Abstract
Obesity is a critical public health issue worldwide. Patients with obesity have markedly increased morbidity and mortality compared to the general population. The increased health risks of obesity in part are due to its close association with each of the other components of the metabolic syndrome, including hypertension, dyslipidemia, and insulin resistance. Accordingly, obese individuals are at particularly increased risk of cardiovascular disease and chronic kidney disease. Modest weight loss results in improvements in serum cholesterol levels, blood pressure, and glycemic profiles. Lifestyle interventions for weight loss have long been the mainstay of treatment in obesity. However, the existing literature demonstrates limited weight loss sustainability and inconsistent cardiovascular and renal benefits using these modalities. In addition to improvements in intermediate risk factors, surgical interventions provide a more lasting impact on long-term cardiovascular and renal outcomes, though carry higher short-term risks due to perioperative complications.
Keywords: Obesity, Metabolic syndrome, Bariatric surgery, Lifestyle interventions, Cardiovascular disease, Chronic kidney disease
Introduction
Obesity is a widespread and constantly evolving public health issue in the United States of America (USA) and other developed countries. Obesity is defined as a body mass index (BMI) ≥30 kg/m2, class I obesity is a BMI of 30–34.9 kg/m2, severe obesity or class II obesity is a BMI of 35–39.9 kg/m2, and class III obesity (previously known as morbid obesity) is a BMI of ≥40 kg/m2 [1]. According to the National Health and Nutrition Examination Survey, the number of obese adults in the US population increased from 22.9 % in 1988 to 1994 to 35.7 % in 2009 to 2010 [2, 3]. Although the rate of obesity in the USA seems to be stabilizing based on the most recent survey results, there continues to be an alarming increase in the prevalence of severe levels of obesity, particularly among adolescents [4, 5]. Patients with class II and III obesity place the greatest burden on the healthcare system. In 2010, the estimated healthcare costs of obesity and comorbidities associated with obesity were 315.8 billion dollars in the USA alone [6]. These costs were substantially greater in patients with a BMI ≥35 kg/m2, particularly in patients with concurrent diabetes [6].
Obesity is a major contributing risk factor to a number of serious morbidities, resulting in increased disability and mortality and in reduced overall quality of life. Specifically, obesity is a strong independent risk factor for the development of each of the other components of the metabolic syndrome, including insulin resistance, dyslipidemia, and hypertension [7, 8]. Obese men are at a 7-fold increased risk, and obese women are at a 12-fold increased risk of developing type 2 diabetes mellitus compared to normal-weight individuals [8]. Obese men and women have a 2-fold and 3-fold increased risk of cardiovascular disease, respectively, a 2-fold increased incidence of hypertension, and a 1.5-fold increased incidence of stroke compared to normal-weight individuals [8].
Additionally, obesity substantially increases the risk for development and progression of kidney disease. Obesity plays a critical role in increasing the rate of progression of chronic kidney disease (CKD) in patients with underlying glomerular disease [9]. The increased risk of kidney disease in obese patients is likely, in part, due to the increased incidence of diabetes and hypertension as these conditions account for the majority of CKD cases in the USA [10]. However, obesity is also a significant independent risk factor for the development of CKD and end-stage renal disease (ESRD) [11, 12]. In 2006, Hsu et al. performed a retrospective cohort study of 320, 252 patients linking the Kaiser Permanente database to the US Renal Data System Registry. After adjusting for diabetes and hypertension, the authors found a 3-fold increased incidence of ESRD in all levels of obesity and a 5-fold increased incidence of ESRD in morbidly obese patients compared to normal-weight individuals [11].
The proposed mechanisms of obesity-associated cardiovascular disease and kidney disease are multifactorial and seem to be closely intertwined (see Fig. 1). When occurring in the setting of excess adipose tissue, these disease processes have each been linked to insulin resistance, inflammatory cytokines (i.e., IL-6 and TNF-α), oxidative stress, increased sympathetic nervous activity, adiponectin deficiency, increased leptin production, and increased renin, angiotensin, and aldosterone activity [13–15]. The effect of directly intervening on these metabolic parameters has not been thoroughly investigated. However, there is a growing body of epidemiologic evidence evaluating the impact of various weight reduction modalities on long-term cardiovascular and renal outcomes.
Fig. 1.
