High-resolution crystal structures of the prostaglandin F synthase enzymes from the protazoa T. cruzi and L. major were solved with and without the cofactor NADP.
Keywords: prostaglandin, infectious diseases, leishmaniasis, Chagas disease, SSGCID
Abstract
The crystal structures of prostaglandin F synthase (PGF) from both Leishmania major and Trypanosoma cruzi with and without their cofactor NADP have been determined to resolutions of 2.6 Å for T. cruzi PGF, 1.25 Å for T. cruzi PGF with NADP, 1.6 Å for L. major PGF and 1.8 Å for L. major PGF with NADP. These structures were determined by molecular replacement to a final R factor of less than 18.6% (R free of less than 22.9%). PGF in the infectious protozoa L. major and T. cruzi is a potential therapeutic target.
1. Introduction
The Seattle Structural Genomics Center for Infectious Disease (SSGCID) is a consortium funded by NIAID to elucidate solutions of protein structures from biodefense organisms, as well as those causing emerging and re-emerging diseases. Prostaglandin F synthase (PGF) from the infectious protozoa Leishmania major (UniProt P22045) and Trypanosoma cruzi (UniProt Q4DJ07) have been identified by the TDR target database as being essential proteins for both organisms and potential therapeutic targets (Magariños et al., 2012 ▶). PGF is an oxidoreductase enzyme that catalyzes the reaction (5Z,13E)-(15S)-9α,11α,15-trihydroxyprosta-5,13-dienoate + NADP+ ⇌ (5Z,13E)-(15S)-9α,15-dihydroxy-11-oxoprosta-5,13-dienoate + NADPH + H+. PGF specifically acts on the CH–OH of the proton donor with NADP as the acceptor (Watanabe et al., 1981 ▶). In humans, PGF (UniProt P42330) can interconvert active androgens, estrogens and progestins with their cognate inactive metabolites (Qin et al., 1993 ▶). In protozoa, PGF is involved in essential lipid-metabolism pathways. The protozoa L. major, the causative agent of leishmaniasis, and T. cruzi, the causative agent of Chagas disease, both affect millions of people and represent major public health issues. Both diseases have very limited treatment options and drug resistance is prevalent (Minodier & Parola, 2007 ▶; Buckner et al., 1998 ▶). Here, we describe the structures of PGF with and without NADP in both organisms and the structural differences between the bound and the unbound state of the enzyme with regard to its cofactor. Previous structural work on PGFs has been performed for Homo sapiens PGF (PDB entry 1ry8; Komoto et al., 2004 ▶) and PGF from T. brucei, another protozoan (PDB entry 1vbj; T. Inoue, unpublished work). PDB entry 1ry8 is a useful structure for determining the selectivity of potential compounds against human PGF, which will need to be taken into consideration (Komoto et al., 2004 ▶). PDB entry 1vbj was used as a model for molecular replacement and has sequence identities of 60% to T. cruzi PGF and 61% to L. major PGF (Fig. 1 ▶).
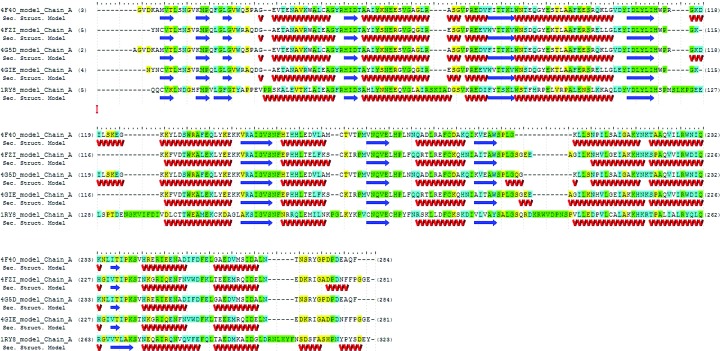
Figure 1.
An alignment of PGFs from H. sapiens (PDB entry 1ry8), T. cruzi (PDB entries 4fzi and 4gie) and L. major (PDB entries 4f40 and 4g5d) with residues that match highlighted in green, residues that are conserved highlighted in blue and residues that are similar highlighted in yellow; residues that are different are not highlighted. The two protozoan proteins have 74% sequence identity to each other. This figure was generated using GeneComposer (Lorimer et al., 2011 ▶).
