Abstract
Small-cell lung cancer (SCLC) is a subgroup of lung cancer with a high frequency of liver metastasis, which is a predictor of poor prognosis. Diffuse liver metastases of SCLC with no visible nodular lesions in the liver when examined using computed tomography (CT) are relatively rare; however, a few cases with rapid progression to acute liver failure that were diagnosed after death have been reported. In this paper, we report a 63-year-old man with diffuse liver metastases of SCLC that were histologically diagnosed using a transjugular liver biopsy while the patient was alive, even though no lesions were visible during a contrast-enhanced CT examination.
Key Words: Diffuse liver metastasis, Acute liver failure, Small-cell lung cancer
Introduction
The liver is one of the most frequent targets of metastasis from primary malignant tumors. Most metastatic liver tumors are diagnosed using imaging techniques such as contrast-enhanced computed tomography (CT); these imaging techniques are often performed to evaluate the stage of malignancy. However, diffuse-type liver metastasis tumors without nodular lesions are considerably rare, even when contrast-enhanced CT examinations are performed; thus, an accurate diagnosis is difficult to obtain prior to death, and some cases may develop acute liver failure or, occasionally, disseminated intravascular coagulation, making a percutaneous liver biopsy difficult to perform. Therefore, most cases are diagnosed histologically as diffuse liver metastasis on a post-mortem basis. Small-cell lung cancer (SCLC) comprises approximately 15% of all lung cancers [1] and is a subgroup of primary lung cancer that is known for its aggressive and rapid growth and early metastasis. SCLC is associated with a poor prognosis and limited treatment options, particularly in cases with liver metastasis [2]. We present the first report of a rare case in whom diffuse liver metastasis of SCLC was diagnosed histologically using a transjugular liver biopsy while the patient was alive, despite the absence of any visible lesions when examined using contrast-enhanced CT. Unfortunately the condition of this patient rapidly progressed to acute liver failure before the indications for chemotherapy could be met.
Case Report
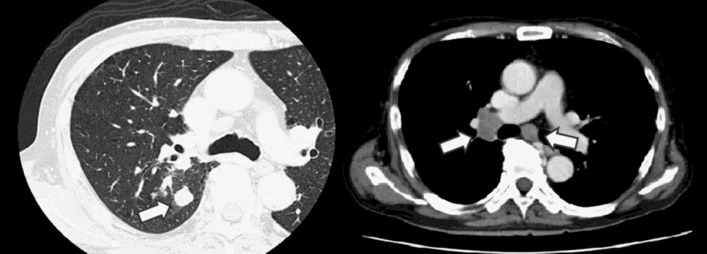
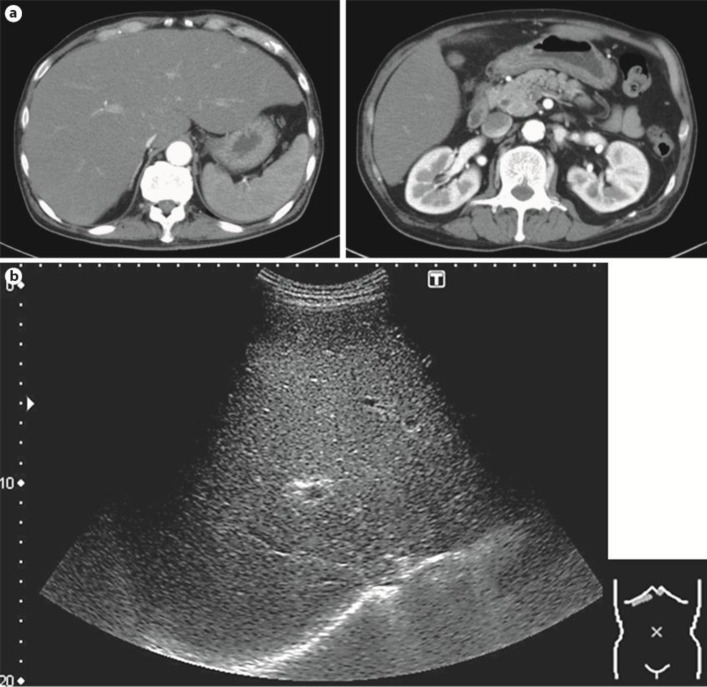
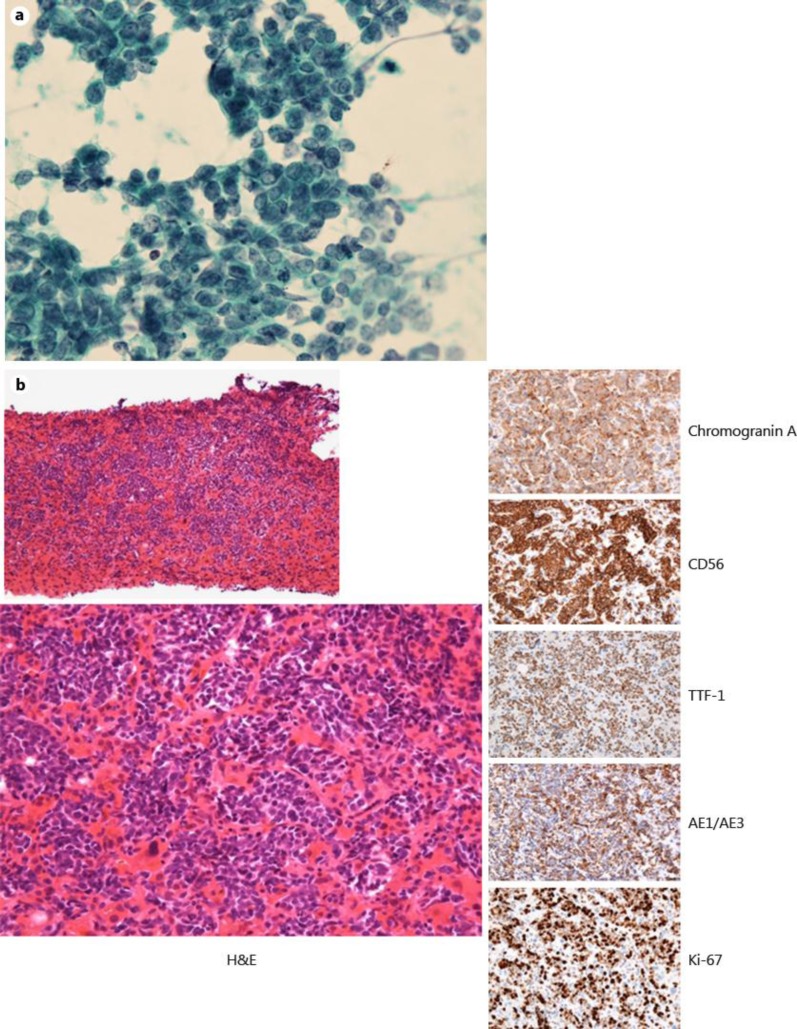
