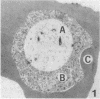
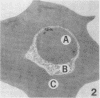
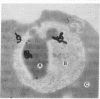
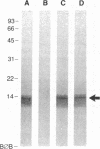
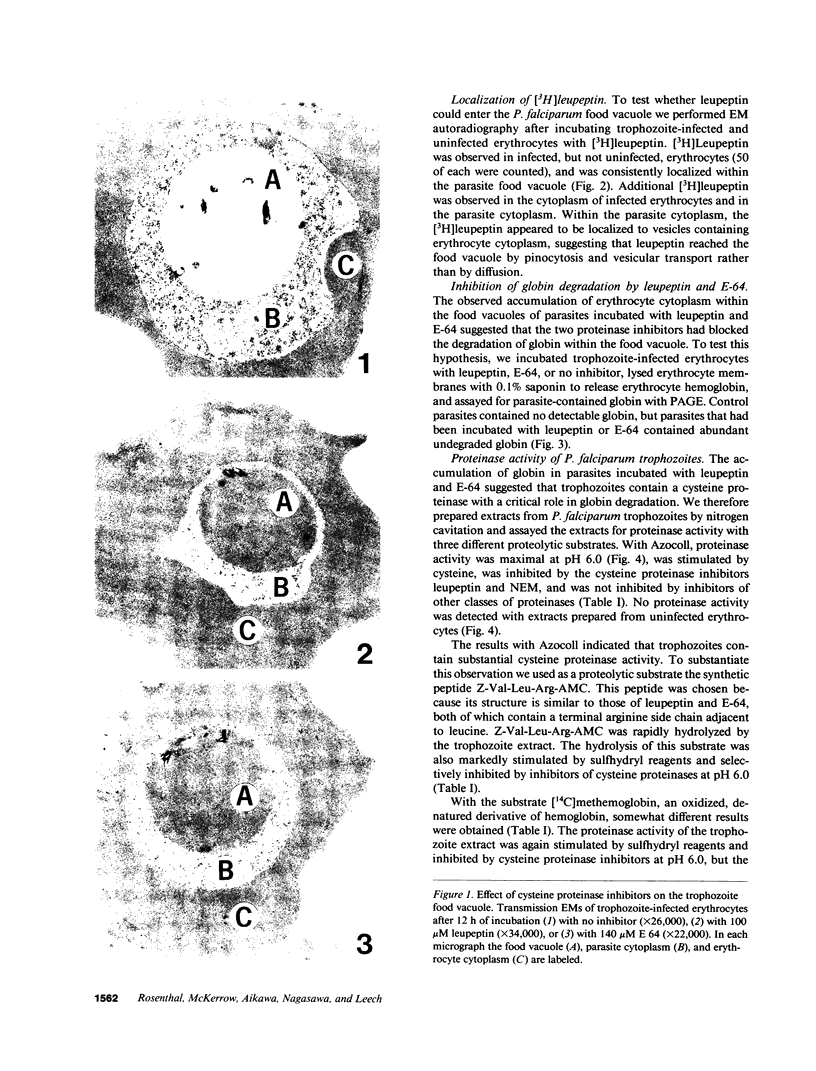
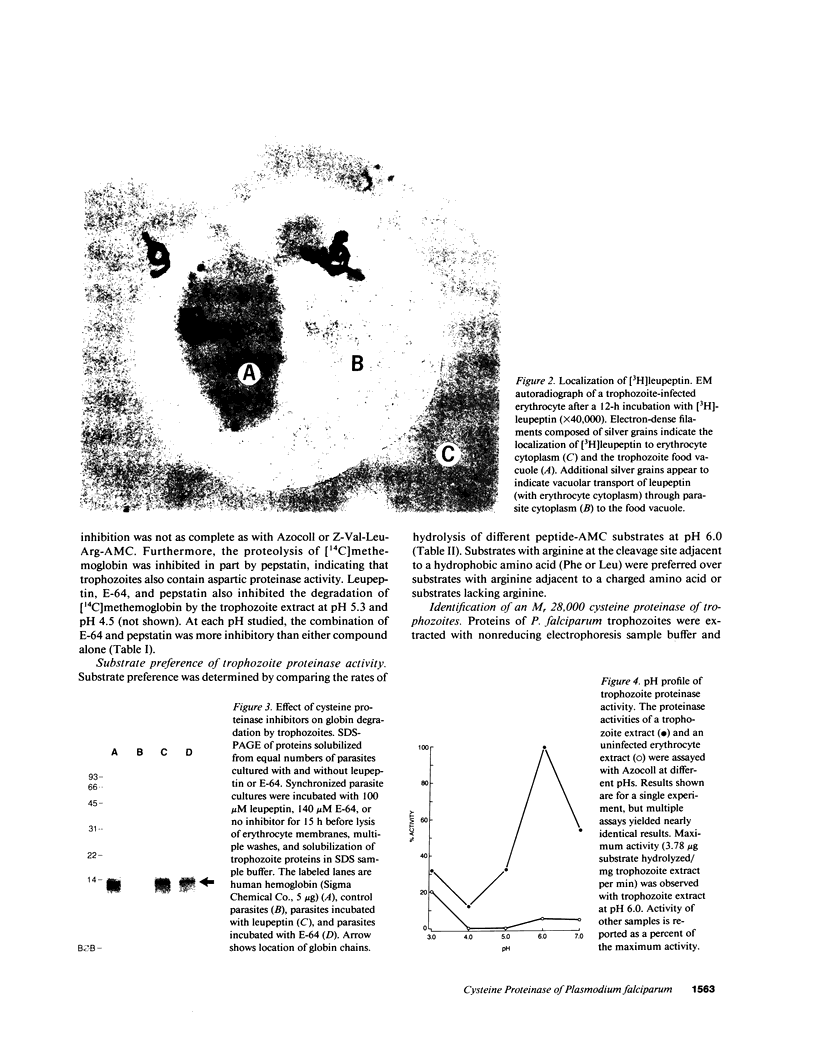
Abstract
To obtain free amino acids for protein synthesis, trophozoite stage malaria parasites feed on the cytoplasm of host erythrocytes and degrade hemoglobin within an acid food vacuole. The food vacuole appears to be analogous to the secondary lysosomes of mammalian cells. To determine the enzymatic mechanism of hemoglobin degradation, we incubated trophozoite-infected erythrocytes with peptide inhibitors of different classes of proteinases. Leupeptin and L-transepoxy-succinyl-leucyl-amido-(4-guanidino)-butane (E-64), two peptide inhibitors of cysteine proteinases, inhibited the proteolysis of globin and caused the accumulation of undegraded erythrocyte cytoplasm in parasite food vacuoles, suggesting that a food vacuole cysteine proteinase is necessary for hemoglobin degradation. Proteinase assays of trophozoites demonstrated cysteine proteinase activity with a pH optimum similar to that of the food vacuole and the substrate specificity of lysosomal cathepsin L. We also identified an Mr 28,000 proteinase that was trophozoite stage-specific and was inhibited by leupeptin and E-64. We conclude that the Mr 28,000 cysteine proteinase has a critical, perhaps rate-limiting, role in hemoglobin degradation within the food vacuole of Plasmodium falciparum. Specific inhibitors of this enzyme might provide new means of antimalarial chemotherapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M. High-resolution autoradiography of malarial parasites treated with 3 H-chloroquine. Am J Pathol. 1972 May;67(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- Aikawa M. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971 Oct;30(2):284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Dennis P. A., Dunn W. A. Metabolism of leupeptin and its effect on autophagy in the perfused rat liver. Acta Biol Med Ger. 1981;40(10-11):1531–1538. [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Hanada K. E-64 [L-trans-epoxysuccinyl-leucyl-amido(4-guanidino)butane] and related epoxides as inhibitors of cysteine proteinases. Acta Biol Med Ger. 1981;40(10-11):1513–1517. [PubMed] [Google Scholar]
