposed that breathing is generated within a very specific region of the brainstem, located in the medulla oblongata. He called this region the “noeud vital” to indicate its vital importance for life (Flourens, 1858). But it was not until another 130 years had passed that Smith et al. (1991) confirmed Flourens’ proposal. Smith et al. discovered not only that lesioning a very specific area within the ventrolateral medulla of neonatal rats abolishes respiratory activity, but also that brainstem slices that contain this area continue to spontaneously generate respiratory activity in isolation. Smith and coworkers (Smith et al., 1991) termed this critical area the “pre-Bötzinger complex” (preBötC) (Fig. 2B and C; Schwarzacher et al., 2011). Located within the same plane of the slice is the hypoglossus motor nucleus (XII), which continues to be rhythmically activated by the preBötC. The XII continues to generate rhythmic motor activity (Telgkamp and Ramirez, 1999) that can be recorded from the XII rootlets (Smith et al., 1991). Since in intact animals, including humans, the XII is activated in phase with inspiration (Ramirez et al., 2013a; Withington-Wray et al., 1988), it is assumed that the rhythmic activity in the slice represents fictive inspiration. This discovery provoked controversies that began in 1991 and continue to this day. A major reason for these controversies was the long-held dogma that different forms of breathing are generated in different brain centers. To this day, some Physiology textbooks teach that breathing (eupneic activity) is generated in the pneumotactic center located within the pons, whereas gasping is generated in a gasping center located within the medulla, as originally proposed by Lumsden (1923). Indeed, the ensuing controversy centered around the question whether the preBötC is the gasping center or a network critical for generating eupneic activity. A series of studies employing various molecular, genetic, and pharmacological tools unambiguously confirmed that even in fully intact adult animals of different species, the preBötC is, indeed, the noeud vital, that is, the center of life that is essential for eupneic breathing (Gray et al., 2001, 2010; Ramirez et al., 1998; Tan et al., 2008). Surprisingly, the location of a “sigh” center was never discussed, possibly because most respiratory physiologists considered the sigh simply as a bigger, that is, “augmented breath” that did not require a separate center.
8 THE CONCEPT OF NETWORK RECONFIGURATION
In 2000, Lieske and coworkers discovered that the network isolated in the pre-Bötzinger generates not just one type of respiratory activity but three distinct, qualitatively and quantitatively different, respiratory activity patterns (Lieske et al., 2000). Lieske et al. (2000) proposed that these three activities constitute the neuronal basis for (a) eupneic (Fig. 4), (b) sighing (Figs. 1B and 2B, D), and (c) gasping activity. This discovery was conceptually a major departure from the long-held belief that different regions of the brain are responsible for the generation of different respiratory patterns (Lumsden, 1923). To explain their finding, (Lieske and coworkers, 2000) introduced the concept of network reconfiguration to the respiratory system and postulated that the preBötC can assume different network states that give rise to eupneic, gasping, and sighing activity depending on the oxygenation and neuromodulatory milieu of the network. Experiments performed several years later confirmed the concept of network reconfiguration in more intact preparations.
Figure 4.
Eupneic activity recorded from an intact animal and as a neuronal signal representing fictive eupneic activity in a transverse slice preparation. (A and B) Simultaneous intracellular recording from an inspiratory neuron within the pre-Bötzinger complex (upper trace) and integrated extracellularly recorded phrenic nerve activity (lower trace). (B) Simultaneous intracellular recording from an inspiratory neuron within the pre-Bötzinger complex (upper trace) and integrated extracellular population activity recorded of the pre-Bötzinger complex (lower trace). Note the remarkable similarities in the intracellularly recorded discharge pattern of the inspiratory neuron both in vivo (A) and in vitro (B). (For the color version of this figure, the reader is referred to the online version of this chapter.)
The concept of network reconfiguration also provided an explanation for how different regions of the brain interact within the wider respiratory network: as many areas within the CNS will reconfigure the respiratory network and vice versa, the respiratory network will reconfigure other areas of the nervous system. From the behavioral perspective, the preBötC is not the only region that participates in breathing. Additional areas can be found in the medulla, the pons, the cerebellum, the cortex, the amygdala, the hypothalamus, and the periaqueductal gray (PAG) (Brannan et al., 2001; Burdakov et al., 2013; Chamberlin and Saper, 1994; Liotti et al., 2001; Masaoka et al., 2012; Nattie and Li, 2012; Ramirez et al., 2012; Smith et al., 2009; Subramanian and Holstege, 2013). The contribution of each of these areas adds different and important aspects to the neuronal control of breathing making breathing one of the most modulated and complex behaviors. As already mentioned, every spoken word in humans is a breathing behavior that emerges through a complex cortical and subcortical orchestration of numerous network interactions (Holstege, 1989; Subramanian and Holstege, 2009, 2013; Subramanian et al., 2008; VanderHorst and Holstege, 1996). In some disorders, such as in Parkinson’s disease, disturbances in language will result from the dysregulation of the complex, reciprocal interactions between cognitive and respiratory functions (Altmann and Troche, 2011; Kemmerer et al., 2013; Skodda, 2011). Similarly, anxiety and panic will be the result of complex interactions between the respiratory network and a variety of areas including the amygdala, hypothalamus, and neocortex (Abelson et al., 2008; Frysztak and Neafsey, 1991; Nattie and Li, 2012). Thus, behaviors associated with breathing depend on the reciprocal interactions between the preBötC and other brainstem areas, such as Kölliker-Fuse in the pons, the RTN/pFRG, and the Bötzinger complex in the medulla, as well as numerous subcortical and cortical areas. There are various areas that can trigger an apnea when lesioned (Song et al., 2010), but the preBötC remains the only area that is not only essential for breathing but also continues to generate three distinct respiratory activities in isolation (Gray et al., 2010; Lieske et al., 2000; Smith et al., 1991; Tan et al., 2008). Thus, any discussion of how different areas influence or are influenced by breathing or the sigh needs to include this noeud vital.
9 HOW IS BREATHING VARIABILITY GENERATED?
As discussed earlier, breathing variability is an essential behavioral property that needs to be homeostatically regulated (Wuyts et al., 2011). Variability is adaptive as it allows the respiratory network to sensitively and quickly react to changes in environmental and behavioral conditions. Thus, how does the nervous system accomplish the important task of generating rhythmic activity patterns that are stable, yet also very flexible and at times extremely variable? In this review, I propose that the neuronal basis of breathing variability and stability is deeply rooted within the synaptic interactions and intrinsic membrane properties of this relatively small neuronal network. This proposal rejects several misconceptions that have confused the field over several decades. An overarching misconception that drove much of the gasping controversy was the notion that the preBötC operates like a simple, hard-wired “clock” or “pacemaker network,” a view that is difficult to reconcile with the enormous variability, flexibility, and plasticity that is required for adaptive and variable eupneic breathing. Sections 9.1–9.5 illustrate why the preBötC is not a simple hard-wired pacemaker network or clock.
9.1 Respiratory Pacemaker Neurons Are Not a Sign of a Primitive Rhythm Generating Network
With the discovery of the preBötC came the demonstration that this network contains autonomously bursting rhythmic neurons that are referred to as “bursting pacemakers” or simply “pacemakers” (Smith et al., 1991). The presence of pacemaker neurons was later confirmed in numerous studies (Johnson et al., 1994; Pena et al., 2004; Thoby-Brisson and Ramirez, 2000; Tryba et al., 2008; Viemari and Ramirez, 2006; Viemari et al., 2011; Fig. 5). These neurons intrinsically generate rhythmic activity much like the cardiomyocytes in the heart (Bradd et al., 2012; Fenske et al., 2011; Sirenko et al., 2013). The demonstration of pacemaker neurons was interpreted by some as a special attribute of a simple rhythm generating gasping network that misses much of the flexibility of eupneic breathing. Perhaps some of this misinterpretation came from the fact that neurons with very similar bursting properties have previously been demonstrated in numerous invertebrate networks (Graubard and Hartline, 1991; Harris-Warrick, 2002; Russell and Hartline, 1978; Soto-Trevino et al., 2005). But this was another misconception, because in invertebrate networks also, pacemaker neurons are everything but simple (Hobbs and Hooper, 2008; Prinz et al., 2003; Ramirez and Pearson, 1991a,b, 1993; Rinberg et al., 2013). Moreover, within the mammalian nervous system, pacemaker neurons are not unique to the respiratory network, and are found in many areas of the CNS; some of these networks are concerned with complex higher functions (Brocard et al., 2013; Kolta et al., 2010; Llano and Sherman, 2009; Marcuccilli et al., 2010; Martell et al., 2010, 2012; Mrejeru et al., 2011; Ramcharan et al., 2005; Ramirez et al., 2004; Ziskind-Conhaim et al., 2010). Indeed, there is probably no region in the mammalian brain that does not contain pacemaker neurons (Ramirez et al., 2011).