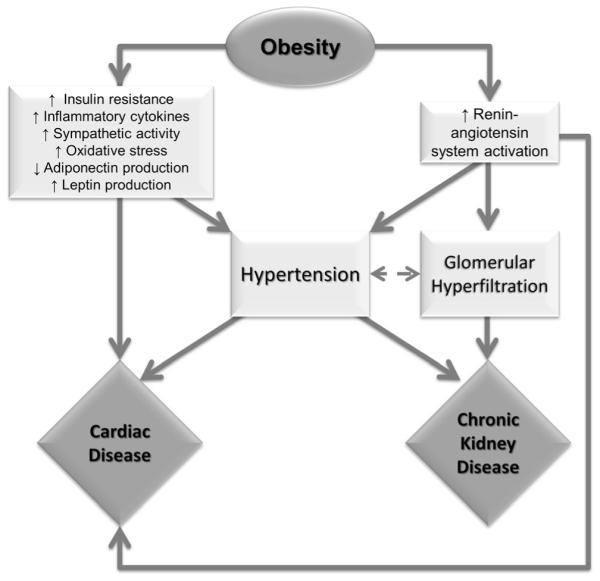
Mechanisms contributing to cardiovascular disease and chronic kidney disease in obesity and the metabolic syndrome
Even modest weight reduction in individuals with a BMI≥ 35 kg/m2 is associated with notable improvements in many of the intermediate comorbidities associated with cardiovascular and renal disease, such as type 2 diabetes mellitus [6]. Multiple international efforts have been implemented to determine the most efficient, cost-effective, sustainable, and safest means of weight reduction among obese patients [1]. Numerous methods of weight reduction have been widely studied, including both discrete use and combinations of diets, exercise regimens, medications (for appetite suppression or decreased fat absorption), and surgical interventions, with variable results with regard to weight loss and reduction of comorbidities [16•, 17, 18].
Cardiovascular Effects of Lifestyle Interventions
Lifestyle interventions have been the mainstay of obesity treatment for the past several decades. However, lifestyle interventions have long been criticized for inadequate effectiveness and durability. Accordingly, a recent Cochrane systematic review of lifestyle modifications in the treatment of obesity revealed that diet, exercise, or both in combination with a counseling model to facilitate behavioral change were not effective at yielding sustained weight loss [18]. Nonetheless, combined lifestyle interventions, including counseling along with diet and/or exercise, reduce intermediate cardiovascular outcomes such as cholesterol and blood pressure. In a meta-analysis of existing interventional studies involving behavioral counseling, Lin et al. demonstrated that total cholesterol decreased by a pooled mean of 4.48 mg/dL (95 % confidence interval (CI) 2.59–6.36), low-density lipoprotein decreased by 3.43 mg/dL (95 % CI 1.49–5.37), systolic blood pressure decreased by 2.03 mmHg (95 % CI 1.15–2.91), and diastolic blood pressure decreased by 1.38 mmHg (95 % CI 0.84–1.92) [19]. However, the included studies were limited to a maximum of 2 years of follow-up and were not exclusive to obese patients. In an observational analysis of 1-year follow-up data from a recent randomized control trial (RCT; Look AHEAD (Action for Health in Diabetes)) of intensive lifestyle interventions in type 2 diabetic patients, Wing et al. found similar improvements in cardiovascular risk factors. In patients who lost 5–10 % of their body weight, there was a 5 mg/dL increase in high-density lipoprotein cholesterol (odds ratio (OR) 1.69, 95 % CI 1.37–2.09), 40 mg/dL decrease in triglycerides (OR 2.20, 95 % CI 1.71–2.83), 5 mmHg decrease in systolic blood pressure (OR 1.56, 95 % CI 1.27–1.91), and 5 mmHg decrease in diastolic blood pressure (OR 1.48, 95 % CI 1.20–1.82) [20]. However, the Look AHEAD trial was stopped early after a median follow-up of 9.6 years due to a failure to detect a difference in the composite outcome of cardiovascular death, myocardial infarction, non-fatal stroke, or hospitalization for angina, despite significantly greater weight reduction in the intervention group [21••].