2. Materials and methods
2.1. Cloning, expression and purification
The gene encoding PGF from L. major strain Friedlin (SSGCID ID LemaA.00019.a.B1) was PCR-amplified from genomic DNA that was kindly provided by Frederick S. Buckner. The gene was amplified using the following primer sequences: FWD primer 5′-CTCACCACCACCACCACCATATGGCTGGCGTTGATAAGGCAAT-3′ and REV primer 5′-ATCCTATCTTACTCACTTAGAACTGCGCCTCATCAGGGTC-3′. Thermal cycling conditions were 371 K for 180 s followed by 34 cycles of 371 K for 30 s, 346 K for 330 s, 360 K for 360 s and 360 K for 360 s followed by a final extension at 351 K for 300 s.
Likewise, the gene encoding PGF from T. cruzi strain CL Brener (SSGCID ID TrcrA.00019.a.B1) was PCR-amplified using the following primer sequences: FWD primer 5′-CTCACCACCACCACCACCATATGAATTGCAATTACAACTGTGTGAC-3′ and REV primer 5′-ATCCTATCTTACTCACTTACTCCTCTCCACCAGGGAAAAAAT-3′. Thermal cycling conditions were 367 K for 180 s followed by 30 cycles of 367 K for 30 s, 333 K for 60 s and 345 K for 120 s followed by a final extension at 345 K for 600 s.
Purified PCR products from both PGF constructs were cloned into a BG1861 expression vector (pET-14b derivative) using ligation-independent cloning (LIC; Aslanidis & de Jong, 1990 ▶). The BG1861 vector contains a T7 promoter, an ampicillin-resistance gene and an N-terminal His6 tag. Plasmid DNA was transformed into Escherichia coli strain BL21 (DE3) for recombinant protein expression.
2 l cultures were grown for both LemaA.00019.a.B1 and TrcrA.00019.a.B1 in a LEX bioreactor (Harbinger) at 293 K in Novagen Overnight Express autoinduction medium. Following 72 h of growth, the cultures were harvested via centrifugation at 5000g for 30 min. The supernatant was discarded and the cell pellets were collected and flash-frozen in liquid nitrogen.
Cell paste for TrcrA.00019.a.B1 and LemaA.00019.a.B1 was resuspended in buffer A [25 mM HEPES pH 7.0, 500 mM NaCl, 5%(v/v) glycerol, 30 mM imidazole, 0.5% CHAPS, 10 mM MgCl2, 1 mM TCEP, 0.01 mg ml−1 lysozyme, 125 U benzonase, 1× EDTA-free protease-inhibitor cocktail from Roche] by stirring at 277 K for 1 h. The cell suspension underwent cell lysis by sonication on ice for 30 min (100 W; cycles of 15 s pulse on and 15 s pulse off). The lysed samples were then clarified via centrifugation at 18 000g for 1 h at 277 K. The supernatant was collected and applied onto a 5 ml HisTrap HP column (GE Healthcare) that had been pre-equilibrated with buffer B [25 mM HEPES pH 7.0, 500 mM NaCl, 5%(v/v) glycerol, 30 mM imidazole, 1 mM TCEP] using an ÄKTAexplorer (GE Healthcare). The column was then washed with buffer B until the A 280 stabilized at background levels. Following the wash step, buffer C [25 mM HEPES pH 7.0, 500 mM NaCl, 5%(v/v) glycerol, 500 mM imidazole, 1 mM TCEP] was applied to elute the target protein from the column. Fractions from nickel-affinity chromatography were analyzed by SDS–PAGE and pooled. Each target was then subjected to further purification by size-exclusion chromatography (SEC) on a Superdex 75 (GE Healthcare) SEC column using buffer D [25 mM Tris pH 8.0, 200 mM NaCl, 1%(v/v) glycerol, 1 mM TCEP]. Fractions were analyzed via SDS–PAGE to determine fractions of interest. Fractions of interest were collected and concentrated to 20 mg ml−1 using a polyethersulfone concentrator with an appropriate molecular-weight cutoff. Protein concentrations were determined with a UV spectrophotometer using the theoretical extinction coefficients determined using the online tool at http://web.expasy.org/protparam/. The final samples were divided into 100 µl aliquots and flash-frozen in liquid nitrogen.
