Abstract
In this study, we examined the possible immune-mediated mechanisms in cardiorenal syndrome (CRS) type 1 pathogenesis. We enrolled 40 patients with acute heart failure (AHF), 11 patients with CRS type 1 and 15 controls. Plasma from the different groups was incubated with monocytes; subsequently, cell apoptosis was evaluated by DNA fragmentation, caspase activity and cytofluorometric assay. Cytokine quantification in plasma and supernatant was performed by ELISA. Monocytes treated with CRS type 1 plasma showed significantly higher apoptosis compared with those treated with AHF and the controls (p < 0.05). Caspase-3 (CRS type 1: 2.20 ng/ml, IQR 2.06-2.33; AHF: 1.48 ng/ml, IQR 1.31-1.56; controls: 0.71 ng/ml, IQR 0.67-0.81) and caspase-8 levels (CRS type 1: 1.49 ng/ml, IQR 1.42-1.57; AHF: 0.94 ng/ml, IQR 0.84-0.98; controls: 0.56 ng/ml, IQR 0.51-0.58) in cells incubated with plasma from these patients demonstrated a significantly higher concentration. We observed a strong upregulation of plasma IL-6 and IL-18 in CRS type 1 compared with AHF and the controls (p < 0.05). Interestingly, we observed a similar concentration of TNF-α in CRS type 1 and AHF. In CRS type 1 patients, IL-6 (52.13 ng/ml, IQR 47.29-66.83) and IL-18 levels (197.75 ng/ml, IQR 120.80-265.49) in supernatant were significantly higher than in AHF patients (IL-6: 28.79 ng/ml, IQR 19.90-36.10; IL-18: 21.98 ng/ml, IQR 15.98-29.85) and controls (IL-6: 5.02 ng/ml, IQR 4.56-6.44; IL-18: 7.91 ng/ml, IQR 5.57-10.62). These findings suggest the presence of a defective regulation of monocyte apoptosis in CRS type 1 patients and the involvement of an immune-mediated mechanism in the pathophysiology of this syndrome.
Key Words: Cardiorenal syndrome, Acute heart failure, Apoptosis, Immune-mediated mechanism, Monocytes, Inflammation
Introduction
‘Organ crosstalk’ is fundamental for the homeostasis and optimal function of all systems. However, in a disease condition, there is a dysregulation of this system, and the diseased organ influences other organs [1,2]. The heart and kidneys are intimately interconnected in a synergistic relationship of heart-kidney crosstalk. A dysfunction of the heart or kidneys often leads to a deterioration in function and damage to the other organ [3]. Given the complex and bidirectional interaction between these organs, the Acute Dialysis Quality Initiative (ADQI) proposed a consensus definition of cardiorenal syndrome (CRS) [4]. This recent definition and classification system has been generated to emphasize the bidirectional relationship between these organs and to include a vast array of acute or chronic conditions [3].
CRS type 1 is characterized by an acute deterioration in cardiac function, such as acute decompensated heart failure, acute coronary syndrome, cardiogenic shock and cardiac surgery, leading to acute kidney injury (AKI) [5,6,7]. In fact, CRS type 1 has been described in 27-45% of hospitalized acute heart failure (AHF) patients [8,9,10]. A significant proportion of AHF patients develop AKI and, thus, CRS type 1 in the first 3-5 days after admission [8,11,12]. Moreover, AKI has been associated with a higher risk of prolonged hospitalization/rehospitalization, cardiovascular events and all-cause mortality, as well as faster progression to chronic kidney disease (CKD) [13,14].
The pathophysiology of CRS type 1 is complex and poorly understood as it involves several factors which are interconnected [15]. It is a multifactorial syndrome involving different mechanisms interplaying between the metabolic, neuroendocrine and immune systems [16]. A better understanding of the complex interaction between the heart and kidneys could improve the management, treatment and prevention of CRS type 1.
In this research paper, we focus on the immune-mediated pathogenesis of CRS type 1, exploring the potential mechanism contributing to the vicious circle leading to this syndrome. The major aim of the study is to investigate the in vitro effect of plasma from CRS type 1 patients on a monocyte cell line. Furthermore, we examine the presence of typical cytokine plasma profiles in CRS type 1 patients to better understand the mechanisms of this syndrome and how inflammation in AHF can lead to immune-mediated acute kidney damage.
Subjects and Methods
Subjects
The study was conducted at the Internal Medicine Department of San Bortolo Hospital, Vicenza, Italy. Patients admitted to the Internal Medicine Department between September 2011 and February 2012 were screened. A total of 80 patients with AHF were further examined, and their medical histories were reviewed for inclusion into the study. Patients with AKI prior to the episode of AHF or with other potential causes of AKI were excluded from this study. Furthermore, patients with an eGFR <45 ml/min/1.73 m2 or with a history of kidney transplantation were excluded.