Correspondingly, changes in surrogate markers of cardiac disease observed in short-term intervention studies did not correlate to cardiovascular outcomes in long-term observational studies of non-surgical weight loss in obese subjects. Several observational studies over the past two decades demonstrated that non-surgical weight reduction was associated with no difference in cardiovascular outcomes [22] or was paradoxically associated with increased cardiovascular morbidity and mortality [23, 24]. That said, a recent meta-analysis by Quinn et al. found that some of the adverse cardiac outcomes associated with weight loss in observational studies seem to be related to unintentional weight loss [25]. The authors suggest that many observational studies capture weight loss that occurred due to unmeasured confounders like malnourishment or severe chronic illness, which may heavily bias the results.
Most RCTs evaluating lifestyle modifications in the treatment of obesity failed to report long-term cardiovascular outcomes [18, 26]. Two RCTs evaluating the effect of lifestyle modification on development of type 2 diabetes mellitus in overweight individuals did report cardiovascular morbidity and mortality [27, 28]. The Finnish Diabetes Prevention Study demonstrated no significant difference in cardiovascular events between the lifestyle intervention group and the control group after 10 years of follow-up (relative risk (RR) 1.02, 95 % CI 0.73–1.42), despite a significant delay in the onset of diabetes [27]. Similarly, the Da Qing Diabetes Prevention Trial, which demonstrated lower incidence of diabetes in the intervention group, showed no significant difference in cardiovascular events (RR 0.98, 95 % CI 0.71–1.37) or cardiovascular mortality (RR 0.83, 95 % CI 0.48–1.40) over 20 years of follow-up [28]. Both of these studies had lower mortality rates than those in patients with similar risk factors in the general population, and the latter study was statistically underpowered to detect differences in cardiovascular outcomes, weakening the generalizability of the results.
Cardiovascular Effects of Surgical Interventions
Based on a recent Cochrane systematic review of RCTs, bariatric procedures have consistently demonstrated more dramatic, persistent weight reduction compared to non-surgical interventions [16•]. Chang et al. recently published a systematic review and meta-analysis chronicling the overall benefits and risks of bariatric surgery in studies published through 2012 [29]. The meta-analysis encompassed almost 162,000 patients across 127 observational studies and 37 RCTs. Of the studies that reported perioperative (≤30 days) and postoperative (>30 days) mortality rates, RCTs and observational studies demonstrated 0.08 and 0.22 % perioperative mortality, respectively, and 0.31 and 0.35 % postoperative mortality, respectively. The greatest mortality rates were appreciated in gastric bypass, followed by sleeve gastrectomy. Sixty-four studies reported complication rates (mean 17 %), including bleeding, stomal stenosis, leak, vomiting, reflux, gastrointestinal symptoms, and nutritional and electrolyte abnormalities, which were similar across all surgical modalities. Adjustable gastric banding had the highest reoperation rates (12 % in RCTs and 7 % in observational studies). Only 11 studies (all observational) reported long-term weight loss outcomes; these studies demonstrated maintained reduction in BMI of 12–17 kg/m2 at 5 years following bariatric surgery [29]. Results from the prospective observational Swedish Obese Subjects (SOS) study demonstrated 6.5 kg/m2 reduction in BMI from baseline at 10 years of follow-up [30] and 7.1 kg/m2 reduction in BMI at 15 years of follow-up [31].
Surgical interventions have a greater effect on intermediate risk factors for cardiovascular disease than non-surgical interventions. In pooled analyses of RCTs evaluating surgical weight loss modalities, 92 % (95 % CI 85–97 %) of patients had remission of diabetes, 75 % (95 % CI 62–86 %) had remission of hypertension, and 76 % (95 % CI 56–91 %) had remission of dyslipidemias, with slightly less dramatic results reported in observational studies [29] and similar results reported in RCTs published after the meta-analysis [32•, 33, 34]. Diabetes remission rates decreased to 30.4 % at 15 years of follow-up in the SOS study; microvascular and macrovascular complications associated with diabetes were significantly reduced compared to non-surgical controls [35••]. A recent meta-analysis by Vest et al. of echocardiographic parameters in patients who underwent bariatric surgery demonstrated regression of left ventricular hypertrophy and improvement in diastolic function over a mean of 20.2 months of follow-up [36]. Data evaluating changes in ejection fraction among patients with systolic heart failure were scarce and were determined to be insufficient for analysis.
