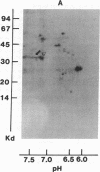
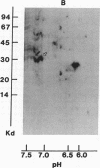
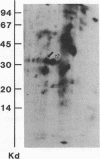
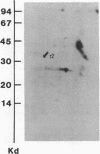
Abstract
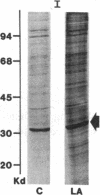
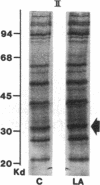
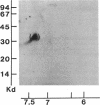
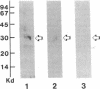
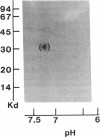
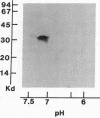
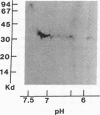
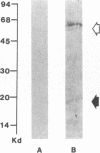
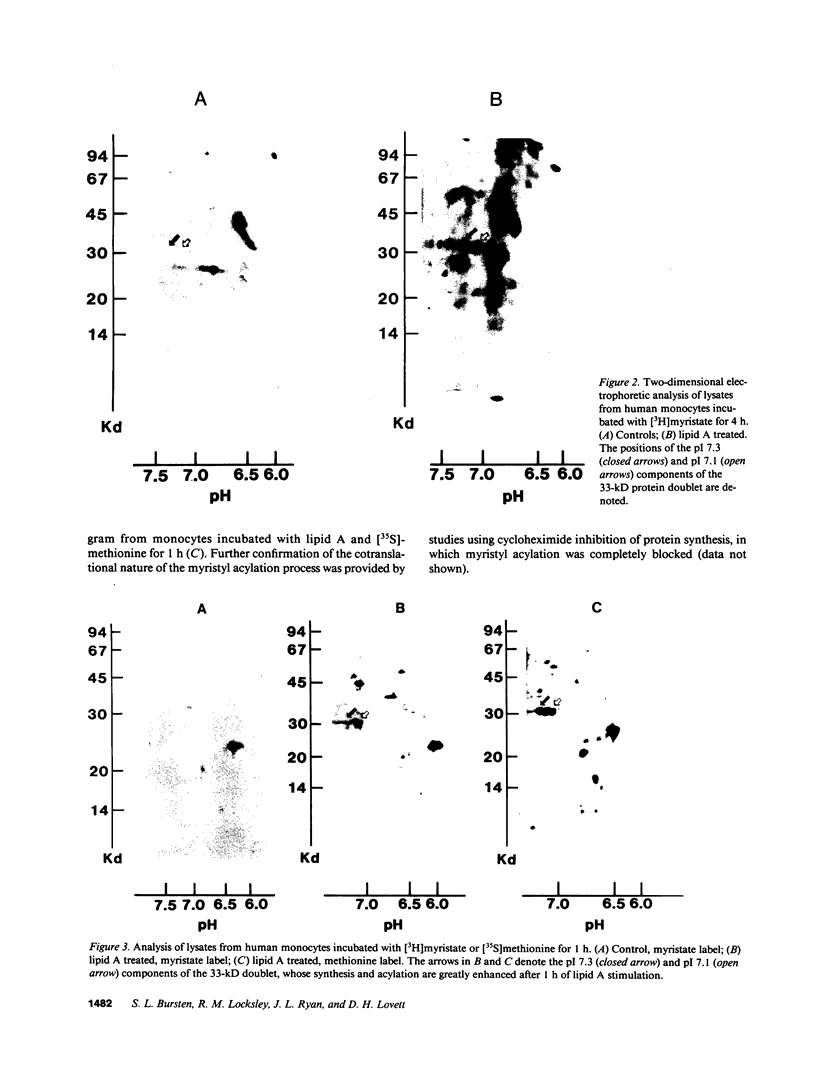
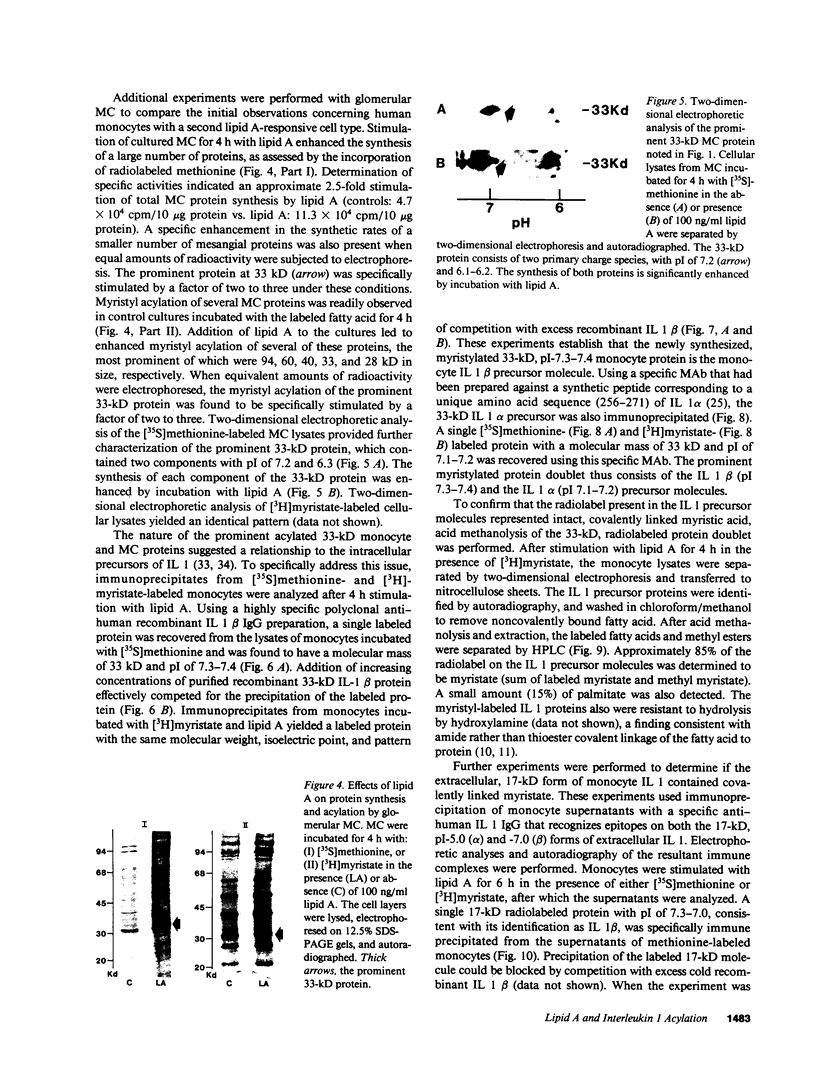
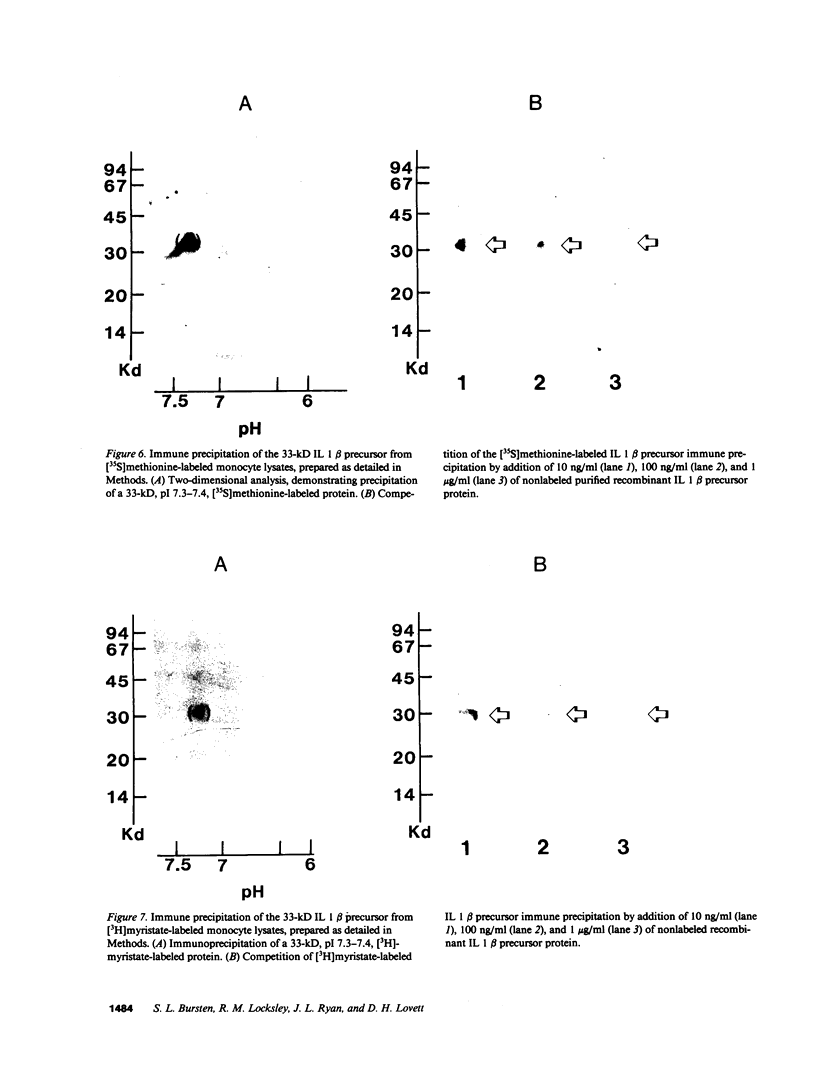