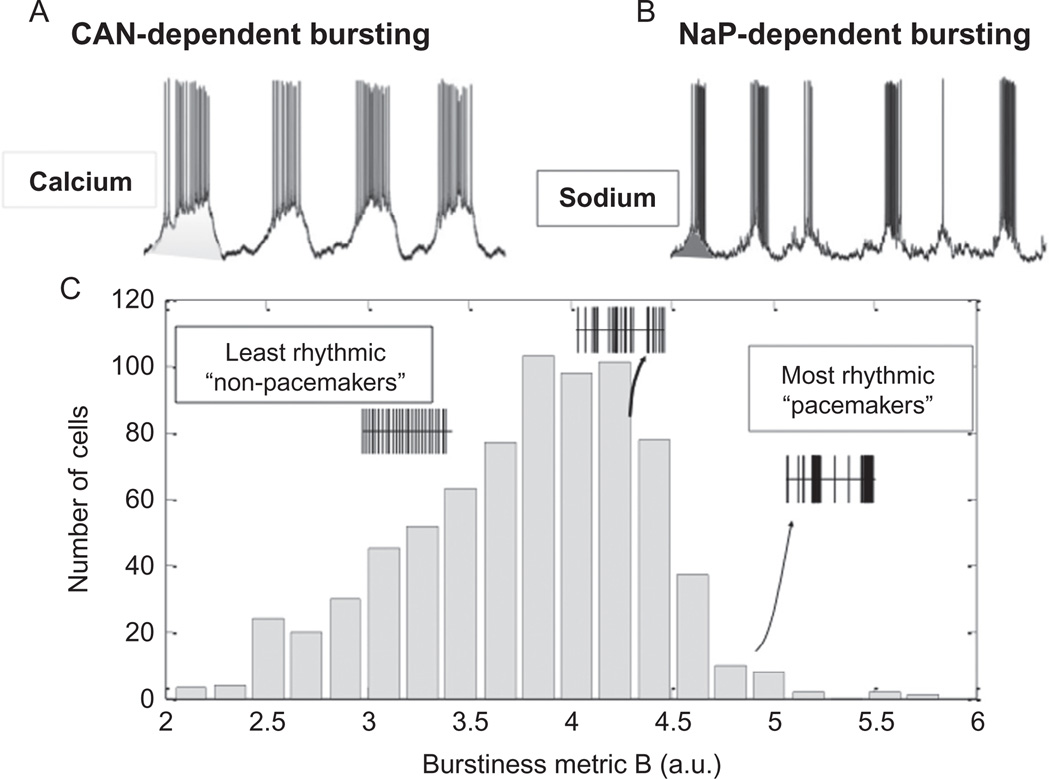
Figure 5.
Pacemaker activity recorded within the pre-Bötzinger complex. (A) Intracellular recording of a synaptically isolated pacemaker neuron in which bursting depends on the activation of the CAN current. (B) Intracellular recording of a synaptically isolated pacemaker neuron in which bursting is driven by the persistent sodium current. (C) The discharge pattern of respiratory neurons within the pre-Bötzinger complex follows a gradient. Some neurons are more bursting (right side) than others. Many neurons are weakly bursting and some are tonically active. This gradient illustrates that pacemaker neurons form not a discrete population. (For the color version of this figure, the reader is referred to the online version of this chapter.)
Modified from (A and B) Pena et al. (2004) and (C) Carroll and Ramirez (2013).
9.2 Pacemaker Neurons Do Not Discharge in a Metronome-Like Manner
The word “pacemaker” may imply to some that these neurons function like a clock and pace a network rhythm in a metronome-like manner. But this is not the case. The discharge of these neurons is variable and highly plastic (Carroll and Ramirez, 2013). In any given neuron, the discharge is determined by a variety of outward, inward, and leak currents (Del Negro et al., 2002). Some of the inward currents are burst-promoting such as the calcium-activated nonspecific CAN (Fig. 5A) or the persistent sodium current (Fig. 5B). However, the complement of these currents varies, and every individual neuron establishes a different dynamic balance of inward and outward currents, a principle that has been well documented for invertebrate neurons (Hudson and Prinz, 2010; Khorkova and Golowasch, 2007; Soofi et al., 2012; Temporal et al., 2012; Zhao and Golowasch, 2012). As a result, some neurons within the respiratory network are more bursting than others; some burst regularly, some are irregular, some discharge tonically, and some are silent (Carroll and Ramirez, 2013; Garcia et al., 2011; Koch et al., 2011; Ramirez et al., 2011, 2012; Viemari and Ramirez, 2006). Bursting depends more on the CAN current in some neurons (Fig. 5A) and on the INap current in others (Fig. 5B; Pena et al., 2004; Thoby-Brisson and Ramirez, 2001), and occasionally respiratory neurons are found that depend on the L-type calcium current (Pena, F. and Ramirez, JM, unpublished data). As a population, the intrinsic activities of preBötC neurons span a continuum that covers the entire spectrum from silent to tonic to bursting activities (Carroll and Ramirez, 2013). This has been quantitatively demonstrated for the intrinsic activities of hundreds of neurons recorded in the preBötC (Fig. 6; Carroll and Ramirez, 2013). Thus, the long-held assumption that the respiratory network contains distinct and concrete, hard-wired populations of pacemaker and non-pacemaker neurons needs to be revisited.
Figure 6.
Role of neuromodulators in determining the bursting characteristics of respiratory neurons. (A) Substance P turns a weakly burstingneuron into a strongly bursting pacemaker neuron. (B) Oxotremorine, an acetylcholine agonist, inhibits bursting in an INap-dependent pacemaker (see hyperpolarization) and induces the generation of a large-amplitude burst. As the modulatory effect weakens, small and large bursts overlap. (For the color version of this figure, the reader is referred to the online version of this chapter.)
9.3 Intrinsic Discharge Properties of any Given Respiratory Neuron Are Plastic
Whether a pacemaker neuron bursts and if it does, how it does so is not established by a fixed genetic program. It is determined by the balance of inward and outward currents, the various receptors, and second messenger systems and to a large extent also by its modulatory and synaptic environment. Neuromodulators released endogenously can induce (Fig. 6A), inhibit, and significantly alter bursting properties in respiratory neurons (Dekin et al., 1985; Doi and Ramirez, 2010; Pena and Ramirez, 2002, 2004; Ptak et al., 2009; Ramirez et al., 2011, 2012; Tryba et al., 2008; Viemari et al., 2011; Fig. 6B). This principle has been well documented in a variety of invertebrate (Crisp et al., 2012; Dickinson and Nagy, 1983; Khorkova and Golowasch, 2007; Ramirez and Pearson, 1991a,b; Zhao and Golowasch, 2012) and other mammalian networks (Kolaj et al., 2007; Mrejeru et al., 2011; Tsuruyama et al., 2013). Within the respiratory network, multiple neuromodulators, such as serotonin, norepinephrine, acetylcholine, or substance P,can alter the distribution of these activities (Doi and Ramirez, 2008; Ramirez et al., 2012; Fig. 6). These neuromodulators target different receptor subtypes, G-proteins, and second messenger systems that will differentially affect the balance between the different inward and outward currents, resulting in a different distribution of these discharge patterns. As an example, norepinephrine acting on the alpha 1 receptor will increase the number of neurons that burst based on the CAN current (Viemari and Ramirez, 2006). But, multiple neuromodulators converge simultaneously onto multiple second messenger systems and G-proteins, which imbue every single neuron within the respiratory network with an enormous flexibility and plasticity (Doi and Ramirez, 2008). The example shown in Fig. 6B demonstrates that the same neuron at the single-cell level can burst in different intrinsic modes, which is reminiscent of the different modes of the network (Fig. 7; Tryba et al., 2008). Indeed, the modulatory effects at the cellular level have important functional consequences at the network level. Norepinephrine acting, for example, on the ICAN current at the level of the single neuron will affect the shape and amplitude of inspiratory activity at the network level, whereas norepinephrine acting on the INap current at the cellular level will affect the respiratory frequency at the network level (Viemari and Ramirez, 2006). Thus, by acting on different receptor subtypes, G-protein-coupled receptors, and different second messenger systems, neuromodulators have an almost unlimited capability to orchestrate intrinsic membrane properties of respiratory neurons and thereby alter different parameters of respiratory activity. These changes can occur not only at the level of intrinsic membrane properties, but modulatory effects will also target synaptic transmission adding another layer of complexity to the respiratory network. Both intrinsic and synaptic properties will engage in a complex interplay that largely depends on the modulatory state (Doi and Ramirez, 2008; Koch et al., 2011; Ramirez et al., 2011,2012).
Figure 7.
The respiratory network in the pre-Bötzinger complex shows a gradient of discharge pattern as illustrated in the multiarray electrode recording (I). (I) The simultaneous multiarray recording from 11 neurons (see ordinate 1–11 in A) represents the cycle-by-cycle spiking behavior for each neuron within a respiratory cycle. Each block in A shows the spike time of a different neuron relative to the onset of inspiratory activity. In addition, the instantaneous spike rates are coded as a heat map from 0 to 55 Hz. C represents the average discharge for each neuron, and in D, the overlay of two pairs of averaged discharge activity shows that neurons have slightly different activation patterns. (II) The onset of discharge relative to the population activity is very variable as shown here for an intracellularly recorded pacemaker neuron. (For the color version of this figure, the reader is referred to the online version of this chapter.)
Modified from Carroll et al. (2013).
9.4 The Modulatory State of the Respiratory Network Is Plastic
Indeed, the modulatory state of the respiratory network is determined by multiple modulators released not only from neurons intrinsic to the network but also from neuromodulators that are released from neurons converging onto the respiratory network from numerous other brain regions. Prominent areas include the hypothalamus, the locus coeruleus, and the raphe nucleus (Doi and Ramirez, 2010). But there are many additional areas that play critical roles under different behavioral, developmental, and environmental conditions. Thus, the modulatory milieu at the network level is not static but defined by the changing behavioral and environmental conditions that the organism encounters or generates. A sleeping animal is defined by a different modulatory state than a waking animal, and different sleep states are characterized by different modulatory states. Whether an animal is anxious, panicking, quiet, concentrated, hypoxic, or hypocapnic will all be reflected in different modulatory states that will directly impact the neuronal discharge properties of the single neurons, and their synaptic interactions at the population level.