2.2. Protein crystallization
All protein crystallization experiments were performed using the sitting-drop vapor-diffusion method in Compact 300 (Rigaku Reagents) crystallization trays at 289 K. Crystallization experiments consisted of 400 nl protein solution and 400 nl precipitant solution in the sample well equilibrated against 80 µl precipitant solution in the reservoir well. Detailed crystallization conditions including protein concentrations and cofactor concentrations are available in Table 1 ▶. All crystals formed in 2–4 weeks.
Table 1. Crystallization.
| Sample | PDB entry | Protein concentation (mgml1) | Cofactor concentration (mM) | Crystallization screen condition | Reservoir composition |
|---|---|---|---|---|---|
| TrcrA.00019.a.B1, apo | 4fzi | 37 | Morpheus H1 | 0.1M MESimidazole pH 6.5, 10% PEG 20000, 20% PEG 550 MME, 0.02M glutamic acid, glycine, serine, alanine and lysine | |
| TrcrA.00019.a.B1 + NADP | 4gie | 37 | 4 | Index G6 | 0.2M ammonium acetate, 0.1M bis-tris pH 5.5, 25% PEG 3350; cryoprotectant 15% ethylene glycol |
| LemaA.00019.a.B1, apo | 4f40 | 25 | JCSG+ A2 | 0.1M sodium citrate pH 5.50, 20% PEG 3000 | |
| LemaA.00019.a.B1 + NADPH | 4g5d | 25 | 2 | JCSG+ B8 | 200mM magnesium chloride, 100mM Tris pH 7.0, 10% PEG 8000 |
The crystals were harvested using mounted cryoloops (Hampton Research) and flash-cooled in pucks immersed in liquid nitrogen for storage until X-ray diffraction data collection. Data were either collected on our in-house FR-E+ SuperBright X-ray source (Rigaku) or on Advanced Light Source (ALS) beamlines 5.0.1 and 5.0.3. All X-ray reflection data were indexed, integrated and scaled using XDS and XSCALE (Kabsch, 2010a ▶,b ▶). Data statistics for all data sets are available in Table 2 ▶.
Table 2. Data collection and processing.
Values in parentheses are for the outer shell.
| TrcrA.00019.a.B1 | LemaA.00019.a.B1 | |||
|---|---|---|---|---|
| Target ID | Apo | NADPH | Apo | NADPH |
| PDB code | 4fzi | 4gie | 4f40 | 4g5d |
| Space group | P21 | C2 | P21 | P21 |
| a, b, c () | 54.46, 66.62, 87.31 | 155.54, 50.34, 37.60 | 94.55, 34.59, 107.20 | 94.66, 34.59, 106.95 |
| , , () | 90, 94.23, 90 | 90, 94.23, 90 | 90, 103.22, 90 | 90, 102.98, 90 |
| Beamline | 5.0.3, ALS | 5.0.1, ALS | 5.0.1, ALS | Rigaku FR-E + SuperBright |
| Wavelength () | 0.9765 | 0.9774 | 0.9774 | 1.54 |
| Data-collection temperature (K) | 100 | 100 | 100 | 100 |
| Resolution range () | 50.02.60 (2.672.60) | 50.01.25 (1.281.25) | 50.01.60 (1.641.60) | 50.01.80 (1.851.80) |
| Unique reflections | 19336 (1416) | 75887 (4767) | 89147 (5975) | 61422 (4020) |
| Completeneess (%) | 99.7 (99.5) | 94.7 (81.0) | 98.7 (90.2) | 96.5 (86.7) |
| Multiplicity | 4.82 (4.95) | 7.61 (7.11) | 4.06 (3.52) | 5.08 (3.30) |
| I/(I) | 17.75 (3.35) | 23.41 (5.29) | 13.33 (2.37) | 13.36 (2.62) |
| R merge | 0.080 (0.529) | 0.062 (0.369) | 0.083 (0.578) | 0.096 (0.515) |
2.3. Data collection and processing
The diffraction data sets for PDB entries 4gie and 4f40 were collected on ALS beamline 5.0.1 with an ADSC Quantum 315 CCD detector. The diffraction data set for PDB entry 4fzi was collected on ALS beamline 5.0.3 with an ADSC Quantum 315 CCD detector. The diffraction data set for PDB entry 4g5d was collected in-house with a Rigaku FR-E+ SuperBright generator using a Rigaku 944+ CCD detector. All data sets were collected at 100 K. The X-ray data were reduced with XDS and XSCALE. Details of the data collections are summarized in Table 2 ▶.