Similar to lifestyle interventions, very few studies of surgical weight loss interventions evaluated long-term cardiovascular morbidity and mortality. Three recent observational studies (and no RCTs) reported cardiovascular events in bariatric surgery patients. In a large retrospective cohort study, Scott et al. found a significant reduction in 5-year rates of myocardial infarction (hazard ratio (HR) 0.54, 95 % CI 0.44–0.67), stroke (HR 0.59, 95 % CI 0.37–0.95), and death (HR 0.60, 95 % CI 0.34–1.07) in morbidly obese bariatric surgery patients compared to morbidly obese patients who underwent unrelated surgeries [37, 38]. Johnson et al. performed a population-based, retrospective, propensity-matched cohort study of obese type 2 diabetic patients; the study demonstrated a significantly lower 5-year rate of composite myocardial infarction, stroke, and all-cause mortality in patients who underwent bariatric surgery compared to controls (HR 0.32, 95 % CI 0.19–0.54) [39]. Recently published 15-year follow-up data from the SOS study showed significantly lower rates of first-time cardiovascular events (myocardial infarction or stroke; HR 0.67, 95 % CI 0.54–0.83) and cardiovascular death (HR 0.47, 95 % CI 0.29–0.76) in obese patients who underwent bariatric surgery versus those who received usual care [40••]. Of note, the usual care group in the SOS study had a significantly higher mean age, systolic and diastolic blood pressure, prevalence of diabetes, and number of smokers at baseline compared to the surgical intervention group, all of which were adjusted for in multivariate analyses [40••]. Thus, in retrospective and prospective observational cohorts, bariatric surgery consistently demonstrated improvements in long-term cardiovascular morbidity and mortality. Further investigation is needed to corroborate these findings in RCT settings and to better understand any morbidity resulting from surgical complications.
Renal Effects of Lifestyle Interventions
Due to the hormonal dysregulation present in obesity, particularly increased activation of the renin-angiotensin system, renal hemodynamics are frequently altered in these patients. The resulting afferent renal arteriolar dilation and efferent renal arteriolar vasoconstriction expose each individual nephron to increased renal plasma flow as body mass increases, causing increased glomerular pressure and glomerular hyperfiltration. These maladaptive changes likely result in the glomerulomegaly, podocytopathy, and focal glomerulosclerosis often observed in renal biopsies in these patients [13, 15, 41]. The podocytopathy coupled with increased intracapillary pressure often results in proteinuria [13, 15].
A number of studies have evaluated the effects of lifestyle interventions on renal outcomes in obese patients, including several small RCTs [42–44]. In the most recent RCT by Straznicky et al., 38 patients with metabolic syndrome and normal baseline renal function were randomized to dietary restriction, diet combined with aerobic exercise, and no intervention. At 12 weeks of follow-up, patients experienced significant weight loss in both intervention groups compared to controls, along with significant decrease in serum creatinine, increase in estimated glomerular filtration rate (GFR), and decrease in albuminuria [44].
In both observational studies and RCTs evaluating weight loss due to dietary interventions in diabetic nephropathy, patients exhibited reduction in proteinuria (mean difference −0.66 to −1.77 g/24 h); patients who underwent combined diet and exercise interventions had a slower decline in renal function compared to control subjects (−9.2 vs. −20.7 mL/min, p<0.001) over 2 years of follow-up [45, 46]. Similar improvement in proteinuria and decelerated loss of renal function were observed in non-diabetic obese patients and patients with more advanced CKD who underwent lifestyle interventions [42, 43, 47]. Improvements in renal measures following non-surgical weight loss have been observed both in conjunction with and independently of remission of hypertension and diabetes [39, 48]. Modest weight loss was associated with decreased muscle sympathetic nerve activity [44] and renin-angiotensin activity [48], potentially contributing to the observed improvements in both hypertension and renal function.
Studies evaluating lifestyle interventions on renal outcomes in obese patients were greatly limited by short durations of follow-up and small sample sizes. No studies have reported long-term renal effects of lifestyle modifications beyond 2 years of follow-up. Also, many of the studies that reported changes in renal function employed estimated GFR equations. These creatinine-based estimating equations can be problematic due to changes in muscle mass with lifestyle interventions. Additionally, many of these equations are normalized to non-obese body surface area (1.73 m2) [49]. Interpretation of the results is further complicated by often misleading, maladaptive elevation in the estimated GFR due to renal hyperfiltration in the early stages of obesity-associated renal injury [15]. No studies have incorporated renal biopsy data following lifestyle interventions. One study demonstrated significant improvement in proteinuria in patients with biopsy-proven obesity-associated kidney disease (defined in the study as glomerulomegaly with or without focal segmental glomerulosclerosis) who succeeded in sustained weight loss following lifestyle modifications after 2 years of follow-up [50]. However, one case report of an obese adolescent girl who lost 30 % of her body weight with diet and exercise demonstrated progression of focal segmental glomerulosclerosis on biopsy after 1 year, despite remission of proteinuria [51].
