Abstract
Background
Therapy for multiple myeloma (MM) has substantially improved in the era of immunomodulatory drugs and bortezomib. However, the prognosis of patients with progressive disease despite treatment with these ‘novel agents’ remains poor. Recently, pomalidomide was approved in this setting, but a median progression-free survival of <4 months still leaves room for improvement. Pomalidomide-based combination therapies are currently under investigation, but data on long-term treatment are lacking.
Case Report
We present the case of a 68-year-old woman with refractory MM who received pomalidomide in combination with various drugs including anthracyclines, alkylators and proteasome inhibitors. Initially, major hematological toxicities and infectious complications including a hepatitis B virus reactivation were encountered. With careful dose adjustments and selection of combination partners, pomalidomide treatment was maintained for over 4 years and led to a sustained partial remission. In particular, the well-tolerated regimen of bortezomib, cyclophosphamide and dexamethasone together with pomalidomide was administered for >30 cycles.
Conclusion
This case illustrates the value of an individualized approach to myeloma care given an increasing availability of ‘novel agents’. Tailored treatment using these drugs as a backbone is essential to achieve long-lasting responses and minimize side effects.
Key Words: Multiple myeloma, Combination therapy, Pomalidomide, Hepatitis B virus reactivation
Introduction
Immunomodulatory drugs (IMiDs) are a distinct group of oral antineoplastic drugs that have gained therapeutic relevance for the treatment of multiple myeloma (MM) since the turn of the millennium [1]. For myeloma patients, the introduction of IMiDs has fundamentally changed treatment options and has significantly improved clinical outcomes [2]. However, in patients failing to respond to treatment with novel agents – including thalidomide and lenalidomide as well as bortezomib – treatment options are limited and survival rates are poor [3].
Pomalidomide (CC-4047), the most recent IMiD, has shown substantial clinical activity in this setting. A randomized phase 2 study showed that the efficacy of pomalidomide is significantly enhanced by concomitant administration of dexamethasone and, moreover, continuous administration of the drug is well tolerated [4]. The MM-003 trial [5] demonstrated a significant increase in progression-free survival for patients receiving pomalidomide plus low-dose dexamethasone compared to patients treated with high-dose dexamethasone alone. The trial eventually led to the approval of pomalidomide for the treatment of patients with refractory and/or relapsed myeloma by the European Medicines Agency (EMA) in August 2013. Combination protocols with bortezomib, carfilzomib, doxorubicin and oral cyclophosphamide are currently under investigation, but data on the long-term treatment with such regimens are lacking.
In this report, we discuss the case of a heavily pretreated female patient with refractory MM who received >30 cycles of an experimental combination of pomalidomide, bortezomib, cyclophosphamide and dexamethasone as ninth-line therapy, resulting in a long-lasting partial remission (PR) and in a good quality of life.
