Abstract
Background
Renal structural alterations have been partially uncovered in cardiorenal syndrome (CRS). Patients with CRS may have evidence of tubular damage, but markers of glomerular damage other than proteinuria have not been thoroughly investigated. The nature of renal damage in CRS may have therapeutic implications, as glomerular damage requires tight blood pressure control and renin-angiotensin-aldosterone system (RAAS) inhibition. The present investigation evaluates patients with CRS type 2 (CRS-2) for direct evidence of glomerular damage as evidenced by the presence of urinary podocin.
Methods
The presence of glomerular damage was assessed in acutely decompensated patients with CRS-2 and healthy controls. Urinary podocin was determined by quantification of a tryptic peptide of podocin with high-performance liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Morning urine samples were collected for podocin, creatinine (Cr), and protein. Urinary podocin was expressed in femtomoles of podocin/milligram of Cr.
Results
The urinary podocin/Cr ratio was greater in patients than in controls (0.37 ± 0.77 vs. 0.06 ± 0.05 fmol podocin/mg Cr, p = 0.04). A total of 40% of the patients had a urinary podocin/Cr ratio greater than the upper limit of normal (>0.2 fmol podocin/mg Cr). Patients with an elevated podocin/Cr ratio were more likely to have received ≤50% of the maximum dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (p = 0.04) than patients with a podocin/Cr ratio in the normal range.
Conclusions
CRS-2 may be associated with glomerular damage as evidenced by an elevated urinary podocin/Cr ratio. Modulators of RAAS may have a protective effect on urinary podocin loss.
Key Words: Cardiorenal syndrome, Podocyturia, Proteinuria
Introduction
The renal structural alterations that underlie cardiorenal syndrome (CRS) [1] have been partially uncovered [2,3,4,5]. Owing to near-hypoxic conditions in tubular cells of the medulla, patients with CRS may present with tubular damage as evidenced by elevated levels of neutrophil gelatinase-associated lipocalin (NGAL) [6,7,8,9].
Proteinuria suggests the presence of glomerular damage and is prevalent in patients with CRS [10]. However, faulty tubular reabsorption rather than glomerular injury may be responsible for proteinuria [11,12]. To our knowledge, patients with CRS have not been studied for the presence of glomerular damage other than proteinuria. In contrast to proteinuria, podocyte urinary excretion reliably ascertains the presence of active glomerular injury in patients with nephropathy and preeclampsia [13,14,15].
CRS type 2 (CRS-2) refers to clinical conditions where chronic abnormalities of cardiac function cause progressive kidney disease. It has been postulated that multiple episodes of acute cardiac decompensation result in the onset followed by the progression of chronic kidney disease in these patients [16]. Several pathophysiological triggers may play a role, including the upregulation of the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS) as well as a chronic inflammatory state. The end result is renal structural injuries, both of the glomeruli (glomerulosclerosis) and the tubuli and interstitium (tubulointerstitial fibrosis). Abnormalities of novel markers of tubular damage, including NGAL, have been documented in several clinical studies [6,7,8,9,16]. The presence of podocyte injury was confirmed in a model of chronic cardiac volume overload (model of aortic regurgitation); 6 months after undergoing a procedure that resulted in aortic regurgitation, Sprague-Dawley rats developed albuminuria and glomerular podocyte injury, as demonstrated by decreases in glomerular nephrin and podocin mRNA levels [17]. However, the effects of episodes of cardiac decompensation on podocyte injury, as the possible mechanism of glomerular injury in CRS-2 patients, have not yet been studied. In addition, while the pivotal role of RAAS activation (both through underfilling of the renal arteries and renal venous hypertension) has been well documented in animal models of heart failure and CRS-2, large congestive heart failure clinical trials of RAAS-modifying therapies have focused on cardiovascular outcomes and have reported limited data with respect to renal prognosis [16]. Consequently, the roles of angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) in the prevention/slowing of the progression of renal/podocyte injury in CRS-2 remain to be determined.