2.4. Structure solution and refinement
Phases for structure determination were obtained via molecular replacement with Phaser (McCoy et al., 2007 ▶) using PDB entry 1vbj as a search model for PDB entry 4f40. After preparing the starting models with CHAINSAW (Stein, 2008 ▶), monomers were used as the search model in molecular replacement. All other structures were solved by molecular replacement using PDB entry 4f40 as the search model. Initial molecular model building was performed using ARP/wARP (Langer et al., 2008 ▶). Models were refined against the X-ray reflection data using either PHENIX (Adams et al., 2010 ▶) and REFMAC (Murshudov et al., 2011 ▶) interspersed with rounds of model building using Coot (Emsley et al., 2010 ▶). TLS groups were chosen using phenix.find_tls_groups within the PHENIX suite (Afonine et al., 2012 ▶). Figures containing molecular graphics were prepared using PyMOL (Schrödinger). Solution and refinement statistics can be found in Table 3 ▶.
Table 3. Structure solution and refinement.
Values in parentheses are for the outer shell.
| PDB code | 4fzi | 4gie | 4f40 | 4g5d |
|---|---|---|---|---|
| Resolution range () | 50.02.60 (2.702.60) | 29.91.25 (1.281.25) | 46.21.60 (1.641.60) | 46.21.80 (1.851.80) |
| Completeness (%) | 99.7 (99.5) | 94.7 (81.0) | 98.7 (90.2) | 96.5 (86.7) |
| Cutoff | F > 0.0(F) | F > 0.0(F) | F > 0.0(F) | F > 0.0(F) |
| No. of reflections, working set | 19335 (1298) | 75882 (4543) | 89114 (2444) | 61405 (2307) |
| No. of reflections, test set | 988 (67) | 3826 (218) | 4466 (135) | 3097 (131) |
| Final R cryst | 0.186 (0.267) | 0.119 (0.170) | 0.154 (0.2151) | 0.172 (0.2219) |
| Final R free | 0.229 (0.354) | 0.136 (0.184) | 0.180 (0.2228) | 0.218 (0.2890) |
| No. of non-H atoms | ||||
| Protein | 4252 | 2309 | 4366 | 4402 |
| Ion | 5 | 4 | 1 | 0 |
| Ligand | 0 | 48 | 98 | 96 |
| Water | 84 | 413 | 674 | 860 |
| Total | 4361 | 2774 | 5139 | 5358 |
| R.m.s. deviations | ||||
| Bonds () | 0.013 | 0.007 | 0.014 | 0.009 |
| Angles () | 1.497 | 1.448 | 1.540 | 1.332 |
| Average B factors (2) | ||||
| Wilson B | 39.8 | 12.3 | 19.7 | 17.6 |
| Protein | 36.1 | 7.4 | 13.1 | 12.1 |
| Ion | 65.6 | 5.8 | 49.9 | |
| Ligand | 4.3 | 27.4 | 9.9 | |
| Water | 25.6 | 20.6 | 26.8 | 27.8 |
| Ramachandran plot | ||||
| Most favoured (%) | 95.74 | 97.43 | 98.33 | 97.60 |
| Allowed (%) | 4.07 | 2.57 | 1.30 | 1.99 |
| Asymmetric unit content | 2 chains | 1 chain | 2 chains | 2 chains |
| B-factor refinement | Isotropic | Anisotropic | Isotropic | Isotropic |
| TLS refinement | Yes, two groups | No | Yes, ten groups | Yes, ten groups |
3. Results and discussion