9.5 Respiratory Neurons Discharge in a Variable Manner Within the Respiratory Cycle
Many computational models assume that respiratory neurons can be classified into a relatively small number of concrete classes of neurons that fire at a fixed time in relation to the respiratory cycle. A recent unexpected discovery suggests that the phase-locked rhythmic activation of respiratory neurons within the preBötC forms a continuum between the different neuron types (Fig. 8-I; Carroll and Ramirez, 2013; Franco et al., 2003, 2010). This continuum consists of neurons that discharge very early, somewhat early, throughout the cycle, somewhat late or very late during inspiration (Fig. 8-I). Some neurons discharge during expiration, or during postin-spiration; some neurons discharge in a ramp-like, bell-shaped, or decrementing manner (Carroll et al., 2013). Moreover, there is remarkable onset variability (Fig. 8-II). Bursting and nonbursting neurons can lead some respiratory cycles, but follow others. This finding leads to the conclusion that the respiratory network is stochastically assembled in a cycle-by-cycle manner (Carroll and Ramirez, 2013; Carroll et al., 2013). In other words, every breath is a new breath with regards to the underlying neuronal mechanisms. Thus, breathing variability is an intrinsic property that is build into the very mechanisms that govern respiratory rhythm generation within the core respiratory network. Based on computational modeling, this variability may be achieved through a sparse connectivity between respiratory neurons (Carroll and Ramirez, 2013). The study by Carroll and Ramirez (2013) compared the onset variability with different degrees of sparse connectivity and found that the higher the onset variability increased, the sparser was the connectivity.
Figure 8.
Neuromodulators can differentially modulate eupneic and sigh activity, characterized as network activity in a functional brainstem slice preparation. The acetylcholine agonist oxotremorine specifically inhibits eupneic activity and activates sigh activity in the isolated pre-Bötzinger complex. (For the color version of this figure, the reader is referred to the online version of this chapter.)
Modified from Tryba et al. (2008).
However, it is important to emphasize that the individual onset variability does not imply that the overall network output must be irregular. Indeed, the rhythmic activity that emerges through this ensemble is regular and stable, yet very flexible (Carroll and Ramirez, 2013). This study also showed that the larger the number of sparsely connected neurons is, the more regular the rhythmic output becomes. At every given moment, it is possible to alter the activation and synchronization of the neuronal elements that give rise to the respiratory activity. However, the sparse connectivity and the multitude of modulatory processes that determine respiratory activity may be at the limits, the price to pay to maximize flexibility. It is conceivable that mechanisms that reduce excitatory synaptic transmission or enhance bursting properties could easily destabilize the network, which could drive the network into random variability. The generation of the sigh is an ideal cellular mechanism to reset this network ensemble. The majority of respiratory neurons within the preBötC are simultaneously activated during the sigh and become simultaneously inhibited after the sigh, giving rise to the postsigh apnea (Fig. 2; Lieske and Ramirez, 2006a,b; Lieske et al., 2000; Orem and Trotter, 1993; Tryba et al., 2008). Thus, the sigh is a neuronal event that supra-maximally activates simultaneously many neurons within the respiratory network. Future experiments will need to test the possibility that this resetting event indeed alters the variability in subsequent cycles at the network level as would be predicted from the hypothesis by Vlemincx and collaborators (Vlemincx et al., 2010b, 2013a; Wuyts et al., 2011).
10 EUPNEIC AND SIGHS ARE GOVERNED BY DISTINCT CELLULAR MECHANISMS
A fascinating, yet unresolved, question is how the preBötC can simultaneously generate two distinct respiratory activities—eupneic and sigh activity within the very same network using the same neurons (Lieske et al., 2000). As stated earlier, the majority of neurons are activated during both eupneic and sigh activity, and this overlap may even be a prerequisite for the hypothesized role of the sigh in resetting the ongoing eupneic network activity. Yet, the timing of eupneic and sigh activities is radically different. One rhythm operates in the range of seconds, the other in a range of several minutes (Lieske et al., 2000). Sigh and eupneic activities also involve different cellular mechanisms. Blockade of PQ-type calcium channels selectively blocks sighs, but not eupneic activity (Lieske and Ramirez, 2006a). The genetic knockout mouse of PQ-type calcium channels continues to breathe but fails to sigh, which is fatal (Koch et al., 2012). Metabotropic glutamate receptors of the subtype 8, group III are critical for the generation of sighs, but not eupneic activity, and eupneic activity is more sensitive to NMDA-dependent modulation than sigh activity (Lieske and Ramirez, 2006b). Not surprisingly, both rhythms can be independently modulated. Acetylcholine acting on muscarinic receptors activates sighs, but inhibits eupneic activities (Fig. 7; Tryba et al., 2008), while serotonin acting on 5-HT2A receptors (Pena and Ramirez, 2002) and substance P acting on NK1 receptors activate both eupneic and sigh activity (Doi and Ramirez, 2010; Pena and Ramirez, 2004). A differential modulation of sighs and eupneic breathing was also described in response to dorsomedial hypothalamic stimulation (Reynolds et al., 2008). The differential modulation at the neuronal level provides a mechanistic explanation how sighs could be differentially activated in the behaving animal.
11 NEURONAL STRUCTURES AND NEUROMODULATORY MECHANISMS LINKING SIGHS WITH ANXIETY AND AROUSAL
As already mentioned, the amygdala is an important integrator that links emotions and respiration (Freire and Nardi, 2012), and an imbalance in amygdalar activity has been implicated in anxiety and panic disorders (Freire et al., 2013; Nardi et al., 2009). Using an in vitro approach, Onimaru and Homma reveal not only strong descending pathways to the respiratory network within the medulla, but also weak ascending pathways from the respiratory network to the amygdala (Fujii et al., 2010, 2011; Onimaru and Homma, 2007). The fact that these ascending pathways are weak is interesting, as this could be a filtering mechanism. Specifically, under control conditions, only sighs that lead to the maximal activation of the respiratory network might be able to activate the amygdala, while inputs associated with normal breathing may be too weak to activate the amygdala. However, at this point, this is pure speculation and probably somewhat simplistic. This is most obvious if one considers a series of elegant human EEG studies that document complex interactions between anxiety and breathing (Masaoka and Homma, 2000). In human subjects feeling anxiety and exhibiting an increased respiratory rate, a positive wave can be triggered from the onset of inspiration, a phenomenon that is called “the respiration-related anxiety potential.” The increase in respiratory rate is related to the anxiety level (Masaoka and Homma, 2000). Moreover, the respiration-related anxiety potential was observed in the right temporal pole in subjects with low anxiety and in the left amygdala in those with high anxiety (Masaoka and Homma, 2000). Patients with lesions in the left amygdala show reduced anxiety levels and respiratory rate during anticipatory anxiety (Masaoka et al., 2003). The authors propose that the left amygdala provides essential excitatory drive to the right amygdala, which is dominantly activated in anxiety (Masaoka et al., 2003).
The amygdala is closely linked with another critical integrator of the limbic response, the PAG. The dorsal PAG, in particular, has a powerful influence on neurons within the preBötC, resulting in tachypnea and hyperventilation (Subramanian, 2013; Subramanian and Holstege, 2013). Interestingly, this area also evokes intense distress, panic terror, and feelings of imminent death, all emotions that are also associated with tachypnea (Del-Ben and Graeff, 2009; Schenberg et al., 2001), but the role in generating sighs has not been specifically investigated. Of clinical interest is that the dorsal PAG also induces the analgesia associated with stressful situation. In humans, analgesia can be electrically stimulated within the dorsal PAG, but it is also associated with strong aversive reactions.
A third structure critical for linking breathing and emotions is the hypothalamus. The hypothalamus has long been implicated in the fight-and-flight response and the etiology of panic and anxiety disorders (Johnson et al., 2012a; Kuwaki and Zhang, 2012; McDougall et al., 2005; McEwen, 2007; Sherin and Nemeroff, 2011). The perifornical and dorsomedial hypothalamus is particularly interesting as this area releases the neuromodulator orexin (Fig. 9). Orexin mediates wakefulness, arousal, and energy homeostasis (Nattie and Li, 2012). A hypoactive orexin system has been implicated in narcolepsy, whereas a hyperactive orexin system evokes panic and states of anxiety (Johnson et al., 2012a,b). Moreover, these neurons also regulate blood pressure and body temperature (Nattie and Li, 2012). But, orexin neurons from the hypothalamus play also critical roles in the neuronal control of breathing, specifically in the regulation of chemosensitivity, predominantly during wakefulness. Most intriguing, orexin has a strong modulatory role specifically activating sighs: an orexin antagonist decreases the generation of sighs (Nattie and Li, 2012). Thus, in a stress situation, this neuromodulator plays a critical role in orchestrating the flight-and-fight response by activating the cardiorespiratory system and, specifically, by activating sighs as well as arousal. If this neuromodulatory system becomes hyperactive, it would lead to a panic-anxiety syndrome that is manifested in not only the emotional but also the respiratory aspects of this disorder.
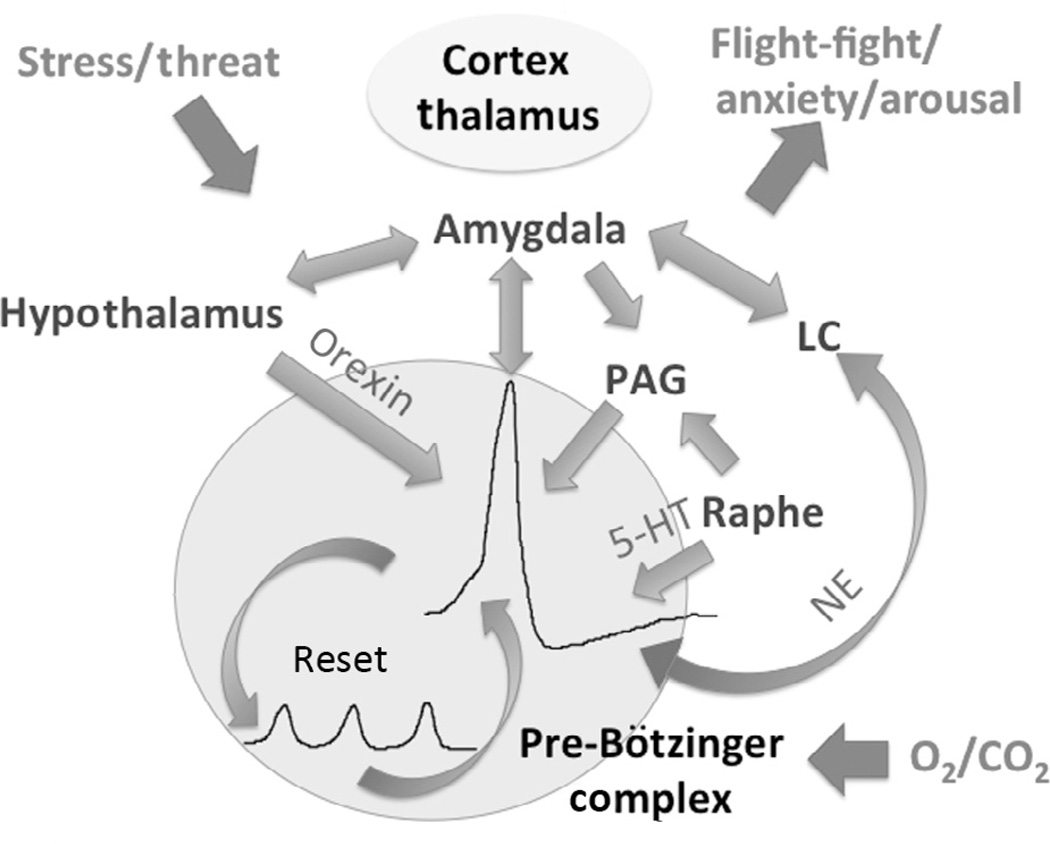
Figure 9.