The aim of the present investigation was to assess patients with CRS-2 for the presence of glomerular damage during an episode of acute cardiac decompensation, owing to its temporal association with increased urinary albumin excretion [18]. The presence of glomerular damage was assessed by quantification of urinary podocyte-specific peptides by liquid chromatography (LC) coupled to tandem mass spectrometry (LC-MS/MS). In addition, we sought to investigate the association between the podocyte damage on one side and clinical parameters and therapeutic strategies on the other.
Methods
Study Population
The study population included 2 cohorts. The first cohort was comprised of 8 healthy subjects. They served as controls for urinary podocin excretion and reproducibility of the urinary podocin excretion measurement. The second cohort consisted of 27 patients with CRS-2. The recruitment of the healthy subjects and patients was carried out in parallel.
Controls
Healthy subjects were recruited from the Tulane faculty and had normal renal function, an estimated glomerular filtration rate (eGFR) >90 ml/min/1.73 m2, as measured by the 4-variable modification of diet on renal disease (MDRD) study equation [19], and had no current or past medical history of glomerular disease and/or hypertension. They were recruited by direct contact, without advertisement/posters or financial remuneration. The healthy subjects were matched for age and sex to patients with CRS-2.
Patients
Patients with CRS-2 were prospectively recruited from a pool of 230 patients with left ventricular (LV) ejection fractions <35% who were receiving treatment for heart failure exclusively at Tulane University for >6 months. All patients had been thoroughly evaluated, including right and left heart catheterization with coronary angiography, and were adherent to medical therapy as defined by the current American College of Cardiology/American Heart Association and Heart Failure Society of America guidelines. Patients who experienced an episode of symptomatic deterioration were screened for the study. The inclusion criteria were: (1) hospitalization for symptomatic decompensation, as evidenced by worsening of shortness of breath, fatigue, or both; clinical evidence of pulmonary and peripheral fluid retention, as confirmed by chest X-ray findings, and an elevated brain natriuretic peptide (BNP) level >200 pg/ml or N-terminal prohormone brain natriuretic peptide (NT-proBNP) level >1,200 pg/ml; (2) no known history or evidence of chronic kidney disease prior to the diagnosis of heart failure; (3) an eGFR ≤60 ml/min/1.73 m2 at the time of hospitalization, and (4) a well-documented history of adherence to a prescribed medical regimen. The exclusion criteria were: (1) a systolic blood pressure >180 mm Hg on admission; (2) poorly controlled type II diabetes mellitus, as evidenced by a HgA1c >7%; (3) active myocardial ischemia, with troponin T >3 times the upper limit of normal values; (4) proteinuria that preceded heart failure onset; (5) urinary tract infection, with urine positive for nitrite or leukocyte esterase; (6) renal transplantation or renal replacement therapy; (7) atrial fibrillation with rapid ventricular response, as indicated by a heart rate >115 beats/min; (8) pregnancy or preeclampsia, and (9) active tobacco use. Routine laboratory data were obtained as clinically indicated. Creatinine (Cr) was measured by standard assay and eGFR was derived using the 4-variable MDRD study equation.
Ethics Statement
The study was approved by the Tulane University and Mayo Clinic Institutional Review Boards. Due to the low risk nature of the study, all eligible patients, except for one, agreed to participate. All patients and healthy subjects signed a written informed consent form.
Urine Collection and Proteinuria Determination
Early-morning urine specimens (100 ml) were collected in sterile cups from patients during the first 12 h of hospitalization only. Urine specimens were collected after patients had received intravenous furosemide at a dose ranging from 40 to 120 mg and prior to the daily administration of ACEI, ARB, or aldosterone receptor inhibitors (ARA). In healthy subjects, early-morning urine specimens were collected on 2 occasions 12-14 days apart.
The concentrations of total urinary protein and Cr were measured using standard methods on a Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis, Ind., USA). A protein/Cr ratio ≥0.15 g of protein per 1 g of Cr in spot urine specimens (or an estimated 24-hour proteinuria of 150 mg) was considered as significant clinical proteinuria. Urine specimens were then centrifuged for 10 min at 1,200 rpm. The supernatants were discarded. Twenty milliliters of methanol was added to each of the urine pellets, and the pellets were then centrifuged for another 10 min at 1,200 rpm. The supernatants were again discarded, and the pellets were frozen at −70°C. The specimens were packaged in dry ice and then shipped to the Mayo Clinic in Rochester, Minn., USA, for quantification of urinary podocin.