Case Presentation
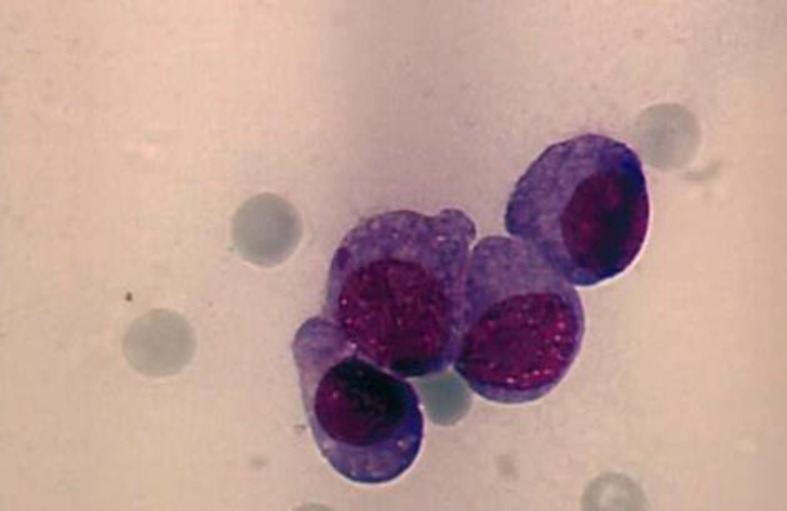
A 59-year-old woman was diagnosed with IgG kappa MM in May 2002, when she presented with Durie-Salmon stage III A disease with lytic destruction of the pelvis. Fluorescence in situ hybridization (FISH) performed on a bone marrow aspirate in 2006 did not reveal any high-risk cytogenetic abnormalities. Induction therapy with vincristine/doxo-rubicin/dexamethasone was followed by double autologous stem cell transplantation after conditioning with high-dose melphalan (200 mg/m2), resulting in a PR that lasted for 2 years. At first relapse, 4 cycles of bortezomib and subsequent bortezomib maintenance were administered and led to a PR for further 3 years. Third-line treatment with lenalidomide, bendamustine and dexamethasone produced only a short-lived response. Therapeutic attempts with the Hsp-90 inhibitor AUY-922 (phase I-Ib/II study) and bortezomib/dexa-methasone resulted in disease progression. Salvage treatment using multidrug combinations of two novel agents (bortezomib plus lenalidomide), alkylating agents (cyclophosphamide or melphalan) and dexamethasone also failed to induce a durable response. With high-grade plasma cell infiltration of the bone marrow (fig. 1), increasingly severe pancytopenia developed, causing chronic fatigue and impaired quality of life and eventually led to treatment discontinuation.
Fig. 1.
Cytomorphology of a bone marrow aspirate smear (×63) performed in August 2010 showing high-grade diffuse infiltration with atypical plasma cells.
In August 2010, at the age of 68 and 8 years after primary diagnosis (fig. 2), we started treatment with pomalidomide (4 mg days 1–21 of a 28-day cycle) and low-dose dexamethasone (40 mg weekly) in combination with doxorubicin (4 mg days 1–4 by continuous infusion). Due to grade 3 thrombocytopenia, the pomalidomide dose was reduced to 2 mg from cycle 2 onwards, resulting in improved tolerability, serological PR and hematological improvement. However, myeloma therapy had to be stopped after six cycles due to the reactivation of a hepatitis B virus (HBV) infection (11.7 × 106 copies/ml), which the patient supposedly acquired in 1945 and which had never emerged during therapy before. Antiviral treatment with entecavir was initiated, which led to a sufficient drop in viral load (9.7 × 102 copies/ml).
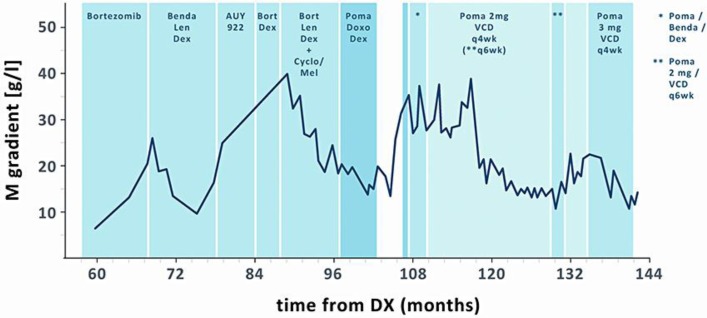
Fig. 2.
Disease course based on M protein levels indicated in months after primary diagnosis (DX). Benda = Bendamustine; Len = lenalidomide; Dex = dexamethasone; Bort = bortezomib; Cyclo = cyclophosphamide; Mel = melphalan; Poma = pomalidomide; VCD = bortezomib/cyclophosphamide/dexa-methasone.