The sigh has important roles in the control of arousal and in resetting breathing variability. The schematic represents some of the network interactions involved in centrally linking the sigh with areas such as the hypothalamus, amygdala, the locus ceruleus (LC), periaqueductal grey (PAG), and the Raphe nucleus involved in the control of the flight-fight response. The cortex and thalamus interact with these areas in a complex manner; the details are not depicted in this illustration for reasons of simplicity, but the reader is referred to the text for more details. Many of the interactions are reciprocal and function via the release of neuromodulators such as orexin, serotonin (5-HT), and norepinephrine (NE) acting on different receptor subtypes. (For the color version of this figure, the reader is referred to the online version of this chapter.)
Certainly, orexin is only one of many neuromodulators that play a role in staging the flight-and-fight response and anxiety. Other important modulators include neuropeptide Y (Eaton et al., 2007; Giesbrecht et al., 2010), neuropeptide S (Okamura and Reinscheid, 2007; Reinscheid and Xu, 2005), corticotropin-releasing factor (Gilpin, 2012), and angiotensin II (Liu et al., 2012). Acetylcholine involving the hippocampus has also been implicated in anxiety (Mineur et al., 2013; Pandya and Yakel, 2013) and the response to mental stress (Meerson et al., 2010). In this context, it is interesting that the same neuromodulator has strong modulatory effects on the sigh as well (Fig. 7, and see also Fig. 6; Koch et al., 2012; Tryba et al., 2008). Well-studied neuromodulators also include norepinephrine and serotonin, both of which have been implicated in the regulation of arousal, anxiety, and emotions (Goddard et al., 2010; Lowry et al., 2005; McEwen, 2007; Ressler and Nemeroff, 2000). The critical areas in the locus ceruleus and raphe nuclei receive strong input from the amygdala (Retson and Van Bockstaele, 2013). These neuromodulators and anatomical regions also play critical roles in modulating breathing and sighing in particular (Pena and Ramirez, 2002; Viemari and Ramirez, 2006; Viemari et al., 2011), and they also regulate anxiety states (Lowry et al., 2005). However, it must be emphasized that neuromodulation is highly complex and the effect on all these behaviors is dependent on the affected receptor subtypes, second messenger systems as well as the modulatory state of the animals. Thus, very detailed information is needed to understand exactly how and under what conditions these aminergic and also peptidergic neuromodulators orchestrate and contribute to the link between breathing and emotion. In the case of many neuromodulators, we still do not know how they influence each other and how they affect the generation of sighs.
12 CONCLUSIONS
The focus of this review is on the close association between the neurobiology, physiology, psychology, and pathology of the sigh. Although it was impossible to consider in sufficient depth many of the important aspects related to this fascinating topic, it is still possible to arrive at several important take-home messages. Breathing, in general, and sighs, in particular, have not only important ventilatory functions but also equally important intrinsic roles within the central nervous system. The sigh plays a role in monitoring brain state changes, controlling arousal, and homeostatically regulating breathing variability. These functions are critical for day-to-day activities. When we speak, exercise, or sleep, breathing requires continued flexibility and adaptability, and the sigh seems to be critical in maintaining a healthy stability. In stressful, threatening, and challenging situations, sighs become essential for staging a flight-and-fight response. But the dual roles in regulating breathing variability and staging a flight-and-fight response can become pathological. Too little sighing and hypoarousal can lead to SIDS, too much sighing and hyperarousal can lead to panic disorders. Understanding how a healthy balance turns into pathologies is a fascinating problem that is clearly understudied. This review raises many open questions. We hope that it will inspire future research aimed at better understanding how breathing and sighing controls not only our physiological but also our emotional health, so that one day we as scientists will be able to catch up with the great artists who have long appreciated the important role of the sigh in regulating our emotions.
Acknowledgment
This work was supported by NIH grants P01HL090554 and R01HL107084.
References
- Abelson JL, Nesse RM, Weg JG, Curtis GC. Respiratory psychophysiology and anxiety: cognitive intervention in the doxapram model of panic. Psychosom. Med. 1996;58:302–313. doi: 10.1097/00006842-199607000-00002. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol. Psychiatry. 2001;49:588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Lyubkin M, Giardino N. Respiratory irregularity and stress hormones in panic disorder: exploring potential linkages. Depress. Anxiety. 2008;25:885–887. doi: 10.1002/da.20317. [DOI] [PubMed] [Google Scholar]
- Abubakr A, Ifeayni I, Wambacq I. The efficacy of routine hyperventilation for seizure activation during prolonged video-electroencephalography monitoring. J. Clin. Neurosci. 2010;17:1503–1505. doi: 10.1016/j.jocn.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Altmann LJ, Troche MS. High-level language production in Parkinson’s disease: a review. Parkinsons Dis. 2011;2011:238956. doi: 10.4061/2011/238956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol. Neonate. 1993;63:139–146. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Dick TE, Orem J. Respiratory responses to tracheobronchial stimulation during sleep and wakefulness in the adult cat. Sleep. 1996;19:472–478. doi: 10.1093/sleep/19.6.472. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Kaul P, Biederman J, Spencer TJ, Hier BO, Hendricks K, Faraone SV. Posttraumatic stress disorder in adult attention-deficit/hyperactivity disorder: clinical features and familial transmission. J. Clin. Psychiatry. 2013;74:e197–e204. doi: 10.4088/JCP.12m07698. [DOI] [PubMed] [Google Scholar]
- Ayas NT, Brown R, Shea SA. Hypercapnia can induce arousal from sleep in the absence of altered respiratory mechanoreception. Am. J. Respir. Crit. Care Med. 2000;162:1004–1008. doi: 10.1164/ajrccm.162.3.9908040. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Jr Origin and regulation of spontaneous deep breaths. Respir. Physiol. 1971;12:230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol. Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell HJ, Haouzi P. The hypoxia-induced facilitation of augmented breaths is suppressed by the common effect of carbonic anhydrase inhibition. Respir. Physiol. Neuro-biol. 2010;171:201–211. doi: 10.1016/j.resp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Ferguson C, Kehoe V, Haouzi P. Hypocapnia increases the prevalence of hypoxia-induced augmented breaths. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R334–R344. doi: 10.1152/ajpregu.90680.2008. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Azubike E, Haouzi P. The “other” respiratory effect of opioids: suppression of spontaneous augmented (“sigh”) breaths. J. Appl. Physiol. 2011;111:1296–1303. doi: 10.1152/japplphysiol.00335.2011. [DOI] [PubMed] [Google Scholar]
- Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J. Appl. Physiol. 1964;19:195–198. doi: 10.1152/jappl.1964.19.2.195. [DOI] [PubMed] [Google Scholar]
- Bergsholm P, Gran L, Bleie H. Seizure duration in unilateral electroconvulsive therapy. The effect of hypocapnia induced by hyperventilation and the effect of ventilation with oxygen. Acta Psychiatr. Scand. 1984;69:121–128. doi: 10.1111/j.1600-0447.1984.tb02475.x. [DOI] [PubMed] [Google Scholar]
- Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Mathur S. Clinic-based retrospective analysis of psychopharmacology for behavior in fragile x syndrome. Int. J. Pediatr. 2012;2012:843016. doi: 10.1155/2012/843016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiten FA. The effects of emotional behaviour on components of the respiratory cycle. Biol. Psychol. 1998;49:29–51. doi: 10.1016/s0301-0511(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Boiten FA, Frijda NH, Wientjes CJ. Emotions and respiratory patterns: review and critical analysis. Int. J. Psychophysiol. 1994;17:103–128. doi: 10.1016/0167-8760(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Bolton DP, Taylor BJ, Campbell AJ, Galland BC, Cresswell C. Rebreathing expired gases from bedding: a cause of cot death? Arch. Dis. Child. 1993;69:187–190. doi: 10.1136/adc.69.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradd AD, Al Abed A, Guo T, Lovell NH, Dokos S. Study of cardiac pacemaker excitation using generic ionic models and realistic cell distribution. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012;2012:195–198. doi: 10.1109/EMBC.2012.6345904. [DOI] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard F, Shevtsova NA, Bouhadfane M, Tazerart S, Heinemann U, Rybak IA, Vinay L. Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron. 2013;77:1047–1054. doi: 10.1016/j.neuron.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci. 1990;13:21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL, Muench F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatr. Clin. North Am. 2013;36:121–140. doi: 10.1016/j.psc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sanche S, Manetti A, Aouizerate B, Ribereau-Gayon R, Charpentier S, Birmes P, Arbus C. Peritraumatic distress but not dissociation predicts posttraumatic stress disorder in the elderly. Int. Psychogeriatr. 2013;25:1007–1012. doi: 10.1017/S1041610213000069. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Karnani MM, Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol. Behav. 2013;121:117–124. doi: 10.1016/j.physbeh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G, Vaschetto R, Turucz E, Dellapiazza F, Colombo D, Blando C, Della Corte F, Maggiore SM, Navalesi P. Influence of lung collapse distribution on the physiologic response to recruitment maneuvers during noninvasive continuous positive airway pressure. Int. Care Med. 2011;37:1095–1102. doi: 10.1007/s00134-011-2239-8. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Sgoifo A, Trombini M, Landgraf R, Neumann ID, Nalivaiko E. Different patterns of respiration in rat lines selectively bred for high or low anxiety. PLoS One. 2013;8:e64519. doi: 10.1371/journal.pone.0064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro CG, Butler J, Dubois AB. Some effects of restriction of chest cage expansion on pulmonary function in man: an experimental study. J. Clin. Invest. 1960;39:573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MS, Ramirez JM. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J. Neurophysiol. 2013;109:296–305. doi: 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MS, Kenny AS, Patwari PP, Ramirez JM, Weese-Mayer DE. Respiratory and cardiovascular indicators of autonomic nervous system dysregulation in familial dysautonomia. Pediatr. Pulmonol. 2012;47:682–691. doi: 10.1002/ppul.21600. [DOI] [PubMed] [Google Scholar]
