Abstract
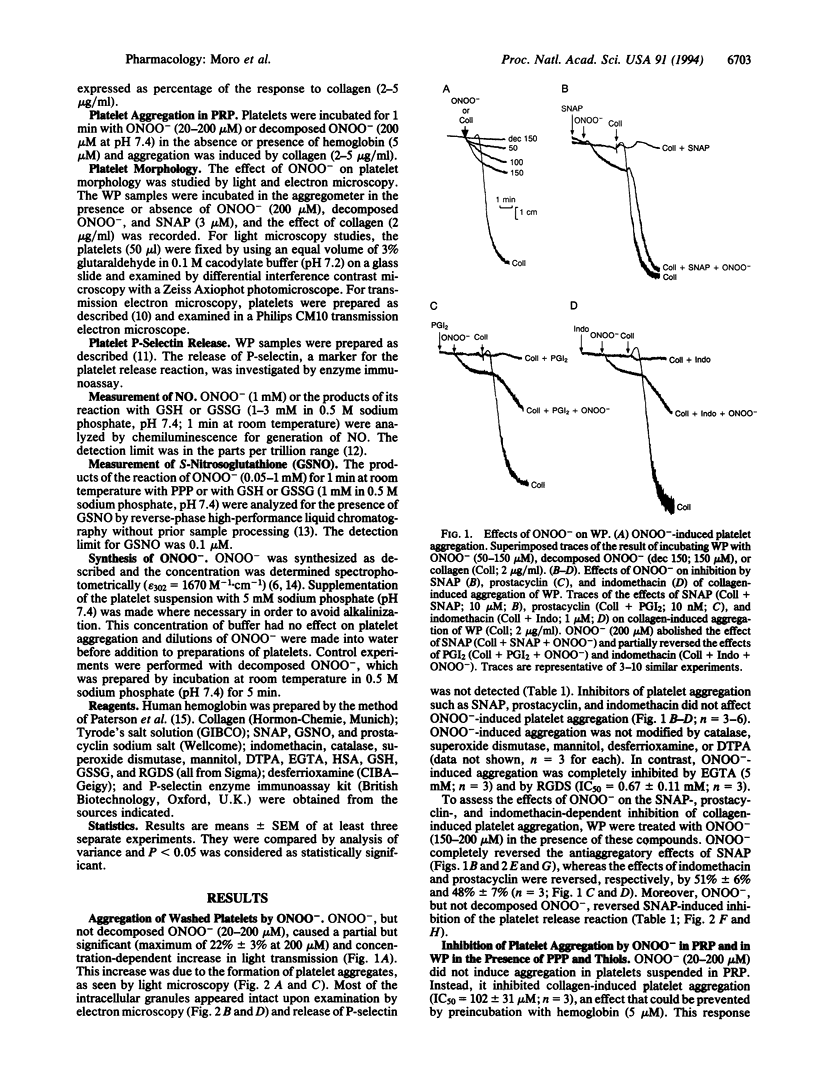
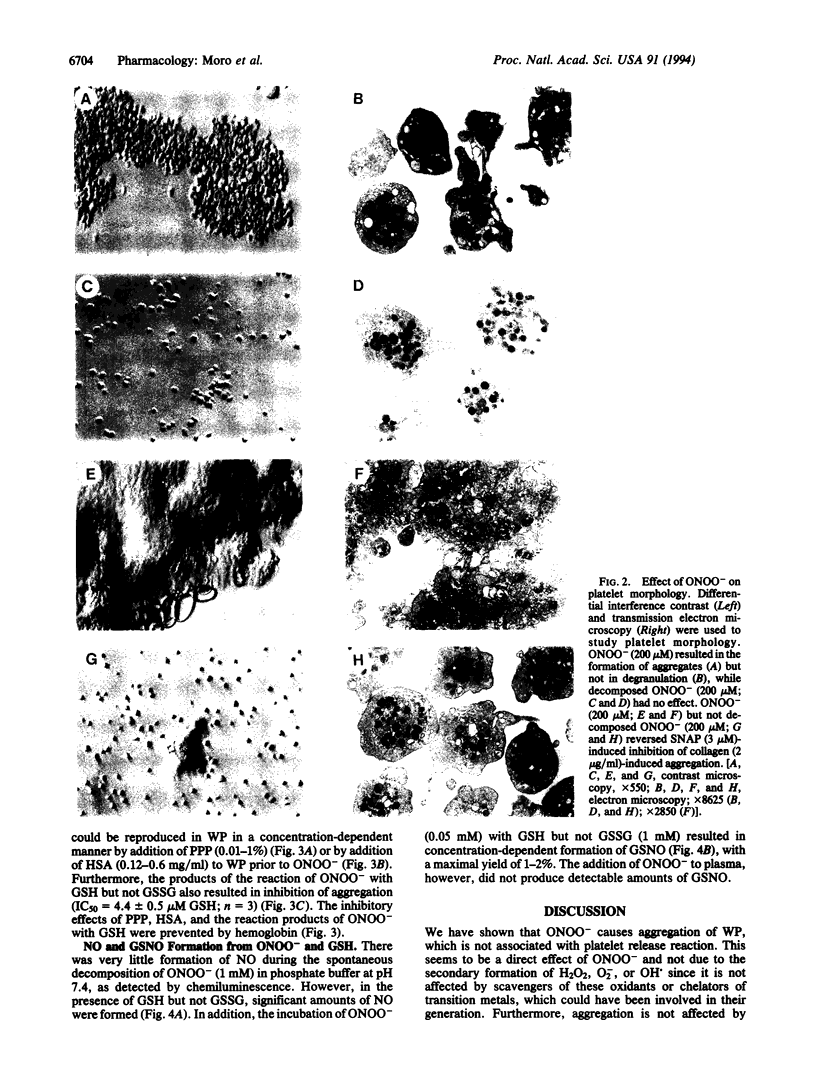
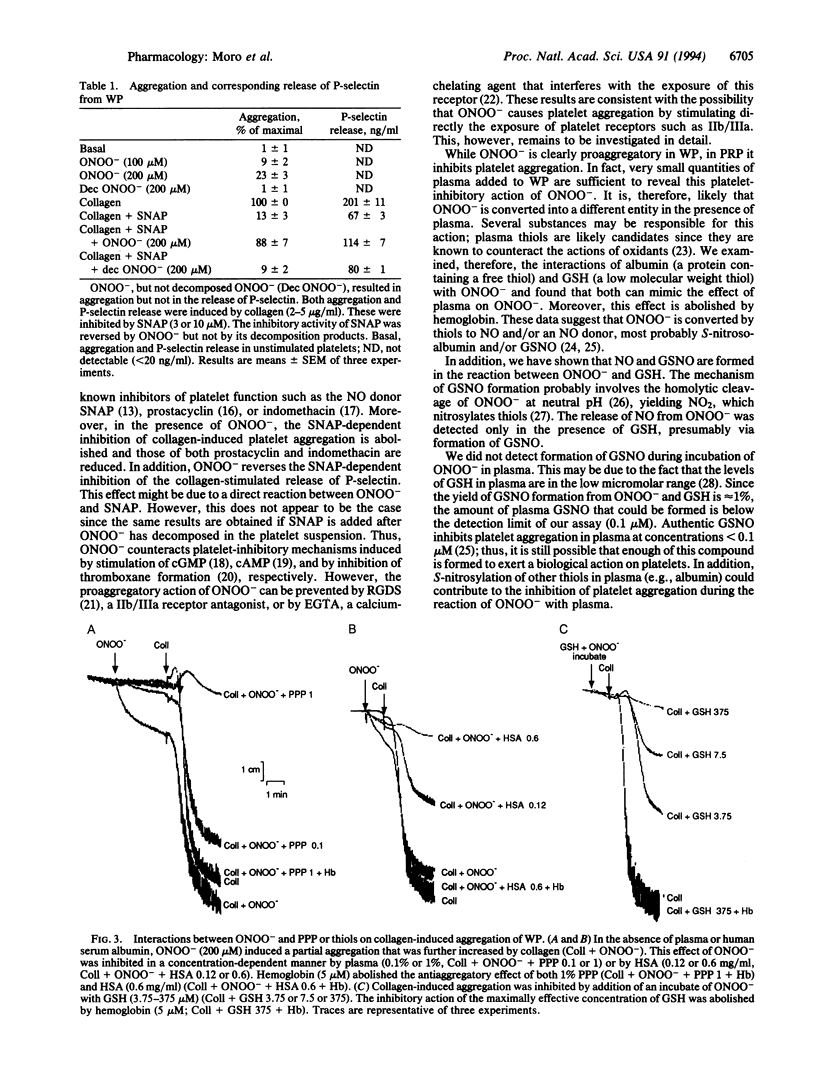
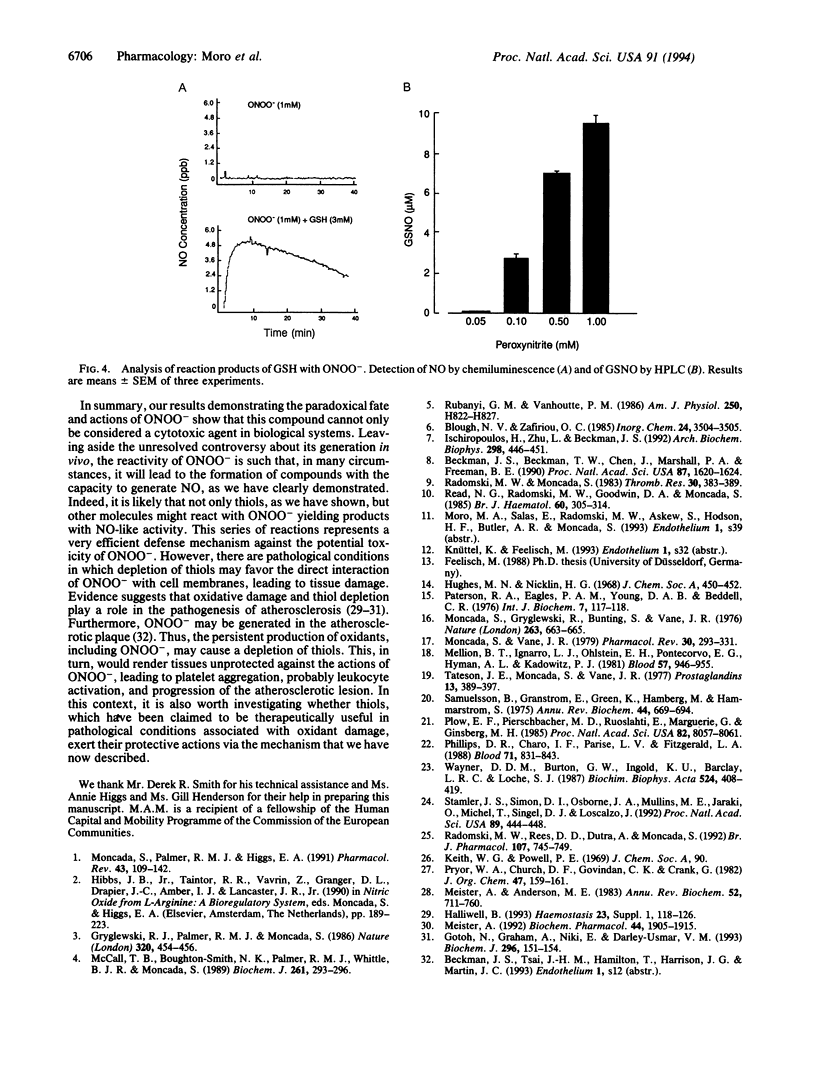
Peroxynitrite (ONOO-), which is formed from the reaction of nitric oxide (NO) and superoxide (O2-), has been suggested to be responsible for some of the cytotoxic effects of these molecules. When protonated, ONOO- gives rise to hydroxyl (OH.) and nitrogen dioxide (NO2) radicals, which are capable of inducing tissue damage. We have investigated the effects of ONOO- on human platelets in vitro in order to explore the potential of this oxidant to contribute to tissue damage. ONOO- caused aggregation of washed platelets and reversed the inhibition of aggregation induced by S-nitroso-N-acetyl-DL-penicillamine (SNAP), prostacyclin, and indomethacin. However, in