Finally, we enrolled 40 AHF patients. Subsequently, we identified 11 of the 40 patients who exhibited AKI at the time of admission for AHF or developed AKI during the course of hospitalization, and those were classified as CRS type 1. CRS type 1 was defined according to the current classification system [3]. The cause of AKI was presumed to be related to the AHF (the exclusion of other plausible causes was based on a review of the clinical course). No patients who were hypotensive or required inotropic support prior to the diagnosis of AKI were included in the study. The remaining 29 patients, with AHF from any cause who did not develop AKI during hospitalization, were analyzed to better understand the contribution of AHF on monocyte apoptosis. In addition, 15 healthy volunteers without AHF or AKI were recruited as controls for this study.
AKI was defined by the Acute Kidney Injury Network (AKIN) criteria [17]. The procedures were in accordance with the Declaration of Helsinki. All patients were informed about the experimental protocol and the objectives of the study before providing informed consent and blood samples. The protocol and consent forms were approved by the Ethics Committee of San Bortolo Hospital, Vicenza.
Sample Collection
Blood samples were collected from the recruited patients on admission to the Internal Medicine ward. We also collected blood samples within 24 h of AKI from patients who developed CRS type 1. The blood samples were collected in EDTA tubes and subsequently centrifuged. Control samples from the healthy volunteers were processed in the same manner.
Biochemical Parameters
Urea, serum creatinine (SCr), albumin, hemoglobin and other biochemical parameters were measured by standard laboratory techniques with an automatic analyzer. SCr was measured by the Jaffe method, and the eGFR was calculated with the 4-variable standardized MDRD study equations. C-reactive protein levels (in mg/dl), white blood cell counts and percentages of neutrophils were assessed as well.
Quantitative determination of NGAL and BNP was performed with an Alere Triage CardioRenal Panel (Alere, San Diego, Calif., USA). Plasma NGAL and BNP levels were measured by fluorescence-based immunoassay with a Triage point-of-care analyzer (Alere), which allows the rapid quantitative measurement of NGAL and BNP concentrations in EDTA plasma; they are expressed as nanograms per milliliter and picograms per milliliter, respectively.
U937 Cell Culture
The human cell line U937 is a monocytic precursor cell line derived from a histiocytic lymphoma [18]. U937 cells were grown in a complete liquid-phase medium (RPMI 1640; International PBI, Milan, Italy) supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma Chemical Co., St. Louis, Mo., USA). The U937 cells were maintained in a controlled-atmosphere incubator (5% CO2) at 37°C.
Induction of Apoptosis
The U937 cells were plated at 2 × 106 cells per well in 48-well plates and incubated with 90% (270 μl) RPMI 1640 medium (with 2 mM L-glutamine, 100 IU/ml penicillin and 100 mg/ml streptomycin) and 10% EDTA plasma (30 μl) from CRS type 1 and AHF patients as well as healthy controls in standard conditions (at 37°C in 5% CO2 for 24 h).
Evaluation of Apoptosis
The U937 cells were treated with plasma from three different groups; the ability of the plasma to induce apoptosis was evaluated at 24 h. Untreated cells were maintained in an identical manner and used as a negative control. The apoptotic DNA fragmentation assay was performed with an Apoptotic DNA Ladder Extraction KIT (BioVision, Milpitas, Calif., USA) according to the manufacturer's protocol. DNA ladder fragmentation was detected by electrophoresis on 1.2% agarose gel staining with SYBR Safe (Invitrogen, Grand Island, N.Y., USA).
The U937 cells were also assayed for activation of caspase-3 and −8. The activities of caspase-3 and −8 were measured with a fluorometric assay (Human Caspase-3 Instant ELISA Kit, eBioscience, San Diego, Calif., USA; TruPoint Caspase-8 Kit, PerkinElmer Life Sciences, Boston, Mass., USA). U937 cells incubated with plasma for 24 h were processed according to the manufacturer's instructions; finally, activities were measured in cell lysates at 450 nm for caspase-3 and at 615 nm for caspase-8 with a VICTOR X4 Multilabel Plate Reader (PerkinElmer Life Sciences, Waltham, Mass., USA). All tests were performed in duplicate.
Quantitative determinations of apoptosis and necrosis were evaluated by cytofluorometric assay with an annexin V-FITC conjugate kit (Beckman Coulter, Brea, Calif., USA). This is an apoptosis/necrosis detection kit based on the binding properties of annexin V to phosphatidylserine and on the DNA-intercalating capabilities of propidium iodide (PI). Cells were washed twice with cold Dulbecco's PBS and then resuspended in 100 μl of PBS at a concentration of 5 × 105 cells/ml. This solution was incubated with 5 μl of annexin V-FITC conjugate and 2.5 μl of PI (Beckman Coulter). The cells were gently vortexed and incubated for 15 min at room temperature (25°C) in the dark; 400 μl of 1× binding buffer was added to each tube. The analysis was performed with a Navios Flow Cytometer (Beckman Coulter) to identify the subpopulations of apoptotic cells within 1 h. Apoptotic cells were gated and enumerated by identifying those cells that exhibited FITC and PI staining. Annexin V-FITC labeling was used to quantitatively determine the percentage of cells that were undergoing apoptosis. PI was used to distinguish necrotic from nonnecrotic cells. A minimum of 20,000 events were collected from each sample.