- Carroll MS, Viemari JC, Ramirez JM. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J. Neurophysiol. 2013;109:285–295. doi: 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack NS, von Euler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol. Scand. 1981;111:349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Chiodini BA, Thach BT. Impaired ventilation in infants sleeping facedown: potential significance for sudden infant death syndrome. J. Pediatr. 1993;123:686–692. doi: 10.1016/s0022-3476(05)80841-8. [DOI] [PubMed] [Google Scholar]
- Crisp KM, Gallagher BR, Mesce KA. Mechanisms contributing to the dopamine induction of crawl-like bursting in leech motoneurons. J. Exp. Biol. 2012;215:3028–3036. doi: 10.1242/jeb.069245. [DOI] [PubMed] [Google Scholar]
- Datto C, Rai AK, Ilivicky HJ, Caroff SN. Augmentation of seizure induction in electroconvulsive therapy: a clinical reappraisal. J.ECT. 2002;18:118–125. doi: 10.1097/00124509-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009:108135. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J. Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Nagy F. Control of a central pattern generator by an identified modulatory interneurone in crustacea. II. Induction and modification of plateau properties in pyloric neurones. J. Exp. Biol. 1983;105:59–82. doi: 10.1242/jeb.105.1.59. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir. Physiol. Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J. Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GC. The chaotic behaviour of resting human respiration. Respir. Physiol. 1992;88:313–321. doi: 10.1016/0034-5687(92)90005-h. [DOI] [PubMed] [Google Scholar]
- Dong JQ, Zhang JH, Qin J, Li QN, Huang W, Gao XB, Yu J, Chen GZ, Tang XG, Huang L. Anxiety correlates with somatic symptoms and sleep status at high altitudes. Physiol. Behav. 2013;112–113:23–31. doi: 10.1016/j.physbeh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Dunne KP, Fox GP, O’Regan M, Matthews TG. Arousal responses in babies at risk of sudden infant death syndrome at different postnatal ages. Ir. Med. J. 1992;85:19–22. [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr. Top. Med. Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog. Cardiovasc. Dis. 2013;55:548–556. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderby P. Disorders of communication: dysarthria. Handb. Clin. Neurol. 2013;110:273–281. doi: 10.1016/B978-0-444-52901-5.00022-8. [DOI] [PubMed] [Google Scholar]
- Fenske S, Krause S, Biel M, Wahl-Schott C. The role of HCN channels in ventricular repolarization. Trends Cardiovasc. Med. 2011;21:216–220. doi: 10.1016/j.tcm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Ferris BG, Jr, Pollard DS. Effect of deep and quiet breathing on pulmonary compliance in man. J. Clin. Invest. 1960;39:143–149. doi: 10.1172/JCI104012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE. Protective responses of the newborn to hypoxia. Respir. Physiol. Neurobiol. 2005;149:243–255. doi: 10.1016/j.resp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J. Physiol. 1984;347:1–16. doi: 10.1113/jphysiol.1984.sp015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flourens M. Nouveau details sur le noeud vital. Compt. Rend. Acad. Sci. 1858;47:803–806. [Google Scholar]
- Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin. Auton. Res. 1998;8:243–249. doi: 10.1007/BF02277969. [DOI] [PubMed] [Google Scholar]
- Franco P, Verheulpen D, Valente F, Kelmanson I, de Broca A, Scaillet S, Groswasser J, Kahn A. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med. 2003;4:569–577. doi: 10.1016/s1389-9457(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Franco P, Kato I, Richardson HL, Yang JS, Montemitro E, Horne RS. Arousal from sleep mechanisms in infants. Sleep Med. 2010;11:603–614. doi: 10.1016/j.sleep.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Freire RC, Nardi AE. Panic disorder and the respiratory system: clinical subtype and challenge tests. Rev. Bras. Psiquiatr. 2012;34(Suppl 1):S32–S41. doi: 10.1590/s1516-44462012000500004. [DOI] [PubMed] [Google Scholar]
- Freire RC, Nascimento I, Valenca AM, Lopes FL, Mezzasalma MA, de Melo Neto VL, Zin WA, Nardi AE. The panic disorder respiratory ratio: a dimensional approach to the respiratory subtype. Rev. Bras. Psiquiatr. 2013;35:57–62. doi: 10.1016/j.rbp.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb. Cortex. 1991;1:418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- Fujii T, Onimaru H, Suganuma M, Homma I. Effects of hypocapnia on spontaneous burst activity in the piriform-amygdala complex of newborn rat brain preparations in vitro. Adv. Exp. Med. Biol. 2010;669:333–336. doi: 10.1007/978-1-4419-5692-7_68. [DOI] [PubMed] [Google Scholar]
- Fujii T, Onimaru H, Homma I. Effects of corticotropin releasing factor on spontaneous burst activity in the piriform-amygdala complex of in vitro brain preparations from newborn rats. Neurosci. Res. 2011;71:134–139. doi: 10.1016/j.neures.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Gallego J. Genetic diseases: congenital central hypoventilation, Rett, and Prader-Willi syndromes. Comp. Physiol. 2012;2:2255–2279. doi: 10.1002/cphy.c100037. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3—networks within networks: the neuronal control of breathing. Prog. Brain Res. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Koschnitzky JE, Ramirez JM. The physiological determinants of Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2013;189:288–300. doi: 10.1016/j.resp.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerez M, Sada A, Tello A. Amygdalar hyperactivity, a fear-related link between panic disorder and mesiotemporal epilepsy. Clin. EEG Neurosci. 2011;42:29–39. doi: 10.1177/155005941104200108. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Rendall D. Evolution of human vocal production. Curr. Biol. 2008;18:R457–R460. doi: 10.1016/j.cub.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J. Neurosci. 2010;30:16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am. Rev. Respir. Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir. Physiol. 1972;16:179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, Ross D, Klein DF. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am. J. Psychiatry. 1994;151:547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. The multiple challenges of obstructive sleep apnea in children: morbidity and treatment. Curr. Opin. Pediatr. 2008;20:654–658. doi: 10.1097/MOP.0b013e328316ec2d. [DOI] [PubMed] [Google Scholar]
- Graubard K, Hartline DK. Voltage clamp analysis of intact stomatogastric neurons. Brain Res. 1991;557:241–254. doi: 10.1016/0006-8993(91)90141-h. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat. Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J. Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groswasser J, Simon T, Scaillet S, Franco P, Kahn A. Reduced arousals following obstructive apneas in infants sleeping prone. Pediatr. Res. 2001;49:402–406. doi: 10.1203/00006450-200103000-00015. [DOI] [PubMed] [Google Scholar]
- Haeck M, Gillmann B, Janouschek H, Grozinger M. Electroconvulsive therapy can benefit from controlled hyperventilation using a laryngeal mask. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261(Suppl. 2):S172–S176. doi: 10.1007/s00406-011-0240-4. [DOI] [PubMed] [Google Scholar]
- Haldane JS, Meakins JC, Priestley JG. The effects of shallow breathing. J. Physiol. 1919;52:433–453. doi: 10.1113/jphysiol.1919.sp001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JA, Haub MD, Walker JJ, Johnston DT, Goff BS, Dretsch MN. Attention deficit hyperactivity disorder subtypes and their relation to cognitive functioning, mood states, and combat stress symptomatology in deploying U.S. soldiers. Mil. Med. 2012;177:655–662. doi: 10.7205/milmed-d-11-00340. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Khou CS, White CN, Ong JC. Gender and cognitive-emotional factors as predictors of pre-sleep arousal and trait hyperarousal in insomnia. J. Psychosom. Res. 2013;74:283–289. doi: 10.1016/j.jpsychores.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM. Voltage-sensitive ion channels in rhythmic motor systems. Curr. Opin. Neurobiol. 2002;12:646–651. doi: 10.1016/s0959-4388(02)00377-x. [DOI] [PubMed] [Google Scholar]
- Haupt ME, Goodman DM, Sheldon SH. Sleep related expiratory obstructive apnea in children. J. Clin. Sleep Med. 2012;8:673–679. doi: 10.5664/jcsm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Zageris DM, Berry-Kravis E, Porges SW. Autonomic regulation in fragile X syndrome. Dev. Psychobiol. 2011;53:785–795. doi: 10.1002/dev.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DR, Bigatello LM. Lung recruitment: the role of recruitment maneuvers. Respir. Care. 2002;47:308–317. discussion 317-308. [PubMed] [Google Scholar]
- Hill AA, Garcia AJ, 3rd, Zanella S, Upadhyaya R, Ramirez JM. Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J. Neurophysiol. 2011;105:625–639. doi: 10.1152/jn.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs KH, Hooper SL. Using complicated, wide dynamic range driving to develop models of single neurons in single recording sessions. J. Neurophysiol. 2008;99:1871–1883. doi: 10.1152/jn.00032.2008. [DOI] [PubMed] [Google Scholar]