A 63-year-old male was referred to our hospital for further care because of a considerable body weight loss (10 kg over a 1-year period), bloody phlegm for a few months and jaundice for a few days. He had visited another hospital because of hypertension and had never exhibited liver dysfunction during a routine health check-up. He had a past medical history of diabetes mellitus and tonsillitis. He also had a history of heavy smoking and alcohol consumption. While his vital signs upon examination were within the normal range (blood pressure 131/70 mm Hg, pulse rate 86/bpm, body temperature 36.3°C), his physical examination showed significant jaundice and hepatomegaly. His laboratory data revealed elevation of serum liver enzyme levels (aspartate aminotransferase 102 IU/l, alanine aminotransferase 88 IU/l, lactate dehydrogenase 650 IU/l, alkaline phosphatase 723 IU/l, γ-glutamyltransferase 835 IU/l) and jaundice (total bilirubin 9.8 mg/dl, direct bilirubin 7.9 mg/dl). His serum albumin level and platelet counts were decreased (albumin 2.5 g/dl, platelets 4.2 × 104/μl). The prothrombin time was slightly prolonged (74.7%). As for tumor markers, the ProGRP level was prominently increased to 24,000 pg/ml. Contrast-enhanced CT scans revealed a right lung tumor with a size of 15 mm and multiple lymph node metastases, pleural dissemination and a suspected left adrenal metastasis (fig. 1). The liver findings only showed hepatomegaly without any intrahepatic nodular lesions when using contrast-enhanced CT (fig. 2a); diffuse minimal high-echoic nodular shadows were visible during ultrasound examination (fig. 2b). Based on a transbronchial needle aspiration of a mediastinal lymph node, he was diagnosed as having stage IV SCLC (fig. 3a). Further examination to evaluate the cause of the liver dysfunction was needed before determining the chemotherapy options, and a transjugular liver biopsy was performed. A percutaneous transhepatic approach was not feasible because of a bleeding tendency. Histologically, the patient was diagnosed as having diffuse metastatic SCLC in the liver, with positive immunohistological findings for chromogranin A, synaptophysin, CD56, TTF-1 and AE1/AE3 and with a Ki-67 index of 80% (fig. 3b). Thereafter, the patient's general condition and liver failure worsened quite rapidly, and best supportive care was selected. He died 13 days after hospital admission.