Determination of Cytokine Levels in Plasma and Supernatant
Quantitative determinations of TNF-α, IL-6 and IL-18 in the plasma of patients and in the supernatant were performed by the Human Instant ELISA Kit (eBioscience). Cytokine assessments were performed according to the manufacturer's protocol and instructions using 50 μl of sample. Optical density was read using a VICTOR X4 Multilabel Plate Reader at 450 nm. The concentrations of these molecules were calculated from the standard curve according to the manufacturer's protocol. All tests were performed in duplicate.
Statistical Analysis
Statistical analysis was performed using the SPSS 15 software package. Categorical variables are expressed as percentages; continuous variables are expressed as means ± SD (parametric variables) or medians and IQR (nonparametric variables). The Mann-Whitney U test or the t test was used for the comparison of coupled data, as appropriate. The Kruskal-Wallis test for multiple comparisons was applied to compare the groups. A p value of <0.05 was considered statistically significant.
Results
Subjects' Baseline Characteristics
Causes of admission for AHF included non-ST segment elevation myocardial infarction (2%), excessive salt and fluid intake (29%), hypertensive crisis (14%) and other causes (45%). The remaining 10% of the patients did not have any recognizable cause of AHF.
The mean age of the 11 patients with CRS type 1 was 74.0 ± 13.1 years, and 45% of these patients were male. The median baseline SCr of the CRS type 1 patients was 0.96 mg/dl (IQR 0.88-1.02). The median eGFR was 62 ml/min/1.73 m2 (IQR 55-75). Seven of the CRS type 1 subjects (63%) had diabetes, and 10 (90%) had hypertension. On the first day of AKI, the median baseline SCr was 1.17 mg/dl (1.08-1.35) and the median eGFR was 46 ml/min/1.73 m2 (IQR 44-64) in the CRS type 1 patients.
The mean age of the 29 patients with AHF was 73.6 ± 9.5 years, and 58% of them were male. The median baseline SCr of the AHF subjects was 0.98 mg/dl (IQR 0.87-1.15), and the median eGFR was 67 ml/min/1.73 m2 (IQR 53-82). Twelve subjects of the AHF group (41%) had diabetes, and 27 (93%) had hypertension. The characteristics of the CRS type 1 and AHF patients are described in table 1. The mean age of the 15 healthy volunteers was 52.0 ± 7.7 years, and 47% of them were male.
Table 1.
Baseline characteristics of the CRS type 1 and AHF patients and clinical parameters
| CRS type 1 patients (n = 11) | AHF patients (n = 29) | |
|---|---|---|
| Age, years | 74.0 ± 13.1 | 73.6 ± 9.5 |
| Weight, kg | 77 (67–85) | 75 (64–88) |
| Diabetes, % | 63 | 41 |
| Hypertension, % | 90 | 93 |
| Peripheral vascular disease, % | 42 | 38 |
| Cardiovascular disease, % | 19 | 16 |
| Obesity, % | 23 | 20 |
| Dyslipidemia, % | 43 | 37 |
| Death, % | 27 | 3.4 |
| Creatinine, mg/dl | 0.96 (0.88–1.02) | 0.98 (0.87–1.15) |
| eGFR, ml/min/1.73 m2 | 62 (55–75) | 67 (53–82) |
| Mean arterial pressure, mm Hg | 100 (89.2–115.8) | 103 (93.3–120.8) |
| Ejection fraction, % | 35 (25.0–51.0) | 35 (24.0–48.0) |
| BNP, pg/ml | 695 (408–1,837) | 632 (398–946) |
| Troponin I, ng/ml | 0.07 (0.04–0.26) | 0.07 (0.04–0.27) |
| Hemoglobin, g/dl | 11.4 (9.7–13.0) | 11.1 (13.6–14.2) |
| Albumin, g/l | 4.3 (4.0–4.4) | 4.0 (4.2–4.4) |
| Urea, mg/dl | 73 (55–109) | 66 (41–91) |
| NGAL, ng/ml | 228 (164–391) | 197 (145–282) |
| White blood cell count, 109/l | 11.6 (7.3–15.5) | 10.3 (8.3–13.3) |
| Neutrophils, % | 79.8 (72.4–84.7) | 74.1 (68.3–81.6) |
| C-reactive protein, mg/dl | 4.0 (2.1–8.1) | 3.3 (1.0–9.7) |
Values denote means ± SD or medians (IQR) unless specified otherwise.