Renal Effects of Surgical Interventions
By meta-analysis, patients who underwent surgical weight loss interventions had a reversal of renal hyperfiltration [52]. More recent observational evidence demonstrated an improvement in proteinuria and normalization of estimated GFR in patients with baseline hyperfiltration, normal estimated GFR, and mildly decreased estimated GFR 1 year following bariatric surgery, with a high proportion of patients experiencing complete or near-complete resolution of microalbuminuria [53–57]. A recent small prospective observational study demonstrated an improvement in urinary and serum inflammatory markers that correlated with normalization of renal function and weight loss 1 year following bariatric surgery [58].
Similar to studies of lifestyle modification, most studies of renal outcomes following bariatric surgery were limited by a short duration of follow-up, the use of creatinine-based estimation equations of renal function, and difficulty in interpreting changes in GFR due to frequent baseline renal hyperfiltration. One recent small prospective study of patients with elevated creatinine levels at baseline demonstrated improvement in adiponectin levels but no change in measured GFR 1 year following bariatric surgery [59]. Although recent results from the SOS study demonstrate a significant overall improvement in diabetic microvascular (i.e., ophthalmologic, peripheral nervous, and renal) complications 15 years following bariatric surgery, specific renal outcomes were not reported [35••]. One case-controlled study followed obese diabetic patients for 10 years after undergoing biliopancreatic diversion [60]. The study reported an increase in estimated GFR overtime in patients who underwent surgical intervention (13.6±24.5 %) compared to a decrease in estimated GFR in those who received usual care (−45.7±18.8 %). In all patients with baseline microalbuminuria who underwent surgical intervention, microalbuminuria resolved and did not recur. No studies have evaluated changes in pathological findings on renal biopsy following bariatric surgery. Case reports and case series of patients with biopsy-proven obesity-associated focal segmental glomerulosclerosis have demonstrated improvement in proteinuria and stabilization of renal function following bariatric surgery [61–63].
Few studies have reported renal-associated perioperative morbidity in bariatric surgery. Based on retrospective data, degree of baseline renal dysfunction directly correlated to risk of perioperative complications surrounding bariatric surgery, independent of underlying hypertension and diabetes [64•]. Also, a recent retrospective study of patients who underwent bariatric surgery demonstrated a relatively high incidence of acute kidney injury (5.8 %) [65]. In the study, higher BMI and diabetes were independently associated with increased odds of perioperative acute injury.
Conclusions
Weight loss in obese patients is critical in controlling long-term morbidity and mortality. Dietary interventions and increased physical activity are reasonably effective in improving all of the components of the metabolic syndrome, which typically serve as reliable intermediate markers of cardiovascular and renal outcomes. However, lifestyle modifications suffer from inadequate durability of weight loss, and no studies have demonstrated long-term cardiovascular or renal benefit using these modalities. Bariatric surgery carries several associated risks. The existing studies have multiple limitations, but the data are nonetheless promising regarding improvements in long-term cardiovascular and renal outcomes following bariatric surgery. More investigations are needed to understand specific factors that predict improved long-term outcomes in these patients, as well as the complex mechanisms contributing to the impact of surgical interventions. Our understanding of long-term outcomes in these patients also hinges on developing more appropriate measurements of disease, particularly in the setting of renal dysfunction, given the limitations of existing estimating equations of glomerular function. In counseling our patients with severe obesity, we need to keep in mind that the chronic benefits of bariatric surgery should be assessed based on the potential acute risks and contributing comorbidities of each individual.