Fig. 1.
Contrast-enhanced CT scans revealed a right lung tumor with a size of 15 mm and multiple lymph node metastases (arrows).
Fig. 2.
a Contrast-enhanced CT scans showed only hepatomegaly without any intrahepatic nodular lesions. b An ultrasound sonography examination revealed only diffuse minimal high-echoic nodular shadows.
Fig. 3.
a Cytology from a mediastinal lymph node by transbronchial needle aspiration revealed SCLC (×400). b Histological examination of the liver revealed diffuse liver metastases of SCLC, all of which were positive for chromogranin A, synaptophysin, CD56, TTF-1 and AE1/AE3 and with a Ki-67 index of 80% (hematoxylin and eosin: ×40, ×400; chromogranin A, CD56, TTF-1, AE1/AE3, Ki-67: ×400).
Discussion
SCLC accounts for approximately 15–18% of all lung cancers; it is the leading cause of cancer death in men worldwide and is strongly associated with smoking. The poor survival rate of SCLC is related to its invasive tendency and its high rate of metastasis, and the identification of liver metastasis has been shown to be a significant indicator of a poor prognosis [2]. Liver metastasis is diagnosed in about 50% of patients with extensive-stage SCLC [3]; however, diffuse parenchymal metastasis correlated with liver failure is an unusual and extremely rare pattern of liver metastasis. CT examinations are generally preferred for the staging of malignancy. However, in cases with diffuse malignant infiltration of the hepatic sinusoids, plain CT imaging can fail to detect gross hepatic nodules [4], while contrast-enhanced CT can reveal diffuse multiple low-density, hypovascular areas in the liver [5]; in these cases, magnetic resonance imaging or FDG-PET may be useful for staging purposes [6]. Furthermore, almost all cases reported as having diffuse liver metastasis of SCLC exhibit rapid progression to acute liver failure and death. Therefore, diffuse liver metastasis is mostly diagnosed after death. As for the underlying mechanism of the progression of hepatic metastasis of SCLC to acute liver failure, massive diffuse sinusoidal infiltration and obstructive invasion of the hepatic vessels by tumor cells as well as replacement of normal liver parenchyma with malignant cells have been reported to result in hepatocyte ischemia and necrosis [5].
Seventeen well-described cases of diffuse liver metastases of SCLC causing acute liver failure have been previously reported [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14], and these cases differ from cases of liver predominant/primary small-cell carcinoma [15]. None of these 17 cases had been histologically diagnosed prior to death, despite the absence of any visible nodular lesions in the liver observed using contrast-enhanced CT. Since coagulation abnormalities usually prohibit liver biopsy, in case of a high risk of bleeding, alternative biopsy techniques such as transjugular liver biopsy should be favored [11]. Thus, as far as we know, the case reported here is the first case of diffuse liver metastasis of SCLC diagnosed histologically based on a transjugular liver biopsy while the patient was alive, with no nodular lesions visible in the liver even using a contrast-enhanced CT scan of the abdomen. However, in our case, appropriate chemotherapy could not be performed because of the severe liver dysfunction at the time of diagnosis.
In conclusion, we report a case of diffuse liver metastasis of SCLC diagnosed histologically based on a transjugular liver biopsy that progressed rapidly to acute liver failure. A liver biopsy may be indicated early during the clinical course of SCLC patients with liver dysfunction and hepatomegaly.