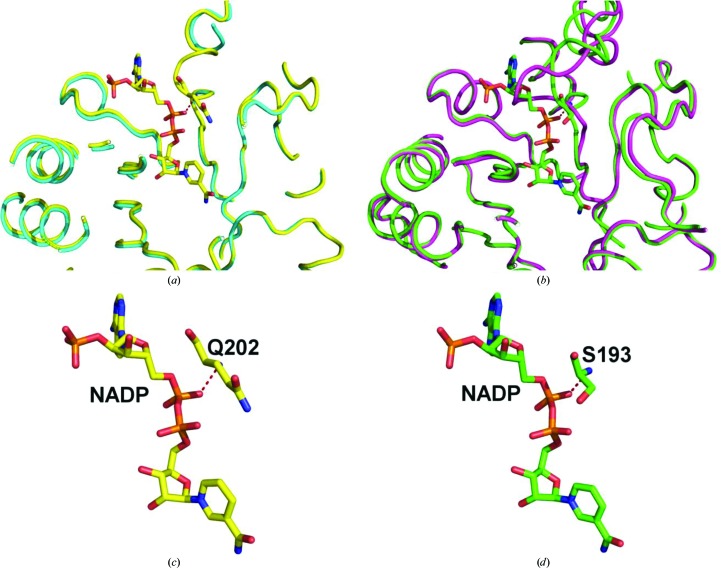
As part of the efforts of the SSGCID as a high-throughput structure-determination consortium our goal is to help enable, through structure determination, therapeutic development or function determination of infectious disease proteins (Myler et al., 2009 ▶). For each of the two proteins two crystallization trials were set up before finding usable crystals. For LemaA.00019.a.B1 JCSG+ and PACT were used. For TrcrA.00019.a.B1 the Morpheus and Index screens were used. The prostaglandin F synthases from both L. major and T. cruzi represent classic NADP-binding Rossmann-fold structural motifs, as are common in oxidoreductases (Rao & Rossmann, 1973 ▶). These high-resolution structures show that the same loop [residues 188–196 in TrcrA.00019.a.B1 and residues 201–205 (disordered) in LemaA.00019.a.B1] has significant movement between the apoenzyme and holoenzyme (Fig. 2 ▶). The L. major loop becomes ordered and a key interaction with one of the phosphates of NADP at Gln202 is likely to stabilize this region (Figs. 3 ▶ a and 3 ▶ c). Similarly, T. cruzi Ser193 makes an interaction with one of the phosphates of NADP that is likely to stabilize the loop in a significantly different orientation (Figs. 3 ▶ b and 3 ▶ d). In the human PGF structure (PDB entry 1ry8), Ser221 makes a hydrogen-bond interaction with one of the phosphates of NADP via the backbone amide and is the equivalent residue to Ser193 and Gln202 in the T. cruzi and L. major structures, respectively. The pairwise Cα r.m.s.d. between the two apo structures of protozoan PGFs is 0.400 Å. The r.m.s.d.s between the two cofactor-bound structures of protozoan PGFs is 0.409 Å. The structures of these proteins with and without their cofactor provide a more robust and clearer understanding of potential binding sites for not only the cofactor but also for the substrate and any subsequent molecules designed for these proteins. Their structural similarity could be useful as a template for structure-based drug-design efforts for therapeutics that target both L. major and T. cruzi. However, the high structural similarity of human PGF (PDB entry 1ry8), with a Cα r.m.s.d. of 0.656 Å for PDB entry 4gie and 0.729 Å for PDB entry 4g5d, represents a potential hurdle for the design of selective molecules even though the sequence identity between human PDF and the T. cruzi and L. major PGFs is 36 and 37%, respectively. As the interaction of residue Ser221 with the cofactor takes place through the backbone amide, the difference in the amino acid at this location in the binding pocket is not likely to be very important.