- Hoch B, Bernhard M, Hinsch A. Different patterns of sighs in neonates and young infants. Biol. Neonate. 1998;74:16–21. doi: 10.1159/000014006. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, Worthington JJ, Pollack MH, Simon NM. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry. 2013;74:786–792. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J. Comp. Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Horne RS, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir. Physiol. Neurobiol. 2005;149:257–271. doi: 10.1016/j.resp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Huber JE, Darling M. Effect of Parkinson’s disease on the production of structured and unstructured speaking tasks: respiratory physiologic and linguistic considerations. J. Speech Lang. Hear. Res. 2011;54:33–46. doi: 10.1044/1092-4388(2010/09-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Darling M, Francis EJ, Zhang D. Impact of typical aging and Parkinson’s disease on the relationship among breath pausing, syntax, and punctuation. Am. J. Speech Lang. Pathol. 2012;21:368–379. doi: 10.1044/1058-0360(2012/11-0059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AE, Prinz AA. Conductance ratios and cellular identity. PLoS Comput. Biol. 2010;6:e1000838. doi: 10.1371/journal.pcbi.1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J. Neurophysiol. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog. Brain Res. 2012a;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol. Behav. 2012b;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J, Vignal JP, Baumann C, Anxionnat JF, Muresan M, Vespignani H, Maillard L. Effect of hyperventilation on seizure activation: potentiation by anti-epileptic drug tapering. J. Neurol. Neurosurg. Psychiatry. 2011;82:928–930. doi: 10.1136/jnnp.2009.200329. [DOI] [PubMed] [Google Scholar]
- Kahn A, Blum D, Rebuffat E, Sottiaux M, Levitt J, Bochner A, Alexander M, Grosswasser J, Muller MF. Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics. 1988;82:721–727. [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–292. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- Kalk NJ, Nutt DJ, Lingford-Hughes AR. The role of central noradrenergic dysregulation in anxiety disorders: evidence from clinical studies. J. Psychopharmacol. 2011;25:3–16. doi: 10.1177/0269881110367448. [DOI] [PubMed] [Google Scholar]
- Kalume F. Sudden unexpected death in Dravet syndrome: respiratory and other physiological dysfunctions. Respir. Physiol. Neurobiol. 2013;189:324–328. doi: 10.1016/j.resp.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Groswasser J, Franco P, Scaillet S, Kelmanson I, Togari H, Kahn A. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am. J. Respir. Crit. Care Med. 2001;164:1464–1469. doi: 10.1164/ajrccm.164.8.2009001. [DOI] [PubMed] [Google Scholar]
- Kato I, Scaillet S, Groswasser J, Montemitro E, Togari H, Lin JS, Kahn A, Franco P. Spontaneous arousability in prone and supine position in healthy infants. Sleep. 2006;29:785–790. doi: 10.1093/sleep/29.6.785. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Miller L, Macpherson MK, Huber J, Tranel D. An investigation of semantic similarity judgments about action and non-action verbs in Parkinson’s disease: implications for the Embodied Cognition Framework. Front. Hum. Neurosci. 2013;7:146. doi: 10.3389/fnhum.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JS, Thach BT. Sudden death in infants sleeping on polystyrene-filled cushions. New Eng. J. Med. 1991;324:1858–1864. doi: 10.1056/NEJM199106273242605. [DOI] [PubMed] [Google Scholar]
- Kemp JS, Kowalski RM, Burch PM, Graham MA, Thach BT. Unintentional suffocation by rebreathing: a death scene and physiologic investigation of a possible cause of sudden infant death. J. Pediatr. 1993;122:874–880. doi: 10.1016/s0022-3476(09)90010-5. [DOI] [PubMed] [Google Scholar]
- Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J. Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Kipman M, Deldonno SR, Weber M. Insomnia-related complaints correlate with functional connectivity between sensory-motor regions. Neuroreport. 2013;24:233–240. doi: 10.1097/WNR.0b013e32835edbdd. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ, Cheong TH, Olha AE, Charbonneau M, Levy RD, Cosio MG, Gottfried SB. Mechanisms of apnea termination in obstructive sleep apnea. Role of chemoreceptor and mechanoreceptor stimuli. Am. J. Respir. Crit. Care Med. 1994;149:707–714. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu. Rev. Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch. Gen. Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Klein DF. Testing the suffocation false alarm theory of panic disorder. Anxiety. 1994;1:1–7. doi: 10.1002/anxi.3070010103. [DOI] [PubMed] [Google Scholar]
- Koch H, Garcia AJ, 3rd, Ramirez JM. Network reconfiguration and neuronal plasticity in rhythm-generating networks. Integr. Compar. Biol. 2011;51:856–868. doi: 10.1093/icb/icr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Zanella S, Elsen G, Smith L, Doi A, Garcia AJ, 3rd, Wei A, Xun R, Kirsch S, Gomez M, Hevner R, Ramirez JM. Stable respiratory activity requires both P/Q-type and N-type voltage-gated channels. J. Neurosci. 2012;33:3633–3645. doi: 10.1523/JNEUROSCI.6390-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Doroshenko P, Yan Cao X, Coderre E, Renaud LP. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience. 2007;147:1066–1075. doi: 10.1016/j.neuroscience.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Kolta A, Morquette P, Lavoie R, Arsenault I, Verdier D. Modulation of rhythmogenic propertiesof trigeminal neurons contributing to the masticatory CPG Prog. Brain Res. 2010;187:137–148. doi: 10.1016/B978-0-444-53613-6.00009-5. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W. Orexin neurons and emotional stress. Vitam. Horm. 2012;89:135–158. doi: 10.1016/B978-0-12-394623-2.00008-1. [DOI] [PubMed] [Google Scholar]
- Lee MH, Lee SA, Lee GH, Ryu HS, Chung S, Chung YS, Kim WS. Gender differences in the effect of comorbid insomnia symptom on depression, anxiety, fatigue, and daytime sleepiness in patients with obstructive sleep apnea. Sleep Breath. 2013 doi: 10.1007/s11325-013-0856-x. [DOI] [PubMed] [Google Scholar]
- Liegeois FJ, Morgan AT. Neural bases of childhood speech disorders: lateralization and plasticity for speech functions during development. Neurosci. Biobehav. Rev. 2012;36:439–458. doi: 10.1016/j.neubiorev.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J. Neurophysiol. 2006a;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II. Intrinsic modulation by metabotropic glutamate receptors. J. Neurophysiol. 2006b;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment] Nat. Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J. Appl. Physiol. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proc. Natl. Acad. Sci. U. S. A. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Havens J, Yu Q, Wang G, Davisson RL, Pickel VM, Iadecola C. The link between angiotensin II-mediated anxiety and mood disorders with NADPH oxidase-induced oxidative stress. Int. J. Physiol. Pathophysiol. Pharmacol. 2012;4:28–35. [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb. Cortex. 2009;19:2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C, Simpson B, MacPherson R. Augmentation strategies in electroconvulsive therapy. J. ECT. 2010;26:202–207. doi: 10.1097/YCT.0b013e3181e48143. [DOI] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: common volitional control for two upper airway functions. NeuroImage. 2007;36:131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lumsden T. The regulation of respiration: part I. J. Physiol. 1923;58:81–91. doi: 10.1113/jphysiol.1923.sp002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin L, Leseche G, Duprey A, Clerici C. Ventilatory chaos is impaired in carotid atherosclerosis. PLoS One. 2011;6:e16297. doi: 10.1371/journal.pone.0016297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuccilli CJ, Tryba AK, van Drongelen W, Koch H, Viemari JC, Pena-Ortega F, Doren EL, Pytel P, Chevalier M, Mrejeru A, Kohrman MH, Lasky RE, Lew SM, Frim DM, Ramirez JM. Neuronal bursting properties in focal and parafocal regions in pediatric neocortical epilepsy stratified by histology. J. Clin. Neurophysiol. 2010;27:387–397. doi: 10.1097/WNP.0b013e3181fe06d8. [DOI] [PubMed] [Google Scholar]
- Martell A, Dwyer J, Koch H, Zanella S, Kohrman M, Frim D, Ramirez JM, van Drongelen W. N-methyl-D-aspartate-induced oscillatory properties in neocortical pyramidal neurons from patients with epilepsy. J. Clin. Neurophysiol. 2010;27:398–405. doi: 10.1097/WNP.0b013e3182007c7d. [DOI] [PubMed] [Google Scholar]
- Martell AL, Ramirez JM, Lasky RE, Dwyer JE, Kohrman M, van Drongelen W. The role of voltage dependence of the NMDA receptor in cellular and network oscillation. Eur. J. Neurosci. 2012;36:2121–2136. doi: 10.1111/j.1460-9568.2012.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JM, Kent JM, Coplan JD, Browne ST, Papp LA, Sullivan GM, Kleber M, Perepletchikova F, Fyer AJ, Klein DF, Gorman JM. Respiratory variability in panic disorder. Depress. Anxiety. 2001;14:232–237. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- Masa JF, Corral J, Martin MJ, Riesco JA, Sojo A, Hernandez M, Douglas NJ. Assessment of thoracoabdominal bands to detect respiratory effort-related arousal. Eur. Respir. J. 2003;22:661–667. doi: 10.1183/09031936.03.00010903. [DOI] [PubMed] [Google Scholar]
- Masaoka Y, Homma I. The source generator of respiratory-related anxiety potential in the human brain. Neurosci. Lett. 2000;283:21–24. doi: 10.1016/s0304-3940(00)00895-8. [DOI] [PubMed] [Google Scholar]
- Masaoka Y, Hirasawa K, Yamane F, Hori T, Homma I. Effects of left amygdala lesions on respiration, skin conductance, heart rate, anxiety, and activity of the right amygdala during anticipation of negative stimulus. Behav. Modif. 2003;27:607–619. doi: 10.1177/0145445503256314. [DOI] [PubMed] [Google Scholar]
- Masaoka Y, Sugiyama H, Katayama A, Kashiwagi M, Homma I. Remembering the past with slow breathing associated with activity in the parahippocampus and amygdala. Neurosci. Lett. 2012;521:98–103. doi: 10.1016/j.neulet.2012.05.047. [DOI] [PubMed] [Google Scholar]