Urinary Podocin
The quantification of urinary podocin was based on the detection of a podocin tryptic peptide by high-performance LC coupled to LC-MS/MS. This methodology has been validated against the gold standard for the detection of viable urinary podocytes, which is based on the overnight culture of urinary sediment and staining for podocyte-specific proteins [20]. The LC-MS/MS methodology is based on the principle that tryptic peptides may serve as surrogate markers for the quantification of their respective proteins [21]. Each of the aliquots of the urine specimens (100 ml) was processed as previously described [20]. The LC-MS/MS technology was performed as follows: recombinant human podocin was obtained from Novus Biologicals and used for determining the proteotypic peptide to be used for the quantification of podocin. The recombinant human podocin was digested with trypsin and analyzed by LC-MS/MS on an AB SCIEX 5600 Q-TOF mass spectrometer operated in IDA mode. MS/MS spectra were searched against the human subset of the SwissProt protein database using ProteinPilot software. The peptide, QEAGPEPSGSGR, was identified as having the highest response of all podocin tryptic peptides and was therefore selected as the proteotypic peptide for quantification.
A synthetic isotope-labeled peptide with the proteotypic sequence, QEAGPEPSGSGR, was synthesized by the peptide synthesis facility at the Mayo Clinic. The synthetic peptide contains two stable isotope-labeled prolines at positions 4 and 6, with each proline containing three 13C and three 15N, for a total mass shift of 12 Da above the molecular mass of the native tryptic peptide. Total nitrogen analysis of the purified product showed that the peptide content was 0.86 g/1.0 g of material by weight. Selected reaction monitoring transitions used for quantification were determined by infusing the stable isotope-labeled peptide at a flow rate of 10 µl/min into the Turbo V ion source of an AB SCIEX API 5000 triple quadrupole mass spectrometer run in positive ESI mode. The transition representing the doubly charged peptide precursor ion with an m/z of 586.60 for the unlabeled peptide, and of 592.60 for the labeled peptide, to the singly charged y6 fragment ion with an m/z of 560.35 for the unlabeled peptide, and of 566.35 for the labeled peptide, was used for quantification. A secondary multiple reaction monitoring transition representing the doubly charged peptide precursor ion to the y10 ion was also monitored to confirm that the ion ratios between the unlabeled peptide in the patient samples and the stable isotope-labeled internal standard peptide remained constant.
Cellular material present in the urine was isolated by centrifugation and then fixed with methanol. The fixed cellular material was then solubilized with a detergent, followed by digestion with trypsin. The proteotypic peptide, QEAGPEPSGSGR, was monitored by selected reaction monitoring and quantified using a single-point isotope dilution experiment. The stable isotope-labeled peptide was spiked into each sample at a known concentration, and the molar ratio of the response from the native peptide in the patient urine to the stable isotope-labeled internal standard peptide was used to determine the concentration of podocin in the pellet. Prior to digestion, the methanol-fixed pellets were resuspended in methanol fixative and then centrifuged at 600 g for 10 min. The supernatant was removed, and the pellet was re-suspended in 50 μl RapidGest™ SF detergent at a concentration of 0.1% in 50 mM ammonium bicarbonate, pH 8.0. The sample was sonicated for 5 min; then, 100 μg of trypsin was added, and the sample was sonicated for another 5 min. The sample was then digested in a shaking incubator at 37°C for 4 h. After digestion, the sample was acidified with 2 μl formic acid and centrifuged for 10 min at 14,000 g. A volume of 18 μl patient digest was put into a well of a 96-well sample tray. A stable isotope-labeled internal standard peptide was added to each sample and then analyzed by LC-MS/MS.
All samples were analyzed using a Thermo TLX-2 HPLC system coupled to an AB SCIEX API 5000 triple quadrupole mass spectrometer. A 20-µl injection was made from each sample, and separations were carried out on a 100 × 3.0 mm Atlantis T3 column, with a 3-µm particle size and a 120-Å pore size, run at a flow rate of 250 µl/min. A gradient consisting of mobile phase A (100% water and 0.1% formic acid) and mobile phase B (100% acetonitrile and 0.1% formic acid) was used to resolve the peptides with a 15-min gradient.