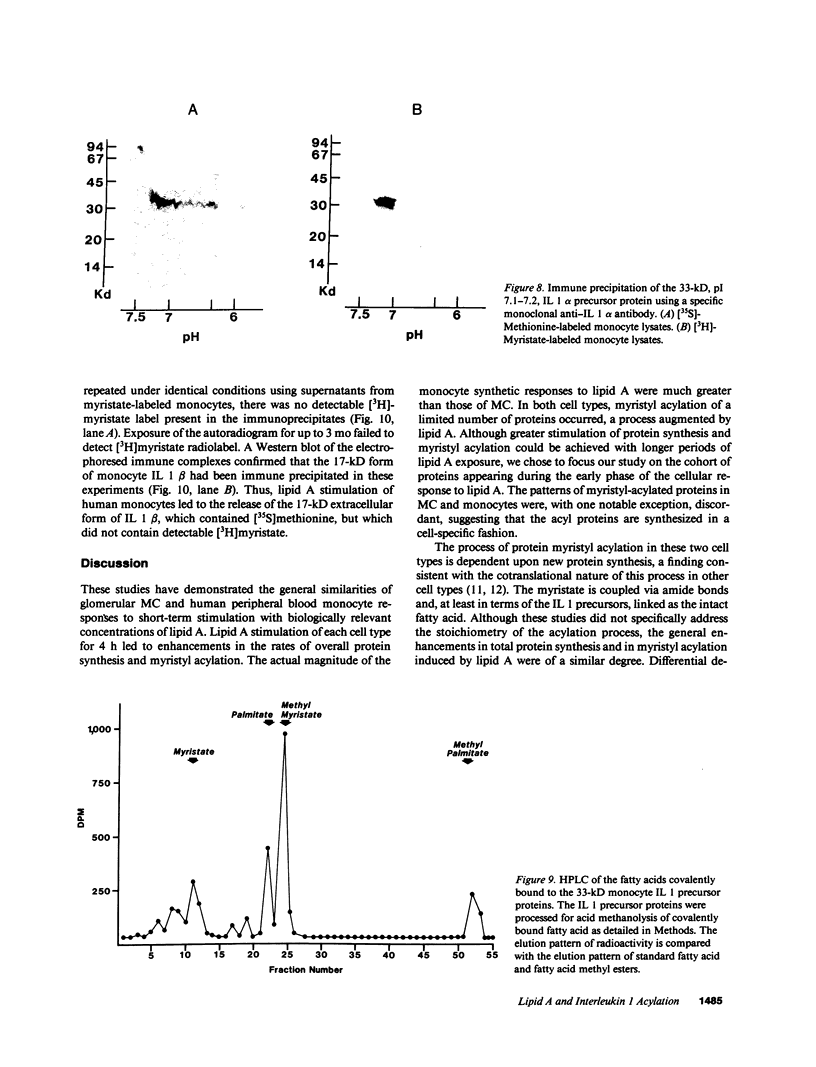
Acylation of cellular proteins with the fatty acids myristate or palmitate represents an important mechanism for the co- or posttranslational modification of proteins. Lipid A, the biologically active component of bacterial endotoxin, exerts a number of biochemical effects on responsive cell types. Evidence is presented that lipid A stimulates the synthesis and subsequent myristyl acylation of intracellular monocyte and glomerular mesangial cell proteins. Two of the myristylated monocyte proteins were identified by specific immunoprecipitation as the 33-kD IL 1 alpha and beta precursors; a similar myristylated protein was found in mesangial cells. The 17-kD secretory form of monocyte IL 1 beta did not contain covalently linked myristate. Myristyl acylation of the IL 1 precursor proteins may facilitate the processing or membrane localization of these proteins, which lack characteristic hydrophobic signal sequences. The acylated 33-kD IL 1 alpha may remain preferentially associated with the membrane in an active form, whereas limited proteolysis may convert the biologically inactive IL 1 beta precursor into the extracellular, nonacylated, active 17-kD protein.