platelet-rich plasma, ONOO- not only did not possess proaggregatory properties but acted as an inhibitor of platelet aggregation. This reversal of the aggregatory effect of ONOO- could also be achieved in washed platelets by adding low concentrations of plasma, human serum albumin, or glutathione and was inhibited by hemoglobin. An analysis of the reaction products of ONOO- and glutathione revealed the presence of both NO and S-nitrosoglutathione in quantities sufficient to account for the antiaggregatory effects observed. Thus the fate and therefore the actions of ONOO- in biological systems are critically dependent on the biological environment in which this oxidant is present.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Graham A., Nikl E., Darley-Usmar V. M. Inhibition of glutathione synthesis increases the toxicity of oxidized low-density lipoprotein to human monocytes and macrophages. Biochem J. 1993 Nov 15;296(Pt 1):151–154. doi: 10.1042/bj2960151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis. 1993 Mar;23 (Suppl 1):118–126. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992 Nov 17;44(10):1905–1915. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992 Nov;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Read N. G., Radomski M. W., Goodwin D. A., Moncada S. An ultrastructural study of stored human platelets after washing using prostacyclin. Br J Haematol. 1985 Jun;60(2):305–314. doi: 10.1111/j.1365-2141.1985.tb07416.x. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Barclay L. R., Locke S. J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987 Jun 22;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]