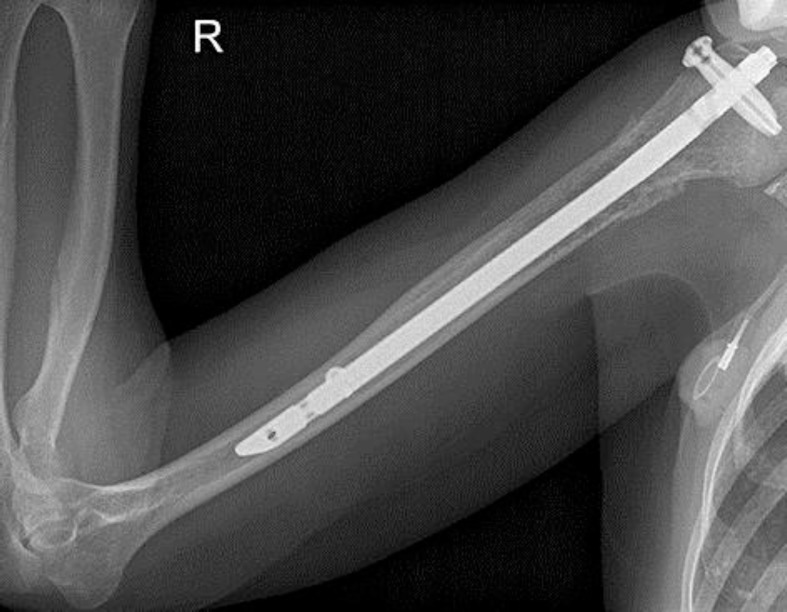
Five months after the interruption of antimyeloma treatment, the patient suffered from serological disease progression and a pathological humeral fracture requiring surgical treatment (fig. 3). To prevent further disease progression, one more cycle of pomalidomide/doxorubicin/dexamethasone was administered. For the subsequent cycles, doxorubicin was replaced by bendamustine (40 mg/m2 days 1–2) in light of the published data reporting an increased risk of HBV reactivation in patients receiving anthracyclines. However, two cycles of the new combination regimen resulted in transfusion-dependent anemia and grade 3 neutropenia, and only stable disease was observed.
Fig. 3.
Radiograph of a pathological humeral fracture corresponding to serological disease progression in June 2011. Imaging after osteosynthesis with a proximally and distally fixed intramedullary nail.
Since the patient was not eligible for treatment within one of the active clinical trials at our institution, individualized therapy was initiated, whereby pomalidomide (2 mg on days 1–21 of a 28-day cycle) was combined with bortezomib (1.3 mg/m2 day 1, 4), cyclophosphamide (250 mg days 1–3) and dexamethasone (10 mg days 1–3, weekly thereafter). As ninth-line therapy, this individualized combination regimen inhibited the progression of the bone lesions, and the patient continued to experience a good quality of life. When the symptoms of sensory polyneuropathy worsened after 18 cycles, bortezomib was reduced to 1.0 mg/m2 and the cycle length was extended to 43 days. Neuropathy improved gradually; however, we observed a steady rise in serologic disease activity. For the subsequent cycles, the pomalidomide dose was increased to 3 mg days 1–21 and the cycles were repeated on day 29, resulting in sustained PR. Due to an extended treatment-free interval after 34 cycles of pomalidomide + VCD (bortezomib/cyclophosphamide/dexamethasone), we observed an increase of serologic markers. More than 12 years after primary diagnosis and 4 years and 4 months after the first treatment with pomalidomide, regrettably, our patient succumbed to a fulminant pneumogenic septicemia in grade 1 neutropenia.
Discussion
Recently, pomalidomide was approved by the EMA for treatment of advanced MM in conjunction with low-dose dexamethasone based on favorable results of a phase III trial [5]. Even though this combination results in further disease control in heavily pretreated myeloma patients, the median gain in the overall survival of roughly 4 months is modest. Triplets of IMiDs, corticosteroids and the first-in-class proteasome inhibitor bortezomib have proven to be effective in various myeloma settings. Therefore, it is tempting to speculate that combining pomalidomide with bortezomib and dexamethasone might be more efficacious than pomalidomide and dexamethasone alone.