Footnotes
This article is part of the Topical Collection on Hypertension and Obesity
Conflict of Interest Jordana B. Cohen and Debbie L. Cohen declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.WHO Consultation on Obesity . Obesity: preventing and managing the global epidemic. Vol. 894. World Health Organization; Geneva, Switzerland: 2000. p. 9. WHO Technical Report Series. [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. doi:10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. National Center for Health Statistics; Hyattsville, MD: 2012. NCHS data brief, no 82. http://www.cdc.gov/nchs/data/databriefs/db82.pdf. [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. doi:10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37(6):889–91. doi: 10.1038/ijo.2012.159. doi:10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. PharmacoEconomics. 2014 doi: 10.1007/s40273-014-0230-2. doi:10.1007/s40273-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126(25):2983–9. doi: 10.1161/CIRCULATIONAHA.112.117333. doi:10.1161/CIRCULATIONAHA.112.117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. doi:10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoux F, Mariat C, Maillard N. Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant. 2013;28(suppl 4):iv160–6. doi: 10.1093/ndt/gft286. doi:10.1093/ndt/gft286. [DOI] [PubMed] [Google Scholar]
- 10.System. URD . USRDS 2014 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. http://www.usrds.org/2014/download/V2_Ch_01_ESRD_Incidence_Prevalence_14.pdf. [Google Scholar]
- 11.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 12.Burton JO, Gray LJ, Webb DR, Davies MJ, Khunti K, Crasto W, et al. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant. 2012;27(5):1860–6. doi: 10.1093/ndt/gfr574. doi:10.1093/ndt/gfr574. [DOI] [PubMed] [Google Scholar]
- 13.Thethi T, Kamiyama M, Kobori H. The link between the reninangiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep. 2012;14(2):160–9. doi: 10.1007/s11906-012-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364–76. doi: 10.1038/nrendo.2014.44. doi:10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33(1):23–33. doi: 10.1016/j.semnephrol.2012.12.003. doi:10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. doi:10.1002/14651858.CD003641.pub4. In this recent Cochrane Review of RCT’s evaluating one to two year outcomes following bariatric surgery, the authors concluded that bariatric surgery results in greater, more sustained reduction in body weight and obesity-associated comorbidities compared to non-surgical interventions. The authors also noted that adverse events associated with bariatric surgery and re-operation rates are poorly reported in the literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD003817. doi: 10.1002/14651858.CD003817.pub3. doi:10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastellos N, Gunn LH, Felix LM, Car J, Majeed A. Transtheoretical model stages of change for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2014;2:CD008066. doi: 10.1002/14651858.CD008066.pub3. doi:10.1002/14651858.CD008066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle for cardiovascular disease prevention in persons with cardiovascular risk factors: an updated systematic evidence review for the U.S. Preventive Services Task Force. US Preventive Services Task Force Evidence Synthesis. 2014 13-05179-EF-1. [PubMed] [Google Scholar]
- 20.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6. doi: 10.2337/dc10-2415. doi:10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. doi:10.1056/NEJMoa1212914. This large, multi-center RCT randomized patients with type 2 diabetes to lifestyle interventions (including calorie-restricted diet and increased physical activity) versus diabetes support and education, with plans to follow patients up to 13.5 years. The study was discontinued early on the basis of futility because of a failure to detect a difference in cardiovascular events between the treatment and control groups after a median follow up of 9.6 years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker M, Wannamethee G, Whincup PH, Shaper AG. Weight change and risk of heart attack in middle-aged British men. Int J Epidemiol. 1995;24(4):694–703. doi: 10.1093/ije/24.4.694. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson PM, Nilsson JA, Hedblad B, Berglund G, Lindgarde F. The enigma of increased non-cancer mortality after weight loss in healthy men who are overweight or obese. J Intern Med. 2002;252(1):70–8. doi: 10.1046/j.1365-2796.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- 24.Pamuk ER, Williamson DF, Serdula MK, Madans J, Byers TE. Weight loss and subsequent death in a cohort of U.S. adults. Ann Intern Med. 1993;119(7):744–8. doi: 10.7326/0003-4819-119-7_part_2-199310011-00023. Pt 2. [DOI] [PubMed] [Google Scholar]