Full text
PDF

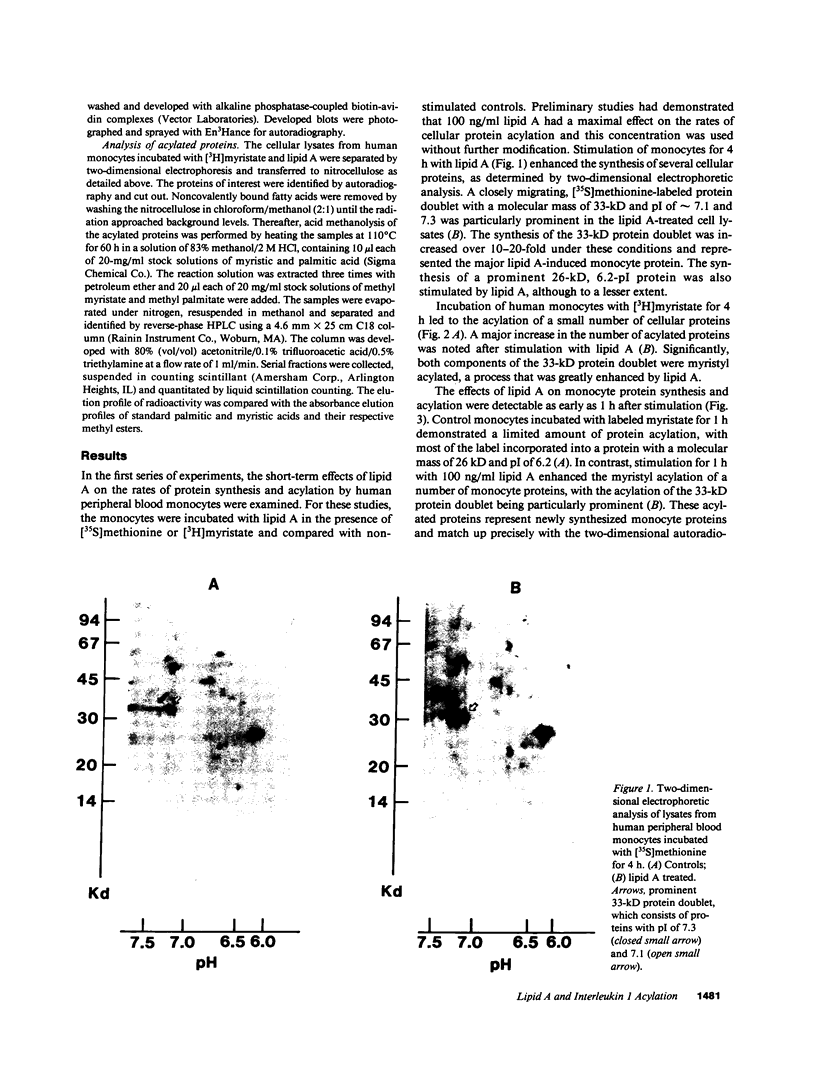



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Keum M. M., Pure E., Cohn Z. A. Bacterial lipopolysaccharides, phorbol myristate acetate, and zymosan induce the myristoylation of specific macrophage proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5817–5821. doi: 10.1073/pnas.83.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron P. E., Warner S. J., Webb A. C., Cannon J. G., Bernheim H. A., McAdam K. J., Rosenwasser L. J., LoPreste G., Mucci S. F., Dinarello C. A. Studies on the molecular nature of human interleukin 1. J Immunol. 1987 Mar 1;138(5):1447–1456. [PubMed] [Google Scholar]
- Bakouche O., Brown D. C., Lachman L. B. Liposomes expressing IL 1 biological activity. J Immunol. 1987 Jun 15;138(12):4256–4262. [PubMed] [Google Scholar]
- Bakouche O., Brown D. C., Lachman L. B. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J Immunol. 1987 Jun 15;138(12):4249–4255. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Conlon P. J., Grabstein K. H., Alpert A., Prickett K. S., Hopp T. P., Gillis S. Localization of human mononuclear cell interleukin 1. J Immunol. 1987 Jul 1;139(1):98–102. [PubMed] [Google Scholar]
- Giri J. G., Lomedico P. T., Mizel S. B. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985 Jan;134(1):343–349. [PubMed] [Google Scholar]
- Goldsmith M. R., Rattner E. C., Koehler M. M., Balikov S. R., Bock S. C. Two-dimensional electrophoresis of small-molecular-weight proteins. Anal Biochem. 1979 Oct 15;99(1):33–40. doi: 10.1016/0003-2697(79)90041-1. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Collier E., Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987 Jan 25;262(3):954–957. [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Introna M., Hamilton T. A., Kaufman R. E., Adams D. O., Bast R. C., Jr Treatment of murine peritoneal macrophages with bacterial lipopolysaccharide alters expression of c-fos and c-myc oncogenes. J Immunol. 1986 Oct 15;137(8):2711–2715. [PubMed] [Google Scholar]
- Kaufman J. F., Krangel M. S., Strominger J. L. Cysteines in the transmembrane region of major histocompatibility complex antigens are fatty acylated via thioester bonds. J Biol Chem. 1984 Jun 10;259(11):7230–7238. [PubMed] [Google Scholar]