Eight years after initial diagnosis and following five lines of treatment, the patient in this report had been refractory to multidrug regimens containing both bortezomib and lenalidomide in combination with alkylating agents and dexamethasone. Having exhausted therapeutic options and with an increasing need for treatment, the idea of re-intensification using pomalidomide and dexamethasone as a backbone was intriguing.
Combinations of IMiDs and doxorubicin have previously shown promising clinical activity with an acceptable toxicity profile in advanced MM [6]. Preliminary results from an ongoing phase I/II study suggest that using pomalidomide together with anthracyclines and dexamethasone is feasible but carries the risk of substantial hematotoxicity [7]. Similarly, in our patient, grade 3 thrombocytopenia was observed after treatment with pomalidomide at 4 mg together with doxorubicin and dexamethasone. Following a pomalidomide dose reduction to 2 mg, the combination was well tolerated and resulted in a rapid serological response and hematologic improvement.
Interestingly, a HBV reactivation occurred after six cycles of pomalidomide/doxo-rubicin/dexamethasone treatment in our patient with an initially unknown HBV carrier status. This had not been observed after previous administration of highly immunosuppressive high-dose melphalan prior to autologous stem cell transplantation [8]. Whilst there are clinical [9] and preclinical [10] data indicating an increased risk of HBV reactivation for doxorubicin-containing treatments, the role of IMiDs remains unclear [11]. Furthermore, severe immune dysfunction associated with advanced myeloma might also have predisposed our patient to virus reactivation [12].
With MM being a disease characterized by intraclonal heterogeneity [13] and with novel agents generally having bearable side effects, the use of combination regimens of three or more drugs, even in the advanced disease setting, becomes more and more enticing. Therefore, we combined pomalidomide/dexamethasone with bortezomib and cyclophosphamide instead of doxorubicin and were able to elicit a long-lasting partial response in this heavily pre-treated refractory patient. Thus, it is tempting to speculate that in a progressive disease setting, substituting various drugs within combination treatments might help in regaining disease control.
Besides the known hematological toxicities [14] that were observed at the maximum tolerated dose (MTD) of 4 mg, pomalidomide proved to be well tolerated in our patient for more than 4 years of therapy. This is in line with recently published data from a French trial [15] in which 12% of the patients were still on treatment with pomalidomide/dexa-methasone after 30 months. Similarly, pomalidomide-based combination regimens appear to be suitable for long-term continuous application as illustrated in our case study. Tailored treatment approaches including careful dose adaptations are essential to control side effects and allow a good quality of life.
Unfortunately, the fact of an insufficient disease control after a prolonged treatment-free interval made our patient more susceptible to formerly well-controlled hematological side effects, resulting in neutropenia and a fulminant pneumogenic septicemia. Despite intensive antiinfective treatment, the patient succumbed to the generalized infection shortly after hospitalization.
Results from systematic trials of combination regimens containing pomalidomide are expected in the near future, further helping to guide therapeutic decisions in the setting of advanced MM. In the meantime, the use of pomalidomide/dexamethasone as a backbone in antimyeloma combination treatment has proven to be effective in a heavily pretreated refractory patient and has demonstrated a good long-term tolerability.
Disclosure Statement
S.D., M.S., S.S. and S.K. have received travel grants from Celgene. S.K. and H.E. have received honoraria and advisory board fees from Celgene. H.E. has received research funding from Celgene.
Acknowledgements
Medical writing support was provided by Juliane Schreier and Marc Esser (co.faktor GmbH, Berlin) and was funded by Celgene.