The amount of urinary podocin in the early-morning urine specimens from patients with CRS-2 and from healthy subjects was expressed as the ratio of urinary podocin (fmol) to urinary Cr (mg). Analyst™ software version 1.4.2 (Applied Biosystems/Life Technologies, Grand Island, N.Y., USA) was used to acquire and process the data.
Statistical Analysis
Data were expressed as means ± SD. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, N.C., USA). The baseline characteristics of the patients with elevated podocin/Cr ratios and of those with normal-range podocin/Cr ratios were compared using the Student t test. A two-sided p value <0.05 was considered statistically significant. Pearson's correlation was used to determine the strength of the relationship between the urinary podocin/Cr and eGFR, urine protein/Cr ratio, and the presence of diabetes mellitus, with each evaluated as a separate variable.
Results
The healthy cohort consisted of 8 subjects (5 men and 3 women) with an average age of 55 ± 9 years. The average urinary podocin/Cr ratio was 0.06 ± 0.05 fmol/mg in the healthy subjects (range 0.011-0.187). Measurements of the podocin/Cr ratio were repeated 12-14 days apart and were reproducible, with a coefficient of correlation of 0.73 (p = 0.02).
The CRS-2 cohort consisted of 27 patients (15 men and 12 women). Nineteen of these (70%) were African-Americans, and the remaining 8 subjects were Caucasians. The clinical characteristics of the CRS-2 cohort are summarized in table 1. The average age was 57 ± 11 years. In 24 of the 27 patients, the baseline functional class before decompensation was compatible with New York Heart Association (NYHA) functional class III. The remaining 3 patients were in NYHA functional class II. The etiology of the LV systolic dysfunction was coronary artery disease in 8 patients. A history of hypertension was present in 21 patients (77%), and 10 patients (37%) were treated for type II diabetes mellitus. The mean eGFR was 47 ± 13 ml/min/1.73 m2. Fifteen of the 27 patients had proteinuria, as evidenced by a urinary protein/Cr ratio ≥0.15.
Table 1.
Baseline characteristics (n = 27)
| Age, years | 57 ± 11 |
| Male gender | 15 (55) |
| White/African-American | 8 (30)/19 (70) |
| LV ejection fraction | 18 ± 5 |
| NYHA functional class (II/III) | 3/24 |
| Nonischemic/ischemic etiology | 19 (70)/8 (30) |
| Hypertension | 21 (77) |
| Diabetes mellitus | 10 (37) |
| Atrial fibrillation | 10 (37) |
| Obstructive sleep apnea | 3 (11) |
| Systolic blood pressure, mm Hg | 112 ± 17 |
| Heart rate, bpm | 88 ± 12 |
| BMI | 30 ± 5 |
| eGFR, ml/min/1.73 m2 | 47 ± 13 |
| Serum Cr, mg/dl | 1.5 ± 0.6 |
| Proteinuria | 15 (55) |
| β-Blockers | 27 (100) |
| ACEI | 20 (74) |
| ARB | 6 (22) |
| ARA | 7 (26) |
| ICD | 19 (70) |
Values are numbers of patients, means ± SD, or n (%).
BMI = Body mass index (calculated as weight in kilograms divided by height in meters squared); ICD = implantable cardioverter defibrillator.
The mean urinary podocin/Cr ratio was significantly greater in the patients with CRS-2 than in the age- and sex-matched healthy subjects (0.37 ± 0.77 vs. 0.06 ± 0.05 fmol podocin/mg Cr, p = 0.04) (fig. 1). Of the 27 patients with CRS-2, 11 (40%) had urinary podocin/Cr ratios that were outside the range of those from the healthy subjects. In these 11 patients, the urinary podocin/Cr ratio averaged 0.84 fmol podocin/mg Cr (range 0.28-3.77). In the remaining 16 patients, the urinary podocin/Cr ratio averaged 0.05 fmol podocin/mg Cr (range 0.001-0.19).