- Kobayashi Y., Appella E., Yamada M., Copeland T. D., Oppenheim J. J., Matsushima K. Phosphorylation of intracellular precursors of human IL-1. J Immunol. 1988 Apr 1;140(7):2279–2287. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Largen M. T., Tannenbaum C. S. LPS regulation of specific protein synthesis in murine peritoneal macrophages. J Immunol. 1986 Feb 1;136(3):988–993. [PubMed] [Google Scholar]
- Lovett D. H., Haensch G. M., Goppelt M., Resch K., Gemsa D. Activation of glomerular mesangial cells by the terminal membrane attack complex of complement. J Immunol. 1987 Apr 15;138(8):2473–2480. [PubMed] [Google Scholar]
- Lovett D. H., Larsen A. Cell cycle-dependent interleukin 1 gene expression by cultured glomerular mesangial cells. J Clin Invest. 1988 Jul;82(1):115–122. doi: 10.1172/JCI113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Kashgarian M., Ryan J. L. Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int. 1983 Feb;23(2):342–349. doi: 10.1038/ki.1983.25. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- Magee A. I., Schlesinger M. J. Fatty acid acylation of eucaryotic cell membrane proteins. Biochim Biophys Acta. 1982 Nov 30;694(3):279–289. doi: 10.1016/0304-4157(82)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Fatty acylation of cellular proteins. Temporal and subcellular differences between palmitate and myristate acylation. J Biol Chem. 1986 Feb 15;261(5):2458–2466. [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987 May;61(5):1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Myristoylation and the post-translational acquisition of hydrophobicity by the membrane immunoglobulin heavy-chain polypeptide in B lymphocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7654–7658. doi: 10.1073/pnas.84.21.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Hall G. L., Limjuco G., Chin J., Schmidt J. A. Interleukin 1 beta is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988 Feb 1;167(2):389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Weiel J. E., Hamilton T. A., Adams D. O. LPS induces altered phosphate labeling of proteins in murine peritoneal macrophages. J Immunol. 1986 Apr 15;136(8):3012–3018. [PubMed] [Google Scholar]
- Wilcox C., Hu J. S., Olson E. N. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987 Nov 27;238(4831):1275–1278. doi: 10.1126/science.3685978. [DOI] [PubMed] [Google Scholar]