None of the patients had had exposure to radiocontrast media in the 72 h preceding AKI. None of them developed a need for mechanical ventilation and renal replacement therapy. Urea, hemoglobin, albumin, NGAL and BNP levels were not significantly different at admission in CRS type 1 and AHF patients. Medical treatments were similar in the CRS type 1 and AHF patients. In particular, the amount of diuretics administered was similar in these two groups. The level of maximum SCr during hospitalization was significantly different between CRS type 1 and AHF patients (p < 0.05). The inflammatory profile was overlapping in the two groups (table 1).
Apoptotic Effect of CRS Type 1 Plasma on U937 Cells
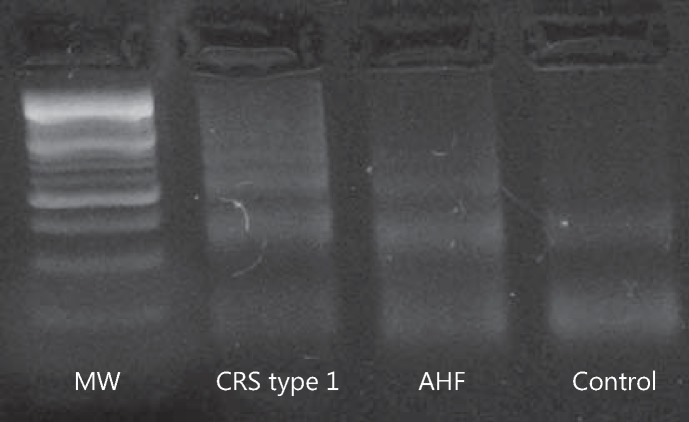
Since cell growth inhibition and apoptotic body formation are indicative of apoptosis induction, we examined the initiation of DNA ladder formation and other characteristic features of apoptosis by the methods described above. In monocyte cell lines treated for 24 h with CRS type 1 plasma, the results showed DNA ladder formation with different molecular-weight fractions, suggesting the presence of apoptotic events (fig. 1).
Fig. 1.
Detection of apoptotic DNA fragmentation induced by plasma from CRS type 1 and AHF patients as well as controls in U937 cells by the separation of agarose gel electrophoresis. MW = 100-bp marker.
Fragmentation of the genomic DNA is a biochemical hallmark of apoptosis, an irreversible event that commits the cell to die. In many systems, this DNA fragmentation has been shown to result from activation of an endogenous nuclear endonuclease. This enzyme selectively cleaves DNA at sites located between nucleosomal units generating mono- and oligonucleosomal DNA fragments. On agarose gel electrophoresis, these DNA fragments reveal a distinctive ladder pattern consisting of multiple DNA subunits.
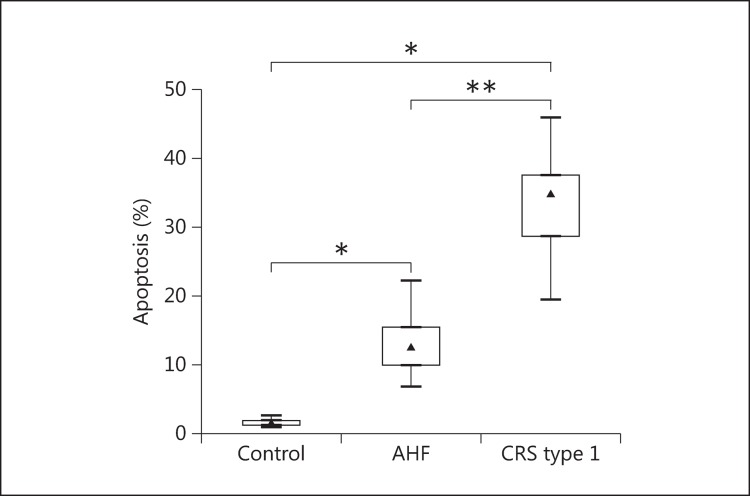
The quantitative analysis of apoptosis by flow cytometry confirmed that U937 cells incubated with plasma from CRS type 1 patients had significantly higher apoptosis rates than those incubated with plasma from AHF patients and controls (p < 0.001 and p < 0.05, respectively). The level of apoptosis detected after 24 h of incubation was 34.95% (IQR 28.78-37.65) for the CRS type 1 patients, while it was 12.50% (IQR 9.98-15.50) in the AHF group and 1.39% (IQR 1.23-1.82) in the controls (fig. 2).
Fig. 2.
Evaluation of the percentage of apoptosis in U937 cells after incubation with plasma from controls as well as AHF and CRS type 1 patients. * p < 0.05, ** p < 0.001.