- 25.Pack QR, Rodriguez-Escudero JP, Thomas RJ, Ades PA, West CP, Somers VK, et al. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc. 2014;89(10):1368–77. doi: 10.1016/j.mayocp.2014.04.033. doi:10.1016/j.mayocp.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieland LS, Falzon L, Sciamanna CN, Trudeau KJ, Brodney S, Schwartz JE, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev. 2012;8:CD007675. doi: 10.1002/14651858.CD007675.pub2. doi:10.1002/14651858.CD007675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uusitupa M, Peltonen M, Lindstrom J, Aunola S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—secondary analysis of the randomized trial. PLoS One. 2009;4(5):e5656. doi: 10.1371/journal.pone.0005656. doi:10.1371/journal.pone.0005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–9. doi: 10.1016/S0140-6736(08)60766-7. doi:10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 29.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. doi:10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi: 10.1056/NEJMoa035622. doi:10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 31.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(Suppl 7):S93–7. doi: 10.1038/ijo.2008.244. doi:10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 32.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. doi:10.1056/NEJMoa1401329. This single center RCT of obese type 2 diabetics randomized 150 patients to intensive medical therapy versus bariatric surgery (Roux-en-Y gastric bypass or gastric sleeve). At three years of follow up, patients who underwent surgical intervention had significantly higher rates of remission of type 2 diabetes compared to those randomized to medical therapy alone. Those surgical patients who remained on antiglycemic medications were on less aggressive regimens compared to the medical intervention group. Patients who underwent bariatric surgery had much more dramatic weight loss and improved quality of life compared to medical therapy alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 34.Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–26. doi: 10.1001/jamasurg.2014.514. doi:10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. doi: 10.1001/jama.2014.5988. doi:10.1001/jama.2014.5988. This important publication from the Swedish Obese Subjects prospective matched cohort study demonstrated diminished diabetes remission rates fifteen years following bariatric surgery, though remission rates remained significantly higher than patients who did not undrego surgical intervention. Patients who underwent bariatric surgery benefited from significant reductions in long-term microvascular and macrovascular diabetic complications compared to usual care. [DOI] [PubMed] [Google Scholar]
- 36.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24):1763–77. doi: 10.1136/heartjnl-2012-301778. doi:10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 37.Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–8. doi: 10.1016/j.ijcard.2014.02.026. doi:10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Scott JD, Johnson BL, Blackhurst DW, Bour ES. Does bariatric surgery reduce the risk of major cardiovascular events? A retrospective cohort study of morbidly obese surgical patients. Surg Obes Relat Dis. 2013;9(1):32–9. doi: 10.1016/j.soard.2011.09.002. doi:10.1016/j.soard.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Johnson BL, Blackhurst DW, Latham BB, Cull DL, Bour ES, Oliver TL, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545–56. doi: 10.1016/j.jamcollsurg.2012.12.019. doi:10.1016/j.jamcollsurg.2012.12.019. discussion 56–8. [DOI] [PubMed] [Google Scholar]
- 40.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. doi:10.1001/jama.2011.1914. Another important publication from the Swedish Obese Subjects prospective matched cohort study, this analysis exhibited significant 15-year decrease in cardiovascular mortality, myocardial infarction, and stroke in patients who underwent bariatric surgery compared to usual care. [DOI] [PubMed] [Google Scholar]
- 41.Zhuo JL, Li XC. New insights and perspectives on intrarenal reninangiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32(7):1551–65. doi: 10.1016/j.peptides.2011.05.012. doi:10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41(2):319–27. doi: 10.1053/ajkd.2003.50039. doi:10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 43.Praga M, Hernandez E, Andres A, Leon M, Ruilope LM, Rodicio JL. Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron. 1995;70(1):35–41. doi: 10.1159/000188541. [DOI] [PubMed] [Google Scholar]
- 44.Straznicky NE, Grima MT, Lambert EA, Eikelis N, Dawood T, Lambert GW, et al. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29(3):553–64. doi: 10.1097/HJH.0b013e3283418875. doi:10.1097/HJH.0b013e3283418875. [DOI] [PubMed] [Google Scholar]