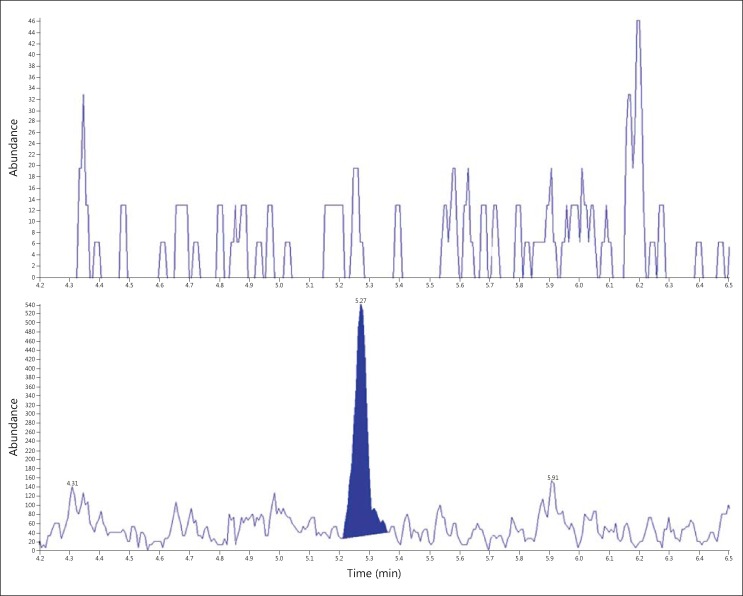
Fig. 1.
LC-MS/MS chromatograms of fixed cells from an age- and sex-matched control (top) and a congestive heart failure patient (bottom), with a calculated tryptic peptide concentration of 0.002 vs. 1.459 fmol podocin/mg Cr, respectively.
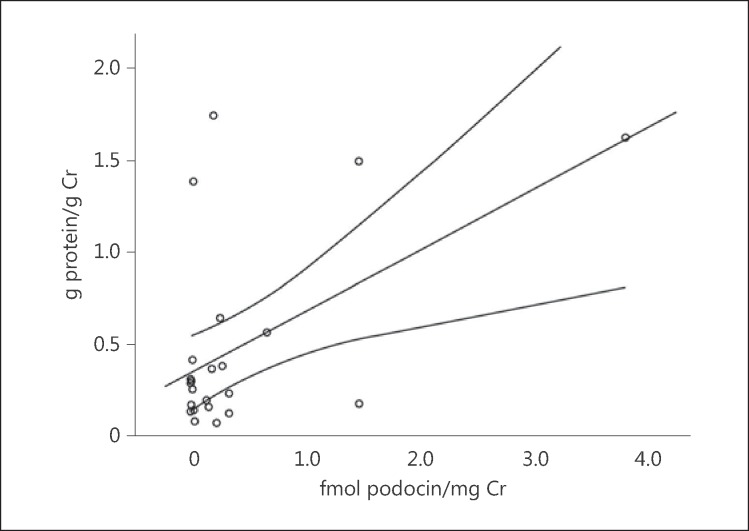
The clinical characteristics of the CRS-2 patients with elevated and normal urinary podocin/Cr ratios are detailed in table 2. Of the 11 patients with elevated urinary podocin/Cr ratios, 6 had proteinuria and 5 did not. Of the 10 patients with diabetes, 3 had an elevated urinary podocin/Cr ratio and 7 had a urinary podocin/Cr ratio in the normal range. CRS-2 patients with elevated podocin/Cr ratios were less likely to have received double therapy (p = 0.05), defined as treatment including at least 2 of the following: ACEI, ARB, and/or ARA; these patients were also more likely to have received ≤50% of the maximum ACEI or ARB dose (p = 0.04). In patients with both elevated urinary podocin/Cr ratios and proteinuria, these two parameters were positively correlated (r = 0.540, p = 0.007), thus suggesting a positive mechanistic link (fig. 2).
Table 2.