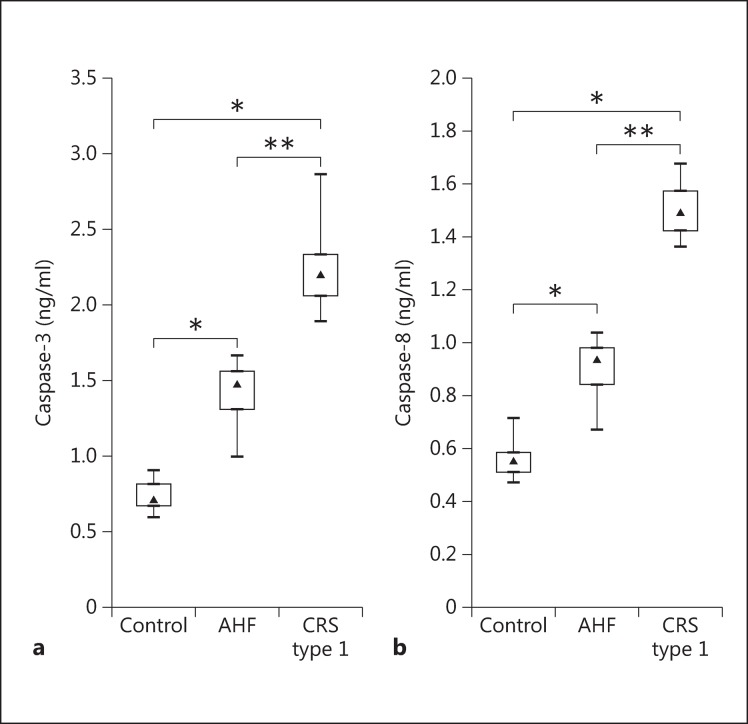
The concentrations of caspase-3 and −8 were measured in U937 cells treated for 24 h with plasma from the different groups. In concordance with the apoptosis rate, U937 cells incubated with plasma from CRS type 1 patients demonstrated significantly higher caspase-3 and −8 activities compared with the AHF and control groups (p < 0.001 and p < 0.05, respectively). The level of caspase-3 detected after 24 h of incubation was 2.20 ng/ml (IQR 2.06-2.33) for the CRS type 1 patients, while it was 1.48 ng/ml (IQR 1.31-1.56) in the AHF group and 0.71 ng/ml (IQR 0.67-0.81) in the control group. The activity of caspase-8 detected after 24 h of incubation was 1.49 ng/ml (IQR 1.42-1.57) for the CRS type 1 patients, while it was 0.94 ng/ml (IQR 0.84-0.98) in the AHF group and 0.56 ng/ml (IQR 0.51-0.58) in the control group (fig. 3).
Fig. 3.
Evaluation of caspase-3 (a) and −8 levels (b) in U937 cell lysate after incubation with plasma from controls as well as AHF and CRS type 1 patients. * p < 0.05, ** p < 0.001.
Inflammatory Cytokines in Plasma
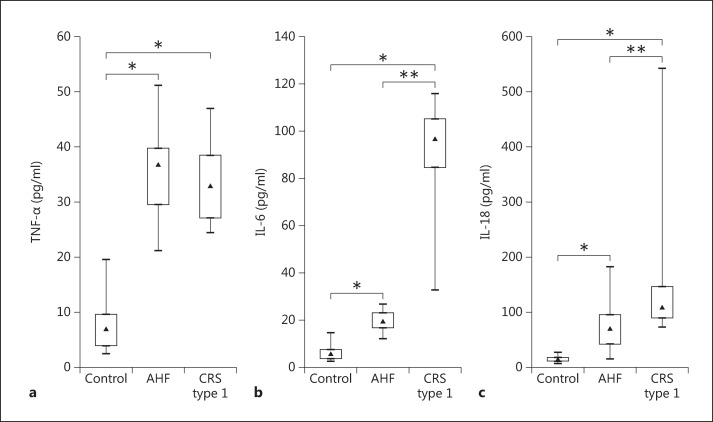
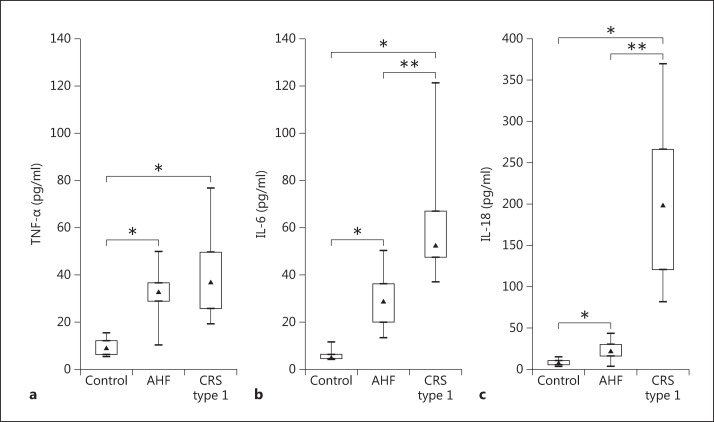
IL-6 and −18 levels were significantly higher in patients with CRS type 1 when compared with patients with AHF (p < 0.001) (table 2; fig. 4). Specifically, the median value for IL-6 in the CRS type 1 patients was 96.78 pg/ml (IQR 84.61-105.21), and in the AHF patients, it was 19.41 pg/ml (IQR 16.55-22.90). The median IL-18 level in the CRS type 1 patients was 108.24 pg/ml (IQR 88.78-146.09) and 69.16 pg/ml (IQR 41.84-95.88) in the AHF patients. No significant difference was found in plasma TNF-α concentrations between CRS type 1 and AHF patients (fig. 4). However, TNF-α levels in plasma were significantly elevated both in CRS type 1 (32.87 pg/ml, IQR 27.09-38.51) and in AHF (36.74 pg/ml, IQR 29.53-39.74) when compared with the controls (6.89 pg/ml, IQR 3.84-9.53; both p < 0.05) (table 2; fig. 4).