- 45.Van Huffel L, Tomson CR, Ruige J, Nistor I, Van Biesen W, Bolignano D. Dietary restriction and exercise for diabetic patients with chronic kidney disease: a systematic review. PLoS One. 2014;9(11):e113667. doi: 10.1371/journal.pone.0113667. doi:10.1371/journal.pone.0113667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55(1):69–76. doi: 10.1053/j.ajkd.2009.09.011. doi:10.1053/j.ajkd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82–98. doi: 10.1093/ndt/gft302. doi:10.1093/ndt/gft302. [DOI] [PubMed] [Google Scholar]
- 48.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 49.Eriksen BO, Melsom T, Mathisen UD, Jenssen TG, Solbu MD, Toft I. GFR normalized to total body water allows comparisons across genders and body sizes. J Am Soc Nephrol. 2011;22(8):1517–25. doi: 10.1681/ASN.2010121321. doi:10.1681/ASN.2010121321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen WW, Chen HM, Chen H, Xu F, Li LS, Liu ZH. Obesity-related glomerulopathy: body mass index and proteinuria. Clin J Am Soc Nephrol. 2010;5(8):1401–9. doi: 10.2215/CJN.01370210. doi:10.2215/CJN.01370210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgaki-Angelaki H, Stergiou N, Manolaki N, Nakopoulou L, Syriopoulou VP, Roma-Giannikou E. Histological deterioration of obesity-related glomerulopathy despite the loss of proteinuria with weight reduction. Pediatr Nephrol. 2010;25(8):1573–4. doi: 10.1007/s00467-010-1475-4. doi:10.1007/s00467-010-1475-4. [DOI] [PubMed] [Google Scholar]
- 52.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–74. doi: 10.2215/CJN.02250409. doi:10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou CC, Shyu RS, Lee WJ, Ser KH, Lee YC, Chen SC. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013;9(2):202–6. doi: 10.1016/j.soard.2012.10.005. doi:10.1016/j.soard.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Reid TJ, Saeed S, McCoy S, Osewa AA, Persaud A, Ahmed L. The effect of bariatric surgery on renal function. Surg Obes Relat Dis. 2014;10(5):808–13. doi: 10.1016/j.soard.2014.02.048. doi:10.1016/j.soard.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 55.Miras AD, Chuah LL, Lascaratos G, Faruq S, Mohite AA, Shah PR, et al. Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care. 2012;35(12):e81. doi: 10.2337/dc11-2353. doi:10.2337/dc11-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amor A, Jimenez A, Moize V, Ibarzabal A, Flores L, Lacy AM, et al. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg Endosc. 2013;27(6):2046–51. doi: 10.1007/s00464-012-2708-3. doi:10.1007/s00464-012-2708-3. [DOI] [PubMed] [Google Scholar]
- 57.Saliba J, Kasim NR, Tamboli RA, Isbell JM, Marks P, Feurer ID, et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147(2):282–7. doi: 10.1016/j.surg.2009.09.017. doi:10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fenske WK, Dubb S, Bueter M, Seyfried F, Patel K, Tam FW, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559–68. doi: 10.1016/j.soard.2012.03.009. doi:10.1016/j.soard.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Navaneethan SD, Malin SK, Arrigain S, Kashyap SR, Kirwan JP, Schauer PR. Bariatric surgery, kidney function, insulin resistance, and adipokines in patients with decreased GFR: a cohort study. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.09.018. doi:10.1053/j.ajkd.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iaconelli A, Panunzi S, De Gaetano A, Manco M, Guidone C, Leccesi L, et al. Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care. 2011;34(3):561–7. doi: 10.2337/dc10-1761. doi:10.2337/dc10-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander JW, Goodman HR, Hawver LR, Cardi MA. Improvement and stabilization of chronic kidney disease after gastric bypass. Surg Obes Relat Dis. 2009;5(2):237–41. doi: 10.1016/j.soard.2008.08.016. doi:10.1016/j.soard.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24(4):851–5. doi: 10.1007/s00467-008-1024-6. doi:10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 63.Huan Y, Tomaszewski JE, Cohen DL. Resolution of nephrotic syndrome after successful bariatric surgery in patient with biopsyproven FSGS. Clin Nephrol. 2009;71(1):69–73. doi: 10.5414/cnp71069. [DOI] [PubMed] [Google Scholar]
- 64.Turgeon NA, Perez S, Mondestin M, Davis SS, Lin E, Tata S, et al. The impact of renal function on outcomes of bariatric surgery. J Am Soc Nephrol. 2012;23(5):885–94. doi: 10.1681/ASN.2011050476. doi:10.1681/ASN.2011050476. This retrospective database study evaluated surgical complications in patients with underlying renal disease. Increased severity of CKD directly predicted complication rates associated with bariatric surgery; this relationship persisted after adjusting for underlying hypertension and diabetes. [DOI] [PubMed] [Google Scholar]
- 65.Weingarten TN, Gurrieri C, McCaffrey JM, Ricter SJ, Hilgeman ML, Schroeder DR, et al. Acute kidney injury following bariatric surgery. Obes Surg. 2013;23(1):64–70. doi: 10.1007/s11695-012-0766-1. doi:10.1007/s11695-012-0766-1. [DOI] [PubMed] [Google Scholar]


