Abstract
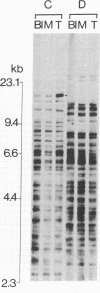
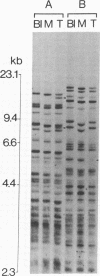
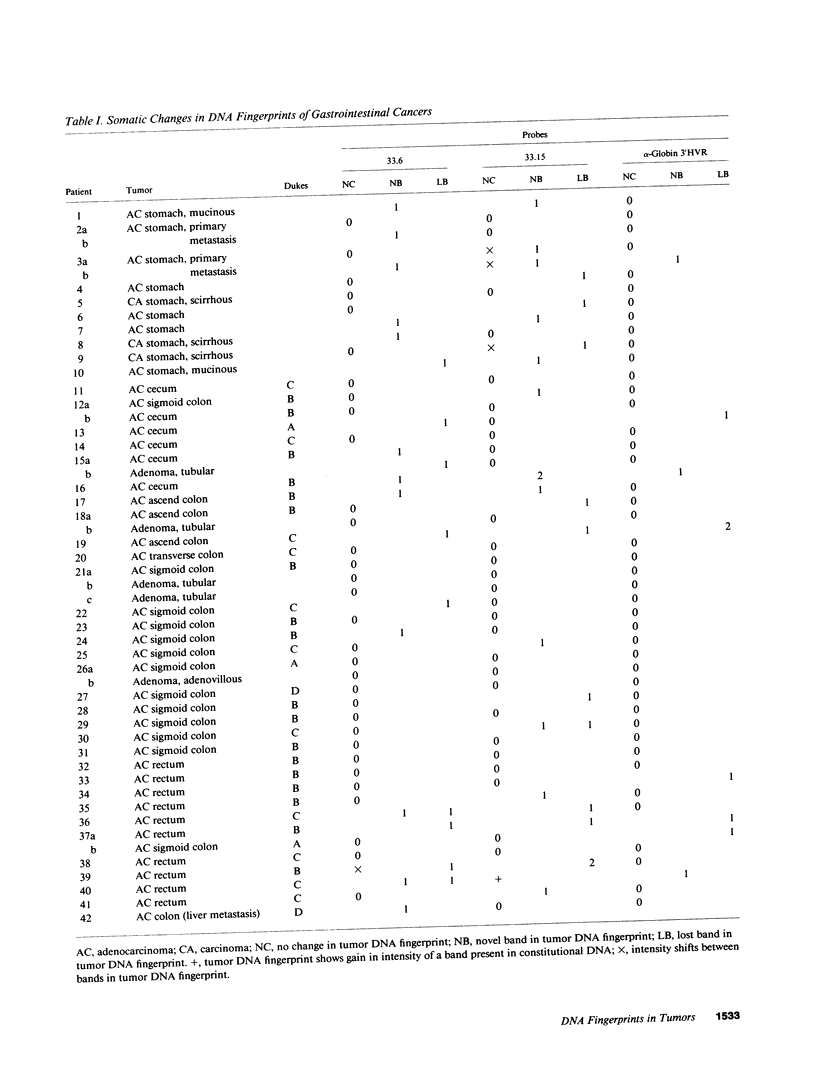
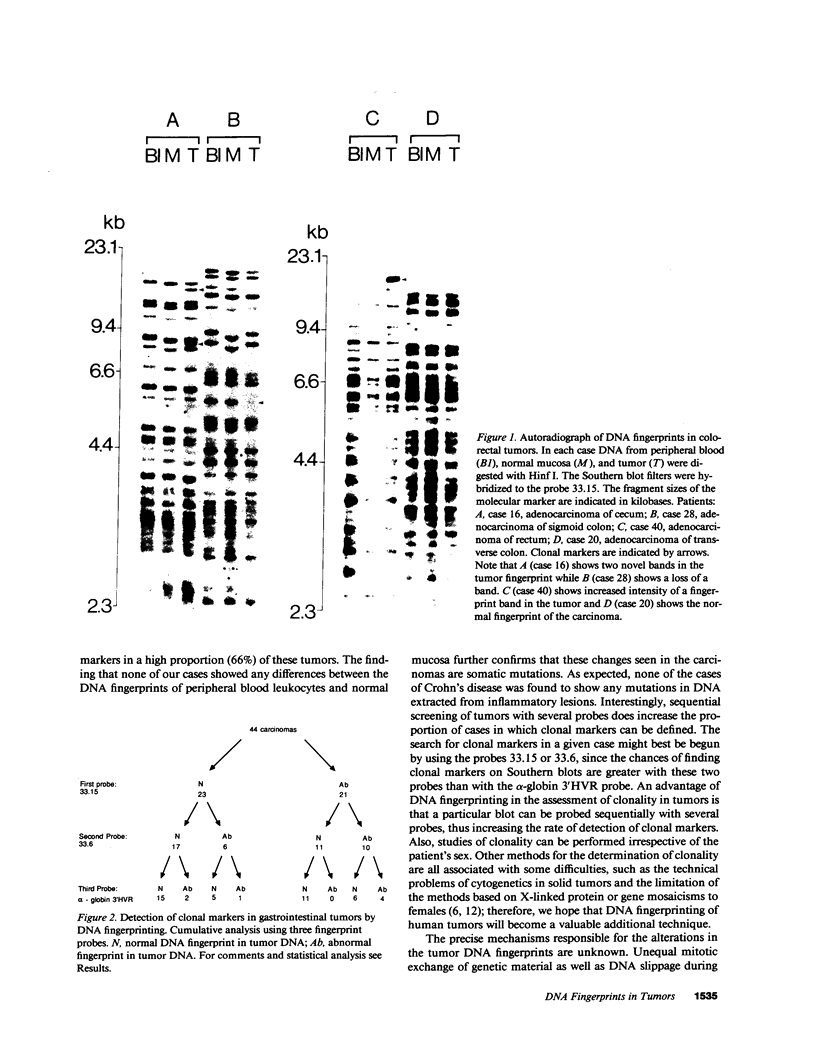
DNA fingerprinting with three different probes (33.15, 33.6, and alpha-globin 3'HVR) was investigated as a method for the determination of clonality in gastrointestinal tumors. In 29/44 carcinomas the tumor DNA showed clonal somatic mutations that were not seen in the corresponding peripheral blood and normal mucosa samples. The changes consisted of either novel fingerprint bands, losses of bands, or both. The probe 33.15 yielded the highest rate of abnormal DNA fingerprints (21/44 carcinomas). Sequential use of the probes increased the number of cases where clonal fingerprint markers could be detected. One out of five colorectal adenomas also showed a clonal loss of a fingerprint band. In two cases of gastric cancer, DNA from the metastatic tumor had a different DNA fingerprint from that found in the primary carcinoma. DNA fingerprinting offers a novel approach to determining clonality in tumors and may prove useful for the study of tumor progression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C. Current concepts in immunology: Cell-surface markers in lymphoproliferative disease. N Engl J Med. 1981 Feb 5;304(6):331–336. doi: 10.1056/NEJM198102053040606. [DOI] [PubMed] [Google Scholar]
- Alexander P. Do cancers arise from a single transformed cell or is monoclonality of tumours a late event in carcinogenesis? Br J Cancer. 1985 Apr;51(4):453–457. doi: 10.1038/bjc.1985.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Beutler E., Collins Z., Irwin L. E. Value of genetic variants of glucose-6-phosphate dehydrogenase in tracing the origin of malignant tumors. N Engl J Med. 1967 Feb 16;276(7):389–391. doi: 10.1056/NEJM196702162760706. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gartler S. M., Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Sagebiel R. W., Gartler S. M., Rimoin D. L. Multiple cell origin of hereditary neurofibromas. N Engl J Med. 1971 Feb 11;284(6):298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- Hsu S. H., Luk G. D., Krush A. J., Hamilton S. R., Hoover H. H., Jr Multiclonal origin of polyps in Gardner syndrome. Science. 1983 Sep 2;221(4614):951–953. doi: 10.1126/science.6879192. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Nicholls R. D., Weatherall D. J., Clegg J. B., Higgs D. R. Molecular characterisation of a hypervariable region downstream of the human alpha-globin gene cluster. EMBO J. 1986 Aug;5(8):1857–1863. doi: 10.1002/j.1460-2075.1986.tb04437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L., Weatherall D. J., Ponder B. A. DNA "fingerprints" and segregation analysis of multiple markers in human pedigrees. Am J Hum Genet. 1986 Jul;39(1):11–24. [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Nelson M. The effect of site specific methylation on restriction endonuclease digestion. Nucleic Acids Res. 1985;13 (Suppl):r201–r207. doi: 10.1093/nar/13.suppl.r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Townsend D. E., Sparkes R. S. Genetic variants of glucose-6-phosphate dehydrogenase in the study of carcinoma of the cervix. Cancer. 1971 Aug;28(2):529–532. doi: 10.1002/1097-0142(197108)28:2<529::aid-cncr2820280235>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Jeffreys A. J., Gooi H. C., Cotter F., Flint J., O'Connor N. T., Weatherall D. J., Wainscoat J. S. Detection of somatic changes in human cancer DNA by DNA fingerprint analysis. Br J Cancer. 1987 Apr;55(4):353–356. doi: 10.1038/bjc.1987.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F., Ansell J. D., Forbes G. M., Gordon J. C., Burton D. I., Micklem H. S. Clonal interaction in tumours. Nature. 1982 Oct 28;299(5886):822–824. doi: 10.1038/299822a0. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]