Table 2.
Plasma proinflammatory cytokine levels (pg/ml) in the CRS type 1 and AHF patients as well as in the controls
| Controls | AHF patients | CRS type 1 patients | |
|---|---|---|---|
| TNF-α | 6.89 (3.84–9.53) | 36.74 (29.53–39.74) | 32.87 (27.09–38.51) |
| IL-6 | 5.56 (3.47–7.31) | 19.41 (16.55–22.90) | 96.78 (84.61–105.21) |
| IL-18 | 13.32 (10.66–16.47) | 69.18 (41.84–95.88) | 108.24 (88.78–146.09) |
Values denote medians (IQR).
Fig. 4.
Evaluation of TNF-α (a), IL-6 (b) and IL-18 levels (c) in plasma from controls as well as AHF and CRS type 1 patients. * p < 0.05, ** p < 0.001.
Cytokine Values in Monocyte Supernatant
Cytokine levels were measured by ELISA in monocyte supernatant after 24 h of incubation to examine the potential immune mediators involved in the immune-mediated damage in CRS type 1 pathogenesis (table 3; fig. 5). TNF-α levels were significantly elevated both in CRS type 1 (36.87 pg/ml, IQR 25.55-49.54) and in AHF (32.66 pg/ml, IQR 28.88-36.61) when compared with the controls (8.85 pg/ml, IQR 6.13-11.89; both p < 0.05). However, no significant difference was found in supernatant TNF-α concentrations between the CRS type 1 and AHF groups (fig. 5). Furthermore, in the CRS type 1 patients, proinflammatory cytokine (IL-6 and −18) levels were significantly higher than in the AHF patients and control subjects (p < 0.001 and p < 0.05, respectively). Specifically, the median values for IL-6 (CRS type 1: 52.13 pg/ml, IQR 47.29-66.83; AHF: 28.79 pg/ml, IQR 19.90-36.10) and IL-18 (CRS type 1: 197.75 pg/ml, IQR 120.80-265.49; AHF: 21.98 pg/ml, IQR 15.98-29.85) in U937 cell supernatant were more than 2 times higher in the CRS type 1 patients than in the AHF subjects (table 3; fig. 5).
Table 3.
Cytokine levels (pg/ml) in monocyte supernatant
| Controls | AHF patients | CRS type 1 patients | |
|---|---|---|---|
| TNF-α | 8.85 (6.13–11.89) | 32.66 (28.88–36.61) | 36.87 (25.55–49.54) |
| IL-6 | 5.02 (4.56–6.44) | 28.79 (19.90–36.10) | 52.13 (47.29–66.83) |
| IL-18 | 7.91 (5.57–10.62) | 21.98 (15.98–29.85) | 197.75 (120.80–265.49) |
Values denote medians (IQR).
Fig. 5.
Evaluation of TNF-α (a), IL-6 (b) and IL-18 levels (c) in U937 cell lysates after incubation with plasma from controls as well as AHF and CRS type 1 patients. * p < 0.05, ** p < 0.001.
Discussion
A concurrent dysfunction of the heart and kidneys is due to the cellular and molecular crosstalk between these organs. Activation of an immune response, a cellular response and inflammation as well as of apoptotic pathways is implicated in the pathogenesis of CRS type 1 [16]. Immune-mediated damage, alterations in the immune response with apoptosis, cytokine release, inflammation and changes in immune cell functions have been postulated as potential mechanisms involved in the pathogenesis of CRS [3]. In this study, we examined the possible role of the immune-mediated mechanisms involved in the pathogenesis of CRS type 1. In fact, we analyzed the in vitro response of monocytes incubated with plasma from CRS type 1 patients in terms of apoptosis and cytokine release. The secondary aim of this study was to evaluate the presence of cytokines in the plasma of CRS type 1 patients to better understand the mechanism of this syndrome.