- McCulloch K, Brouillette RT, Guzzetta AJ, Hunt CE. Arousal responses in near-miss sudden infant death syndrome and in normal infants. J. Pediatr. 1982;101:911–917. doi: 10.1016/s0022-3476(82)80009-7. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE, Lawrence AJ. Central autonomic integration of psychological stressors: focus on cardiovascular modulation. Auton. Neurosci. 2005;123:1–11. doi: 10.1016/j.autneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, London MS, Baker TL, Stevenson M, Hoppenbrouwers T, Harper RM, Sterman MB, Hodgman J. Sleep apnea in normal kittens. Sleep. 1979;1:393–412. [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J. Appl. Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J. Mol. Neurosci. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory biofeedback-assisted therapy in panic disorder. Behav. Modif. 2001;25:584–605. doi: 10.1177/0145445501254006. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Wei A, Ramirez JM. Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J. Physiol. 2011;589:2497–2514. doi: 10.1113/jphysiol.2011.206631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Galloway-Long HS, Frick PJ, Nigg JT. Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(163–171):e162. doi: 10.1016/j.jaac.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respir. Physiol. Neurobiol. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog. Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep. 2005;28:747–752. doi: 10.1093/sleep/28.6.747. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Holbrook CR, Vanderlaan M, Amiel J, Gozal D. Autonomic function in children with congenital central hypoventilation syndrome and their families. Chest. 2005;128:2478–2484. doi: 10.1378/chest.128.4.2478. [DOI] [PubMed] [Google Scholar]
- Okamura N, Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007;10:221–226. doi: 10.1080/10253890701248673. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Spontaneous oscillatory burst activity in the piriform-amygdala region and its relation to in vitro respiratory activity in newborn rats. Neuroscience. 2007;144:387–394. doi: 10.1016/j.neuroscience.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J. Appl. Physiol. 1993;74:761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Cevolani D, Nucifora V, Borlimi R, Agati R, Leonardi M, De Plato G, Brighetti G. Amygdala responses to masked and low spatial frequency fearful faces: a preliminary fMRI study in panic disorder. Psychiatry Res. 2012;203:159–165. doi: 10.1016/j.pscychresns.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Pandya AA, Yakel JL. Activation of the alpha7 nicotinic ACh receptor induces anxiogenic effects in rats which is blocked by a 5-HT(1)a receptor antagonist. Neuropharmacology. 2013;70:35–42. doi: 10.1016/j.neuropharm.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow PM, Harding R, Cranage SM, Adamson TM, Horne RS. Ventilatory responses preceding hypoxia-induced arousal in infants: effects of sleep-state. Respir. Physiol. Neurobiol. 2003;136:235–247. doi: 10.1016/s1569-9048(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Paterson DS. Serotonin gene variants are unlikely to play a significant role in the pathogenesis of the sudden infant death syndrome. Respir. Physiol. Neurobiol. 2013;189:301–314. doi: 10.1016/j.resp.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J. Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Perez LG, Abrams MP, Lopez-Martinez AE, Asmundson GJ. Trauma exposure and health: the role of depressive and hyperarousal symptoms. J. Trauma. Stress. 2012;25:641–648. doi: 10.1002/jts.21762. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, West P, Kryger MH. Sighs during sleep in adult humans. Sleep. 1983;6:234–243. doi: 10.1093/sleep/6.3.234. [DOI] [PubMed] [Google Scholar]
- Pine DS, Weese-Mayer DE, Silvestri JM, Davies M, Whitaker AH, Klein DF. Anxiety and congenital central hypoventilation syndrome. Am. J. Psychiatry. 1994;151:864–870. doi: 10.1176/ajp.151.6.864. [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New Eng. J. Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Porges WL, Hennessy EJ, Quail AW, Cottee DB, Moore PG, McIlveen SA, Parsons GH, White SW. Heart-lung interactions: the sigh and autonomic control in the bronchial and coronary circulations. Clin. Exp. Pharmacol. Physiol. 2000;27:1022–1027. doi: 10.1046/j.1440-1681.2000.03370.x. [DOI] [PubMed] [Google Scholar]
- Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:603–612. doi: 10.1016/j.pnpbp.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Thirumalai V, Marder E. The functional consequences of changes in the strength and duration of synaptic inputs to oscillatory neurons. J. Neurosci. 2003;23:943–954. doi: 10.1523/JNEUROSCI.23-03-00943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Gallego J. Congenital central hypoventilation syndrome. Respir. Physiol. Neurobiol. 2013;189:272–279. doi: 10.1016/j.resp.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Pearson KG. Octopamine induces bursting and plateau potentials in insect neurones. Brain Res. 1991a;549:332–337. doi: 10.1016/0006-8993(91)90477-d. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Pearson KG. Octopaminergic modulation of interneurons in the flight system of the locust. J. Neurophysiol. 1991b;66:1522–1537. doi: 10.1152/jn.1991.66.5.1522. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Pearson KG. Alteration of bursting properties in interneurons during locust flight. J. Neurophysiol. 1993;70:2148–2160. doi: 10.1152/jn.1993.70.5.2148. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. J. Physiol. 1998;507(Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr. Opin. Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Koch H, Garcia AJ, 3rd, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J. Biol. Phy. 2011;37:241–261. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Doi A, Garcia AJ, 3rd, Elsen FP, Koch H, Wei AD. The cellular building blocks of breathing. Comp. Physiol. 2012;2:2683–2731. doi: 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Garcia AJ, 3rd, Anderson TM, Koschnitzky JE, Peng YJ, Kumar G, Prabhakar N. Central and peripheral factors contributing to obstructive sleep apneas. Respir. Physiol. Neurobiol. 2013a;189:344–353. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Ward CS, Neul JL. Breathing challenges in Rett Syndrome: lessons learned from humans and animal models. Respir. Physiol. Neurobiol. 2013b;189:280–287. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees K, Spence DP, Earis JE, Calverley PM. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am. J. Respir. Crit. Care Med. 1995;152:1016–1021. doi: 10.1164/ajrccm.152.3.7663777. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL. Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 2005;272:5689–5693. doi: 10.1111/j.1742-4658.2005.04982.x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety. 2000;12(Suppl. 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Retson TA, Van Bockstaele EJ. Coordinate regulation of noradrenergic and serotonergic brain regions by amygdalar neurons. J. Chem. Neuroanat. 2013;52:9–19. doi: 10.1016/j.jchemneu.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Vujisic K, Davenport PW, Hayward LF. Disinhibition of the dor-somedial hypothalamus increases the frequency of augmented breaths in the anesthetized rat. Adv. Exp. Med. Biol. 2008;605:274–278. doi: 10.1007/978-0-387-73693-8_48. [DOI] [PubMed] [Google Scholar]
- Rinberg A, Taylor AL, Marder E. The effects of temperature on the stability of a neuronal oscillator. PLoS Comput. Biol. 2013;9:e1002857. doi: 10.1371/journal.pcbi.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Gomolla A, Meuret AE, Alpers GW, Handke EM, Wilhelm FH. High altitudes, anxiety, and panic attacks: is there a relationship? Depress. Anxiety. 2002;16:51–58. doi: 10.1002/da.10059. [DOI] [PubMed] [Google Scholar]
- Russell DF, Hartline DK. Bursting neural networks: a reexamination. Science. 1978;200:453–456. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- Sangkatumvong S, Coates TD, Wood JC, Meiselman HJ, Kato R, Detterich JA, Bush A, Khoo MC. Time-varying analysis of autonomic control in response to spontaneous sighs in sickle cell anemia. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010;2010:1626–1629. doi: 10.1109/IEMBS.2010.5626649. [DOI] [PubMed] [Google Scholar]
- Sangkatumvong S, Khoo MC, Kato R, Detterich JA, Bush A, Keens TG, Meiselman HJ, Wood JC, Coates TD. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am. J. Respir. Crit. Care Med. 2011;184:474–481. doi: 10.1164/rccm.201103-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Kato I, Franco P, Sottiaux M, Kadhim H, Shimizu S, Groswasser J, Togari H, Kobayashi M, Nishida H, Sawaguchi A, Kahn A. Apnea, glial apoptosis and neuronal plasticity in the arousal pathway of victims of SIDS. Forensic Sci. Int. 2005;149:205–217. doi: 10.1016/j.forsciint.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Schechtman VL, Harper RM, Wilson AJ, Southall DP. Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics. 1992;89:865–870. [PubMed] [Google Scholar]
- Schenberg LC, Bittencourt AS, Sudre EC, Vargas LC. Modeling panic attacks. Neurosci. Biobehav. Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Goetz RR, Klein DF, Endicott J, Gorman JM. Tidal volume of respiration and “sighing” as indicators of breathing irregularities in panic disorder patients. Anxiety. 1996;2:145–148. doi: 10.1002/(SICI)1522-7154(1996)2:3<145::AID-ANXI6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- Schwenke DO, Cragg PA. Carotid bodies and the sigh reflex in the conscious and anaesthetised guinea-pig. Adv. Exp. Med. Biol. 2000;475:801–813. doi: 10.1007/0-306-46825-5_81. [DOI] [PubMed] [Google Scholar]