Clinical characteristics of the CRS-2 patients with elevated and normal-range urinary podocin/Cr ratios
| Elevated podocin/Cr ratio (n = 11) | Normal-range podocin/Cr ratio (n = 16) | p value | |
|---|---|---|---|
| Characteristics | |||
| Age, years | 58 ± 14 | 57 ± 9 | 0.80 |
| Male | 6 (55) | 9 (56) | 0.93 |
| White/African-American | 5 (45)/6 (55) | 3 (16)/13 (84) | 0.07 |
| Medical history | |||
| Diabetes mellitus | 3 (27) | 7 (43) | 0.40 |
| Hypertension | 9 (81) | 12 (81) | 0.68 |
| Atrial fibrillation | 4 (36) | 6 (37) | 0.95 |
| Ischemic etiology | 3 (27) | 5 (31) | 0.94 |
| Cr, mg/dl | 1.37 ± 0.4 | 1.59 ± 0.67 | 0.34 |
| Proteinuria, % | 54 | 56 | 0.71 |
| eGFR, ml/min/1.73 m2 | 48 ± 9 | 46 ± 17 | 0.75 |
| Treatment | |||
| β-Adrenergic blockade | 11 (100) | 16 (100) | |
| ACEI | 8 (72) | 12 (75) | 0.90 |
| ARB | 1 (9) | 5 (31) | 0.17 |
| ARA | 1 (9) | 6 (37) | 0.10 |
| Double therapy1 | 1 (9) | 7 (44) | 0.05 |
| Double therapy with either ACEI or ARB at ≥50% of the max. dose | 0 (0) | 5 (31) | 0.04 |
| ICD | 8 (72) | 11 (68) | 0.82 |
Values are means ± SD or n (%), unless otherwise indicated.
ICD = Implantable cardioverter defibrillator.
Combined therapy consisting of at least two of the following: ACEI, ARA, or ARB.
Fig. 2.
Correlation with 95% confidence interval between urinary podocin/Cr ratio and proteinuria in patients with CRS-2 (r = 0.540, p = 0.007). Patients with advanced nephrotic range proteinuria (proteinuria ≥3.5 g/24 h) were excluded (n = 2), as advanced proteinuria may be a marker of global podocyte depletion.
Discussion
The present investigation provides the first direct evidence of podocyte damage in acutely decompensated patients with CRS-2. Of note, active glomerular damage is commonly demonstrated by the presence of viable podocytes in urinary sediments [22]. We inferred the presence of glomerular damage from elevation in the urinary podocin/Cr ratio as measured by LC-MS/MS technology. Podocin, an abundant protein in the body of podocytes, is a known reliable marker of urinary podocyte loss [20]. We have previously shown that the quantification of urinary podocin – based on the detection of a podocin tryptic peptide – correlates with the gold standard for the detection of viable urinary podocytes, i.e. the overnight culture of urinary sediment and staining for podocyte-specific proteins [20]. Therefore, based on the assumption that the podocin tryptic peptide originates from podocytes present in the urine, we used the low-speed spin to isolate as many cellular components as possible. In total, 40% of the patients had an elevated urinary podocin/Cr ratio. Podocin, a podocyte-specific protein, was quantified with the LC-MS/MS technology, which is operator independent and highly reproducible. In addition, the modulators of RAAS demonstrated a protective effect on urinary podocin loss.
Uncovering glomerular damage may have implications for the treatment of CRS. Lowering intrarenal pressure, preferably through medications that downregulate the RAAS, such as ACEI, ARB, and ARA, may reduce podocyte injury and glomerular damage in the juxtamedullary nephrons [23,24,25]. However, lowering intrarenal pressure may aggravate tubular damage in the superficial nephrons [9,24]. Thus, an improved understanding of glomerular damage may have significant therapeutic implications in CRS.