Our results indicate significantly higher apoptosis levels in monocytes incubated with CRS type 1 plasma when compared with the levels in monocytes incubated with plasma from AHF patients and controls. Moreover, the caspase-3 and −8 levels confirmed a strong activation of the apoptotic pathway. We also report a strong upregulation of plasma proinflammatory cytokines in CRS type 1 patients compared with AHF patients and controls. Furthermore, we observed that high levels of IL-6 and −18 as well as TNF-α were produced by monocytes incubated with CRS type 1 plasma and then released in the cell supernatant.
The mechanisms by which the onset of AHF leads to AKI are multiple and complex. All experimental models of AKI, such as ischemia-reperfusion, sepsis-endotoxemia and nephrotoxic models, are associated with a strong inflammatory activity [19]. Recently, Lee et al. [20] reported a central role for cytokines in the pathophysiology of AKI. The multiple factors involved in the development of AKI during heart failure describe a pathogenesis of AKI accounting for multiple pathways. Recently, humoral signaling and the inflammatory pathways have been reported as new interesting mechanisms to explain distant organ damage and, in particular, kidney injury in heart failure [21].
A marked apoptotic activity, highlighted by DNA fragmentation, the asymmetric distribution of phosphatidylserine (detected by annexin V) and caspase activation, was observed in the monocytes incubated with plasma from CRS type 1 patients. In particular, we found a significantly higher level of in vitro apoptosis in monocytes incubated with plasma from CRS type 1 patients compared with the levels in monocytes incubated with plasma from AHF patients and controls.
We studied caspase-3 and −8 activation and observed a significantly higher activity in cells incubated with CRS type 1 plasma than in those incubated with plasma from AHF patients and controls, which was associated with a higher extent of apoptotic death. It is likely that in U937 cells, the observed apoptosis may be due to the presence of proapoptotic factors in the plasma of CRS type 1 patients. This finding suggests that CRS type 1 might have an inflammatory pattern due to various cardiorenal mediators that induce an early pathological apoptosis in monocytes.
We found twice the levels of IL-18 and more than twice the levels of IL-6 in the plasma of CRS type 1 patients compared with AHF patients. Furthermore, we observed a similar plasma concentration of TNF-α in these two groups, but significantly elevated levels of TNF-α when compared with the controls. In fact, TNF-α levels are directly related to progression and prognosis in patients with heart failure [22,23]. We found a comparable level of TNF-α in CRS type 1 and AHF patients, and we hypothesized that, differently from other cytokines, TNF-α could not be implicated in the renal damage in CRS type 1 but only in the pathophysiological mechanism of AHF.
Based on our results, we postulate that an immune-mediated mechanism and a loss of the normal balance of the immune system may play a role in the pathogenesis of CRS type 1. The loss of the normal balance of the immune system and an altered humoral profile may induce the deleterious impact of AHF on kidney function. Factors such as inflammation and oxidative stress may be involved in an altered immune regulation that induces monocyte apoptosis as well as tissue and organ damage.
Monocytes may mediate apoptosis in other cells through the production of proinflammatory cytokines and other mediators. In our in vitro system, monocytes were capable of producing proinflammatory cytokines after receiving a signal present in the plasma of CRS type 1 patients. These mediators may induce apoptosis as well as tissue and organ damage via endocrine and paracrine actions. Monocytes are exposed to proinflammatory and proapoptotic factors present in the plasma of patients, and this leads to an upregulation of the production and release of proinflammatory cytokines that can lead to a vicious circle of CRS and the crosstalk between organs [24].
Our results are similar to those obtained by Virzì and colleagues. In particular, in a recent study [25], they observed that the plasma of CRS type 1 patients induces apoptosis in U937 cells. However, this study had a strong limitation: all 15 patients included with CRS type 1 had CKD prior to their enrollment in the study. In particular, 1 patient had CKD stage 2, 4 patients had CKD stage 3, 6 had CKD stage 4, and 4 had CKD stage 5 (not yet on renal replacement therapy). CKD could be a confounding factor and partly contribute to the apoptosis [26]. Indeed, in previous studies, de Cal and colleagues [27,28] had shown that the plasma of patients with CKD by itself induces apoptosis in these cells. In fact, Virzì et al. [25] reported that apoptosis appeared to increase across CKD stages 1-4 and that this was associated with increased proinflammatory activity.
In this study, we investigated the role of immune-mediated mechanisms in CRS type 1 patients with AKI related to AHF with only normal-to-moderate CKD. Therefore, the observed apoptosis is not principally due to CKD but only due to the inflammatory and apoptotic mechanisms of CRS. These findings suggest that the presence of proinflammatory cytokines and proapoptotic factors in CRS type 1 plasma causes a defective regulation of monocyte apoptosis in those patients. Furthermore, these preliminary results suggest that inflammatory pathways have a central role in the pathogenesis of CRS type 1.