- Shea SA, Horner RL, Banner NR, McKenzie E, Heaton R, Yacoub MH, Guz A. The effect of human heart-lung transplantation upon breathing at rest and during sleep. Respir. Physiol. 1988;72:131–149. doi: 10.1016/0034-5687(88)90001-1. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011;13:263–278. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko S, Yang D, Li Y, Lyashkov AE, Lukyanenko YO, Lakatta EG, Vinogradova TM. Ca(2)(+)-dependent phosphorylation of Ca(2)(+) cycling proteins generates robust rhythmic local Ca(2)(+) releases in cardiac pacemaker cells. Sci. Signal. 2013;6 doi: 10.1126/scisignal.2003391. ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S. Aspects of speech rate and regularity in Parkinson’s disease. J. Neurol. Sci. 2011;310:231–236. doi: 10.1016/j.jns.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Small M, Judd K, Lowe M, Stick S. Is breathing in infants chaotic? Dimension estimates for respiratory patterns during quiet sleep. J. Appl. Physiol. 1999;86:359–376. doi: 10.1152/jappl.1999.86.1.359. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sody AN, Kiderman A, Biton A, Furst A. Sigh syndrome: is it a sign of trouble? J. Fam. Pract. 2008;57:E1–E5. [PubMed] [Google Scholar]
- Soltysik S, Jelen P. In rats, sighs correlate with relief. Physiol. Behav. 2005;85:598–602. doi: 10.1016/j.physbeh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS. Bilateral lesions of pontine Kolliker-Fuse nuclei provoke apnea instead of apneusis in anesthetized adult rats. Adv. Exp. Med. Biol. 2010;669:185–188. doi: 10.1007/978-1-4419-5692-7_37. [DOI] [PubMed] [Google Scholar]
- Soofi W, Archila S, Prinz AA. Co-variation of ionic conductances supports phase maintenance in stomatogastric neurons. J. Comput. Neurosci. 2012;33:77–95. doi: 10.1007/s10827-011-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Trevino C, Rabbah P, Marder E, Nadim F. Computational model of electrically coupled, intrinsically distinct pacemaker neurons. J. Neurophysiol. 2005;94:590–604. doi: 10.1152/jn.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: fatal postictal respiratory and arousal mechanisms. Respir. Physiol. Neurobiol. 2013;189:315–323. doi: 10.1016/j.resp.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH. Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo. J. Physiol. 2013;591:109–122. doi: 10.1113/jphysiol.2012.245217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J. Neurosci. 2009;29:3824–3832. doi: 10.1523/JNEUROSCI.0607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Stimulation of the midbrain periaqueductal gray modulates preinspiratory neurons in the ventrolateral medulla in the rat in vivo. J. Comp. Neurol. 2013;521:3083–3098. doi: 10.1002/cne.23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J. Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat. Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teigen KH. Is a sigh “just a sigh”? Sighs as emotional signals and responses to a difficult task. Scand. J. Psychol. 2008;49:49–57. doi: 10.1111/j.1467-9450.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J. Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Temporal S, Desai M, Khorkova O, Varghese G, Dai A, Schulz DJ, Golowasch J. Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J. Neurophysiol. 2012;107:718–727. doi: 10.1152/jn.00622.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT. Graded arousal responses in infants: advantages and disadvantages of a low threshold for arousal. Sleep Med. 2002;3(Suppl. 2):S37–S40. doi: 10.1016/s1389-9457(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Thach BT, Lijowska A. Arousals in infants. Sleep. 1996;19:S271–S273. doi: 10.1093/sleep/19.suppl_10.s271. [DOI] [PubMed] [Google Scholar]
- Thach BT, Taeusch HW., Jr Sighing in newborn human infants: role of inflation-augmenting reflex. J. Appl. Physiol. 1976;41:502–507. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol. Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J. Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Tobin MJ, Yang KL, Jubran A, Lodato RF. Interrelationship of breath components in neighboring breaths of normal eupneic subjects. Am. J. Respir. Crit. Care Med. 1995;152:1967–1976. doi: 10.1164/ajrccm.152.6.8520764. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J. Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiptsios DI, Howard RS, Koutroumanidis MA. Electroencephalographic assessment of patients with epileptic seizures. Expert. Rev. Neurother. 2010;10:1869–1886. doi: 10.1586/ern.10.175. [DOI] [PubMed] [Google Scholar]
- Tsuruyama K, Hsiao CF, Chandler SH. Participation of a persistent sodium current and calcium-activated non-specific cationic current to burst generation in trigeminal principal sensory neurons. J. Neurophysiol. 2013;110:1903–1914. doi: 10.1152/jn.00410.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VG, Holstege G. A concept for the final common pathway of vocalization and lordosis behavior in the cat. Prog. Brain Res. 1996;107:327–342. doi: 10.1016/s0079-6123(08)61874-9. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J. Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ, 3rd, Doi A, Ramirez JM. Activation of alpha-2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur. J. Neurosci. 2011;33:2228–2237. doi: 10.1111/j.1460-9568.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila J, Guerra P, Munoz MA, Vico C, Viedmadel Jesus MI, Delgado LC, Perakakis P, Kley E, Mata JL, Rodriguez S. Cardiac defense: from attention to action. Int. J. Psychophysiol. 2007;66:169–182. doi: 10.1016/j.ijpsycho.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Vincent N, Walsh K. Hyperarousal, sleep scheduling, and time awake in bed as mediators of outcome in computerized cognitive-behavioral therapy (cCBT) for insomnia. Behav. Res. Ther. 2013;51:161–166. doi: 10.1016/j.brat.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, De Peuter S, Bresseleers J, Bogaerts K, Fannes S, Li W, Van Den Bergh O. Why do you sigh? Sigh rate during induced stress and relief. Psychophysiology. 2009;46:1005–1013. doi: 10.1111/j.1469-8986.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Taelman J, Van Diest I, Van den Bergh O. Take a deep breath: the relief effect of spontaneous and instructed sighs. Physiol. Behav. 2010a;101:67–73. doi: 10.1016/j.physbeh.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, Lehrer PM, Aubert AE, Van den Bergh O. Respiratory variability preceding and following sighs: a resetter hypothesis. Biol. Psychol. 2010b;84:82–87. doi: 10.1016/j.biopsycho.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Taelman J, De Peuter S, Van Diest I, Van den Bergh O. Sigh rate and respiratory variability during mental load and sustained attention. Psychophysiology. 2011;48:117–120. doi: 10.1111/j.1469-8986.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Abelson JL, Lehrer PM, Davenport PW, Van Diest I, Van den Bergh O. Respiratory variability and sighing: a psychophysiological reset model. Biol. Psychol. 2013a;93:24–32. doi: 10.1016/j.biopsycho.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Vigo D, Vansteenwegen D, Van den Bergh O, Van Diest I. Do not worry, be mindful: effects of induced worry and mindfulness on respiratory variability in a nonanxious population. Int. J. Psychophysiol. 2013b;87:147–151. doi: 10.1016/j.ijpsycho.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Schredl M, Delb W. Tinnitus and insomnia: is hyperarousal the common denominator? Sleep Med. Rev. 2013;17:65–74. doi: 10.1016/j.smrv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Kenny AS, Bennett HL, Ramirez JM, Leurgans SE. Familial dysautonomia: frequent, prolonged and severe hypoxemia during wakefulness and sleep. Pediatr. Pulmonol. 2008a;43:251–260. doi: 10.1002/ppul.20764. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr. Pulmonol. 2008b;43:1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gerlach AL, Roth WT. Slow recovery from voluntary hyperventilation in panic disorder. Psychosom. Med. 2001a;63:638–649. doi: 10.1097/00006842-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gevirtz R, Roth WT. Respiratory dysregulation in anxiety, functional cardiac, and pain disorders. Assessment, phenomenology, and treatment. Behav. Modif. 2001b;25:513–545. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Physiologic instability in panic disorder and generalized anxiety disorder. Biol. Psychiatry. 2001c;49:596–605. doi: 10.1016/s0006-3223(00)01000-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep. 2010;33:379–387. doi: 10.1093/sleep/33.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirrell EC, Camfield PR, Gordon KE, Camfield CS, Dooley JM, Hanna BD. Will a critical level of hyperventilation-induced hypocapnia always induce an absence seizure? Epilepsia. 1996;37:459–462. doi: 10.1111/j.1528-1157.1996.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F, Thach BT. Suppression of sigma spindle electroenceph-alographic activity as a measure of transient arousal after spontaneous and occlusion-evoked sighs and startles. Pediat. Res. 1998;44:767–773. doi: 10.1203/00006450-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F, Thach BT. The role of arousal related brainstem reflexes in causing recovery from upper airway occlusion in infants. Sleep. 2008;31:833–840. doi: 10.1093/sleep/31.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts R, Vlemincx E, Bogaerts K, Van Diest I, Van den Bergh O. Sigh rate and respiratory variability during normal breathing and the role of negative affectivity. Int. J. Psychophysiol. 2011;82:175–179. doi: 10.1016/j.ijpsycho.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Wysocki M, Fiamma MN, Straus C, Poon CS, Similowski T. Chaotic dynamics of resting ventilatory flow in humans assessed through noise titration. Respir. Physiol. Neurobiol. 2006;153:54–65. doi: 10.1016/j.resp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chow JC, Tsai JJ, Huang CW. Hyperventilation-induced ictal fear in nonlesional temporal lobe epilepsy. Epilepsy Behav. 2011;21:100–102. doi: 10.1016/j.yebeh.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Zhao S, Golowasch J. Ionic current correlations underlie the global tuning of large numbers of neuronal activity attributes. J. Neurosci. 2012;32:13380–13388. doi: 10.1523/JNEUROSCI.6500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Mentis GZ, Wiesner EP, Titus DJ. Synaptic integration of rhythmogenic neurons in the locomotor circuitry: the case of Hb9 interneurons. Ann. N. Y. Acad. Sci. 2010;1198:72–84. doi: 10.1111/j.1749-6632.2010.05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]