References
- 1.Larkin M. Low-dose thalidomide seems to be effective in multiple myeloma. Lancet. 1999;354:925. doi: 10.1016/S0140-6736(05)75677-4. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P, LeLeu X, Hulin C, Klein SK, Sonneveld P, Siegel D, Bladé J, Goldschmidt H, Jagannath S, Miguel JS, Orlowski R, Palumbo A, Sezer O, Rajkumar SV, Durie BG. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, Chen C, Lonial S, Jakubowiak A, Bahlis N, Song K, Belch A, Raje N, Shustik C, Lentzsch S, Lacy M, Mikhael J, Matous J, Vesole D, Chen M, Zaki MH, Jacques C, Yu Z, Anderson KC. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, Karlin L, Goldschmidt H, Banos A, Oriol A, Alegre A, Chen C, Cavo M, Garderet L, Ivanova V, Martinez-Lopez J, Belch A, Palumbo A, Schey S, Sonneveld P, Yu X, Sternas L, Jacques C, Zaki M, Dimopoulos M. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 6.Knop S, Gerecke C, Liebisch P, Topp MS, Platzbecker U, Sezer O, Vollmuth C, Falk K, Glasmacher A, Maeder U, Einsele H, Bargou RC. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom) Blood. 2009;113:4137–4143. doi: 10.1182/blood-2008-10-184135. [DOI] [PubMed] [Google Scholar]
- 7.Hilger JD, Berenson JR, Klein LM, Bessudo A, Rosen PJ, Eshaghian S, Chamras H, Nassir Y, Swift RA, Vescio RA. A phase I/II study ( NCT01541332) of pomalidomide (POM), dexamethasone (DEX), and pegylated liposomal doxorubicin (PLD) for patients with relapsed/refractory (R/R) multiple myeloma (MM) ASCO Annual Meeting. 2013:8598. [Google Scholar]
- 8.Borentain P, Colson P, Coso D, Bories E, Charbonnier A, Stoppa AM, Auran T, Loundou A, Motte A, Ressiot E, Norguet E, Chabannon C, Bouabdallah R, Tamalet C, Gérolami R. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg-negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem cell transplantation for cancer. J Viral Hepat. 2010;17:807–815. doi: 10.1111/j.1365-2893.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 9.Ling WH, Soe PP, Pang AS, Lee SC. Hepatitis B virus reactivation risk varies with different chemotherapy regimens commonly used in solid tumours. Br J Cancer. 2013;108:1931–1935. doi: 10.1038/bjc.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CH, Hsu HC, Chen HL, Gao M, Yeh PY, Chen PJ, Cheng AL. Doxorubicin activates hepatitis B virus (HBV) replication in HBV-harboring hepatoblastoma cells. A possible novel mechanism of HBV reactivation in HBV carriers receiving systemic chemotherapy. Anticancer Res. 2004;24:3035–3040. [PubMed] [Google Scholar]
- 11.Mya DH, Han ST, Linn YC, Hwang WY, Goh YT, Tan DC. Risk of hepatitis B reactivation and the role of novel agents and stem-cell transplantation in multiple myeloma patients with hepatitis B virus (HBV) infection. Ann Oncol. 2012;23:421–426. doi: 10.1093/annonc/mdr142. [DOI] [PubMed] [Google Scholar]
- 12.Feyler S, Selby PJ, Cook G. Regulating the regulators in cancer-immunosuppression in multiple myeloma (MM) Blood Rev. 2013;27:155–164. doi: 10.1016/j.blre.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Walker BA, Wardell CP, Melchor L, Brioli A, Johnson DC, Kaiser MF, Mirabella F, Lopez-Corral L, Humphray S, Murray L, Ross M, Bentley D, Gutiérrez NC, Garcia-Sanz R, San Miguel J, Davies FE, Gonzalez D, Morgan GJ. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28:384–390. doi: 10.1038/leu.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanan-Khan AA, Swaika A, Paulus A, Kumar SK, Mikhael JR, Rajkumar SV, Dispenzieri A, Lacy MQ. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013;3:e143. doi: 10.1038/bcj.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, Mathiot C, Petillon MO, Macro M, Roussel M, Pegourie B, Kolb B, Stoppa AM, Hennache B, Bréchignac S, Meuleman N, Thielemans B, Garderet L, Royer B, Hulin C, Benboubker L, Decaux O, Escoffre-Barbe M, Michallet M, Caillot D, Fermand JP, Avet-Loiseau H, Facon T. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–02. Blood. 2013;121:1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]