We are aware that there remains a huge knowledge gap with regard to plasma cytokine concentrations, monocyte apoptosis and renal damage in CRS type 1. The measurement of CRS type 1 plasma cytokines together with the evaluation of their effect on monocytes is only one possible way to demonstrate the immune-mediated defective regulation and the involvement of inflammation in this syndrome.
Further investigations are necessary to better define the actual pathophysiological process, to elucidate the paracrine signaling pathways and to determine the factors involved, such as cytokine release and oxidative stress. A better understanding of the pathophysiology has important therapeutic and prognostic implications for CRS type 1 and for heart-kidney crosstalk.
Disclosure Statement
The plasma NGAL assays were donated by Alere. Alere was not involved in protocol development, analysis or the interpretation of the results. C. Ronco is a consultant for Alere and a member of the speakers bureau for Abbot Diagnostics. The other authors declare no conflicts of interest.
References
- 1.Ledoux P. Cardiorenal syndrome (in French) Avenir Med. 1951;48:149–153. [PubMed] [Google Scholar]
- 2.Molls RR, Rabb H. Limiting deleterious cross-talk between failing organs. Crit Care Med. 2004;32:2358–2359. doi: 10.1097/01.ccm.0000145957.97995.55. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase M, Müller C, Damman K, Murray PT, Kellum JA, Ronco C, McCullough PA. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:99–116. doi: 10.1159/000349969. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 7.Virzì GM, Clementi A, Brocca A, de Cal M, Vescovo G, Granata A, Ronco C. The hemodynamic and nonhemodynamic crosstalk in cardiorenal syndrome type 1. Cardiorenal Med. 2014;4:103–112. doi: 10.1159/000362650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz DN. Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis. 2013;20:56–66. doi: 10.1053/j.ackd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 15.Liang KV, Williams AW, Greene EL, Redfield MM. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med. 2008;36(1 suppl):S75–S88. doi: 10.1097/01.CCM.0000296270.41256.5C. [DOI] [PubMed] [Google Scholar]
- 16.Virzì GM, de Cal M, Cruz DN, Bolin C, Vescovo G, Ronco C. Type 1 cardiorenal syndrome and its possible pathophysiological mechanisms (in Italian) G Ital Nefrol. 2012;29:690–698. [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundström C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 19.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI) Clin Nephrol. 2011;76:165–173. doi: 10.5414/cn106921. [DOI] [PubMed] [Google Scholar]
- 21.Goh CY, Vizzì G, de Cal M, Ronco C. Cardiorenal syndrome: a complex series of combined heart/kidney disorders. Contrib Nephrol. 2011;174:33–45. doi: 10.1159/000329233. [DOI] [PubMed] [Google Scholar]
- 22.Charakida M, Halcox JP. Tumor necrosis factor-α in heart failure: more questions than answers (in Spanish) Rev Esp Cardiol. 2005;58:470–472. [PubMed] [Google Scholar]
- 23.Rordorf R, Savastano S, Sanzo A, Spazzolini C, De Amici M, Camporotondo R, Ghio S, Vicentini A, Petracci B, De Regibus V, Taravelli E, Landolina M, Schwartz PJ. Tumor necrosis factor-α predicts response to cardiac resynchronization therapy in patients with chronic heart failure. Circ J. 2014;78:2232–2239. doi: 10.1253/circj.cj-14-0023. [DOI] [PubMed] [Google Scholar]
- 24.Virzi G, Day S, de Cal M, Vescovo G, Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014;18:201. doi: 10.1186/cc13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virzì GM, Torregrossa R, Cruz DN, Chionh CY, de Cal M, Soni SS, Dominici M, Vescovo G, Rosner MH, Ronco C. Cardiorenal syndrome type 1 may be immunologically mediated: a pilot evaluation of monocyte apoptosis. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dounousi E, Koliousi E, Papagianni A, Ioannou K, Zikou X, Katopodis K, Kelesidis A, Tsakiris D, Siamopoulos KC. Mononuclear leukocyte apoptosis and inflammatory markers in patients with chronic kidney disease. Am J Nephrol. 2012;36:531–536. doi: 10.1159/000345352. [DOI] [PubMed] [Google Scholar]
- 27.Bordoni V, Piroddi M, Galli F, de Cal M, Bonello M, Dimitri P, Salvatori G, Ranishta R, Levin N, Tetta C, Ronco C. Oxidant and carbonyl stress-related apoptosis in end-stage kidney disease: impact of membrane flux. Blood Purif. 2006;24:149–156. doi: 10.1159/000089452. [DOI] [PubMed] [Google Scholar]
- 28.de Cal M, Cruz DN, Corradi V, Nalesso F, Polanco N, Lentini P, Brendolan A, Tetta C, Ronco C. HLA-DR expression and apoptosis: a cross-sectional controlled study in hemodialysis and peritoneal dialysis patients. Blood Purif. 2008;26:249–254. doi: 10.1159/000122110. [DOI] [PubMed] [Google Scholar]