This preliminary study enrolled only patients with CRS-2 in whom abnormal cardiac function resulted in chronic renal disease and focused on studying podocyte injury during acute cardiac decompensation. The role of podocyte injury as the mechanism of glomerular injury has been well documented in several animal models of renal disease, but not in human diseases, including CRS. Rat models of transient and continuous glomerular injury have shown that urinary podocyte loss is a widespread phenomenon in glomerular diseases [26]. A mouse model of selective podocyte depletion using diphtheria toxin, in which a single episode of podocyte injury resulted in glomerular destabilization and persistent podocyte loss, suggested that changes in the structure of the kidney at the level of the podocyte barrier continue following the resolution of an acute injury [27], ultimately progressing to podocyte depletion, interstitial scarring, and loss of renal function. Intervention with angiotensin II blockade (combined enalapril and losartan) re-stabilized glomeruli and prevented both continuous podocyte loss and deterioration of renal function [3]. In the present study, we demonstrate novel evidence for the presence of urinary podocin loss in patients with CRS-2 and further establish the protective effect of RAAS blockade. The presence of continuous podocyte loss in CRS and its role in the development and progression of renal disease need to be further investigated. In addition, the protective role of RAAS inhibition with respect to proteinuria has been well demonstrated, while its role with respect to continuous podocyte loss remains to be determined. Our preliminary data indicating a positive correlation between urinary podocin loss and the degree of proteinuria suggest that these are mechanistically related. However, two observations are worthy of further discussion. First is the absence of urinary podocin in patients with established proteinuria. This is consistent with data in the literature suggesting that podocyturia, unlike proteinuria, is limited to phases of ongoing glomerular injury [14] and, as such, may be absent in patients with established chronic proteinuria. Second, based on our recent study indicating that podocyturia predates clinical proteinuria in preeclampsia, patients without proteinuria, but with documented urinary podocin loss may have early subclinical renal injury [22]. Whether urinary podocin loss predates proteinuria in CRS-2 is the subject of our current investigation.
The patients who comprised the study population were carefully selected on the basis of adherence to their medical regimens, an eGFR ≤60 ml/min/1.73 m2, and demonstrated cardiac decompensation without overt precipitating factors. The 40% prevalence of glomerular damage observed in the present study may overestimate the true prevalence of glomerular damage in less highly selected patients with CRS-2, where nonadherence to medications and diet, as well as deterioration in comorbid conditions such as myocardial ischemia, ventricular/supraventricular arrhythmias, and pulmonary/urinary infections, may account for symptomatic decompensation. Determination of the true prevalence of glomerular damage in CRS-2 awaits larger studies in more heterogeneous populations.
As expected, the prevalence of proteinuria was greater in our acutely decompensated patients than that previously reported in stable chronic heart failure patients [28]. During an episode of acute decompensation, increased glomerular pressure may cause proteinuria. In a recent observational study, patients with acute decompensated heart failure were found to have increased urinary albumin excretion that resolved or decreased significantly with standard heart failure treatment [18]. Although diabetes is known to play an important role in the pathogenesis of many glomerular diseases, glomerular damage was not closely related to the presence of diabetes in our patients.
Our study is limited by the low numbers of both controls and patients and by its cross-sectional design. In addition, a single tryptic peptide was used for the identification of podocin. This quantitative measure would be improved by the identification and measurement of an additional tryptic peptide. Additional peptides were analyzed and confirmed using recombinant podocin. However, each of the peptides analyzed had a response factor that was too low to be used for quantification. Despite these limitations, our study provides preliminary results and proof-of-principle that podocyte injury is present in a subset of patients with CRS-2. It sets the stage for future studies aimed at establishing the normal ranges for unaffected individuals across sexes and life span, as well as the temporal relationship between podocyturia and proteinuria and the role of the former as a more sensitive and earlier marker of renal injury in CRS-2. The present investigation did not attempt to elucidate the mechanisms that mediate urinary podocyte loss in CRS-2. Podocytes are highly specialized cells with complex molecular structures that maintain the integrity of the glomerulus. Angiotensin II regulates podocyte number and integrity [23] and may promote podocyte detachment and shedding, as it increases the intraglomerular pressure by preferentially affecting glomerular efferent arterioles [24]. Lowering blood pressure with full RAAS inhibition may lessen glomerular damage in patients with CRS [25]. However, lowering blood pressure may aggravate renal impairment in patients with CRS and exclusive or predominant tubular damage [9]. A positive correlation was observed between urinary podocin/Cr ratios and proteinuria, suggesting a positive mechanistic link. It is plausible that in patients without proteinuria, elevated urinary podocin/Cr ratios may represent an early subclinical marker of renal injury. Our study sets the stage for future research aiming to identify the nature, extent, and progression of structural renal alterations in CRS that may allow for optimal treatment of these patients.