Disclosure Statement
None of the authors has any conflict of interest to declare concerning the material presented in this paper.
Acknowledgments
The skillful technical assistance of Nami Michiaki is gratefully acknowledged. This study was supported in part by a grant for National Center for Global Health and Medicine (26A-107) to Y. Nozaki.
Footnotes
S. Mishima and Y. Nozaki equally contributed to this work.
References
- 1.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Li F, Jiao SC. Prognostic factors for survival of patients with extensive stage small cell lung cancer – a retrospective single institution analysis. Asian Pac J Cancer Prev. 2012;13:4959–4962. doi: 10.7314/apjcp.2012.13.10.4959. [DOI] [PubMed] [Google Scholar]
- 3.Elliott JA, Osterlind K, Hirsch FR, Hansen HH. Metastatic patterns in small-cell lung cancer: correlation of autopsy findings with clinical parameters in 537 patients. J Clin Oncol. 1987;5:246–254. doi: 10.1200/JCO.1987.5.2.246. [DOI] [PubMed] [Google Scholar]
- 4.Ihara N, Yashiro N, Kinoshita T, Yoshigi J, Ouchi T, Narita M, Hattori C, Kaneko N. Diffuse intrasinusoidal liver metastasis of small cell lung cancer causing fulminant hepatic failure: CT findings – a case report. Radiat Med. 2001;19:275–277. [PubMed] [Google Scholar]
- 5.Sato K, Takeyama Y, Tanaka T, Fukui Y, Gonda H, Suzuki R. Fulminant hepatic failure and hepatomegaly caused by diffuse liver metastases from small cell lung carcinoma: 2 autopsy cases. Respir Investig. 2013;51:98–102. doi: 10.1016/j.resinv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:1235–1240. doi: 10.1097/00042737-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Krause EA, Ludwig PW, Sumner HW. Metastatic carcinoma presenting as fulminant hepatic failure. Am J Gastroenterol. 1979;72:651–654. [PubMed] [Google Scholar]
- 8.Harrison HB, Middleton HM, 3rd, Crosby JH, Dasher MN., Jr Fulminant hepatic failure: an unusual presentation of metastatic liver disease. Gastroenterology. 1981;80:820–825. [PubMed] [Google Scholar]
- 9.McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72:133–139. doi: 10.4065/72.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Kaira K, Takise A, Watanabe R, Mori M. Fulminant hepatic failure resulting from small-cell lung cancer and dramatic response of chemotherapy. World J Gastroenterol. 2006;12:2466–2468. doi: 10.3748/wjg.v12.i15.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexopoulou A, Koskinas J, Deutsch M, Delladetsima J, Kountouras D, Dourakis SP. Acute liver failure as the initial manifestation of hepatic infiltration by a solid tumor: report of 5 cases and review of the literature. Tumori. 2006;92:354–357. doi: 10.1177/030089160609200417. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5:405–408. doi: 10.1038/ncpgasthep1154. [DOI] [PubMed] [Google Scholar]
- 13.Miyaaki H, Ichikawa T, Taura N, Yamashima M, Arai H, Obata Y, Furusu A, Hayashi H, Kohno S, Nakao K. Diffuse liver metastasis of small cell lung cancer causing marked hepatomegaly and fulminant hepatic failure. Intern Med. 2010;49:1383–1386. doi: 10.2169/internalmedicine.49.3296. [DOI] [PubMed] [Google Scholar]
- 14.Vaideeswar P, Munot S, Rojekar A, Deodhar K. Hepatic diffuse intra-sinusoidal metastases of pulmonary small-cell carcinoma. J Postgrad Med. 2012;58:230–231. doi: 10.4103/0022-3859.101654. [DOI] [PubMed] [Google Scholar]
- 15.Lo AA, Lo EC, Li H, Zhang W, Liao J, Rao MS, Miller F, Yang GY. Unique morphologic and clinical features of liver predominant/primary small cell carcinoma – autopsy and biopsy case series. Ann Diagn Pathol. 2014;18:151–156. doi: 10.1016/j.anndiagpath.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]