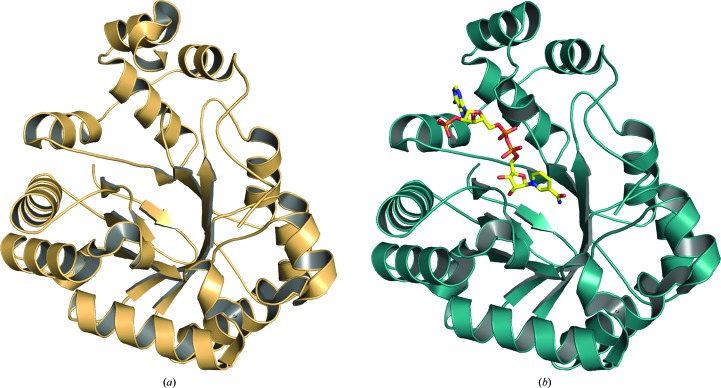
Figure 2.
The overall fold of (a) T. cruzi PGF (PDB entry 4fz1) and (b) T. cruzi PGF with NADP bound (PDB entry 4gie). The appearance of the disordered loop in the NADP-bound structure can be seen.
Figure 3.
(a) Overlay of PDB entry 4f40 (blue) and PDB entry 4g5d (yellow) from L. major showing the NADP binding-site area. (b) Overlay of PDB entry 4fzi (purple) and PDB entry 4gie (green) from T. cruzi showing the NADP binding-site area. (c) NADP from PDB entry 4g5d and its interaction with Gln202. (d) NADP from PDB entry 4gie and its interaction with Ser193.
Supplementary Material
PDB reference: T. cruzi prostaglandin F synthase, apo, 4fzi
PDB reference: complex with NADP, 4gie
PDB reference: L. major prostaglandin F synthase, apo, 4f40
PDB reference: complex with NADPH, 4g5d
Acknowledgments
The authors wish to thank the entire SSGCID team and David M. Dranow. This research was funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, Department of Health and Human Services under Federa Contract Nos. HHSN272201200025C and HHSN272200700057C. The expression plasmids and surplus protein samples can be obtained from either the contact authors or through http://www.ssgcid.org and the raw X-ray diffraction data can be obtained through http://www.csgid.org.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Aslanidis, C. & de Jong, P. J. (1990). Nucleic Acids Res. 18, 6069–6074. [DOI] [PMC free article] [PubMed]
- Buckner, F. S., Wilson, A. J., White, T. C. & Van Voorhis, W. C. (1998). Antimicrob. Agents Chemother. 42, 3245–3250. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010a). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010b). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Komoto, J., Yamada, T., Watanabe, K. & Takusagawa, F. (2004). Biochemistry, 43, 2188–2198. [DOI] [PubMed]
- Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. (2008). Nature Protoc. 3, 1171–1179. [DOI] [PMC free article] [PubMed]
- Lorimer, D., Raymond, A., Mixon, M., Burgin, A., Staker, B. & Stewart, L. (2011). Acta Cryst. F67, 985–991. [DOI] [PMC free article] [PubMed]
- Magariños, M. P., Carmona, S. J., Crowther, G. J., Ralph, S. A., Roos, D. S., Shanmugam, D., Van Voorhis, W. C. & Agüero, F. (2012). Nucleic Acids Res. 40, D1118–D1127. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Minodier, P. & Parola, P. (2007). Travel Med. Infect. Dis. 5, 150–158. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Myler, P. J., Stacy, R., Stewart, L., Staker, B. L., Van Voorhis, W. C., Varani, G. & Buchko, G. W. (2009). Infect. Disord. Drug Targets, 9, 493–506. [DOI] [PMC free article] [PubMed]
- Qin, K.-N., New, M. I. & Cheng, K.-C. (1993). J. Steroid Biochem. Mol. Biol. 46, 673–679. [DOI] [PubMed]
- Rao, S. T. & Rossmann, M. G. (1973). J. Mol. Biol. 76, 241–256. [DOI] [PubMed]
- Stein, N. (2008). J. Appl. Cryst. 41, 641–643.
- Watanabe, K., Shimizu, T. & Hayaishi, O. (1981). Biochem. Int. 2, 603–610.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: T. cruzi prostaglandin F synthase, apo, 4fzi
PDB reference: complex with NADP, 4gie
PDB reference: L. major prostaglandin F synthase, apo, 4f40
PDB reference: complex with NADPH, 4g5d