Conclusion
In addition to previously reported tubular damage, CRS-2 may be associated with glomerular damage. The presence of glomerular damage has therapeutic implications, as patients with glomerular damage may benefit from more stringent control of blood pressure and more complete RAAS inhibition than that routinely achieved.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Liang KV, Williams AW, Greene EL, Redfield MM. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med. 2008;36:S75–S88. doi: 10.1097/01.CCM.0000296270.41256.5C. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40–55. doi: 10.1038/ki.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol. 2009;4:2013–2026. doi: 10.2215/CJN.03150509. [DOI] [PubMed] [Google Scholar]
- 5.Tang WH, Mullens W. Cardiorenal syndrome in decompensated heart failure. Heart. 2010;96:255–260. doi: 10.1136/hrt.2009.166256. [DOI] [PubMed] [Google Scholar]
- 6.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brezis M, Rosen S. Hypoxia of the renal medulla – its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 8.Shrestha K, Shao Z, Singh D, Dupont M, Tang WH. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol. 2012;110:1329–1335. doi: 10.1016/j.amjcard.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winterberg PD, Lu CY. Acute kidney injury: the beginning of the end of the dark ages. Am J Med Sci. 2012;344:318–325. doi: 10.1097/MAJ.0b013e318228aef8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente MA, Damman K, Dunselman PH, Hillege HL, Voors AA. Urinary proteins in heart failure. Prog Cardiovasc Dis. 2012;55:44–55. doi: 10.1016/j.pcad.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis. 2011;54:144–153. doi: 10.1016/j.pcad.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 13.Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, Rose CH, Gavrilova L, Craigo P, Bailey KR, Achenbach J, Schiffer M, Grande JP. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196:320e1–320e7. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 16.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:117–136. doi: 10.1159/000349968. [DOI] [PubMed] [Google Scholar]
- 17.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–1413. doi: 10.1161/CIRCULATIONAHA.111.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otaki Y, Watanabe T, Shishido T, Takahashi H, Funayama A, Narumi T, Kadowaki S, Hasegawa H, Honda S, Netsu S, Ishino M, Arimoto T, Miyashita T, Miyamoto T, Konta T, Kubota I. The impact of renal tubular damage, as assessed by urinary beta2-microglobulin-creatinine ratio, on cardiac prognosis in patients with chronic heart failure. Circ Heart Fail. 2013;6:662–668. doi: 10.1161/CIRCHEARTFAILURE.112.000089. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Garovic VD, Craici IM, Wagner SJ, White WM, Brost BC, Rose CH, Grande JP, Barnidge DR. Mass spectrometry as a novel method for detection of podocyturia in pre-eclampsia. Nephrol Dial Transplant. 2013;28:1555–1561. doi: 10.1093/ndt/gfs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr JR, Maggio VL, Patterson DG, Jr, Cooper GR, Henderson LO, Turner WE, Smith SJ, Hannon WH, Needham LL, Sampson EJ. Isotope dilution – mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem. 1996;42:1676–1682. [PubMed] [Google Scholar]
- 22.Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH, Grande JP, Garovic VD. Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension. 2013;61:1289–1296. doi: 10.1161/HYPERTENSIONAHA.113.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell KN, Raij L, Mundel P. Role of angiotensin II in the development of nephropathy and podocytopathy of diabetes. Curr Diabetes Rev. 2011;7:3–7. doi: 10.2174/157339911794273973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito S. Cardiorenal syndrome: an evolutionary point of view. Hypertension. 2012;60:589–595. doi: 10.1161/HYPERTENSIONAHA.111.188706. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Bakris GL, Abraham WT, Pitt B, Brook RD. Complete renin-angiotensin-aldosterone system (RAAS) blockade in high-risk patients: recent insights from renin blockade studies. Hypertension. 2013;62:444–449. doi: 10.1161/HYPERTENSIONAHA.113.01504. [DOI] [PubMed] [Google Scholar]
- 26.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol. 2009;20:1041–1052. doi: 10.1681/ASN.2007121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]