- Barry D. N. Metabolism of Babesia parasites in vitro. amino acid production by Babesia rodhaini compared to Plasmodium berghei. Aust J Exp Biol Med Sci. 1982 Apr;60(Pt 2):175–180. doi: 10.1038/icb.1982.18. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Cenedella R. J., Rosen H., Angel C. R., Saxe L. H. Free amino-acid production in vitro by Plasmodium berghei. Am J Trop Med Hyg. 1968 Nov;17(6):800–803. doi: 10.4269/ajtmh.1968.17.800. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. Characterization of globin domains: heme binding to the central exon product. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1384–1388. doi: 10.1073/pnas.77.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Sprang S., Fletterick R., Rutter W. J. Intron-exon splice junctions map at protein surfaces. Nature. 1982 Sep 9;299(5879):180–182. doi: 10.1038/299180a0. [DOI] [PubMed] [Google Scholar]
- Dluzewski A. R., Rangachari K., Wilson R. J., Gratzer W. B. Plasmodium falciparum: protease inhibitors and inhibition of erythrocyte invasion. Exp Parasitol. 1986 Dec;62(3):416–422. doi: 10.1016/0014-4894(86)90050-0. [DOI] [PubMed] [Google Scholar]
- FULTON J. D., GRANT P. T. The sulphur requirements of the erythrocytic from of Plasmodium knowlesi. Biochem J. 1956 Jun;63(2):274–282. doi: 10.1042/bj0630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Dynamic aspects of the nitrogen metabolism of Plasmodium gallinaceum in vivo and in vitro. J Infect Dis. 1951 Mar-Apr;88(2):126–150. doi: 10.1093/infdis/88.2.126. [DOI] [PubMed] [Google Scholar]
- Gyang F. N., Poole B., Trager W. Peptidases from Plasmodium falciparum cultured in vitro. Mol Biochem Parasitol. 1982 Apr;5(4):263–273. doi: 10.1016/0166-6851(82)90034-2. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Kovács A. L., Reith A., Seglen P. O. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin or a lysosomotropic amine. Exp Cell Res. 1982 Jan;137(1):191–201. doi: 10.1016/0014-4827(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. Acid-vesicle function, intracellular pathogens, and the action of chloroquine against Plasmodium falciparum. N Engl J Med. 1987 Aug 27;317(9):542–549. doi: 10.1056/NEJM198708273170905. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H., Gluzman I. Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol. 1985 Dec;101(6):2302–2309. doi: 10.1083/jcb.101.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Kim K., McKerrow J. H., Leech J. H. Identification of three stage-specific proteinases of Plasmodium falciparum. J Exp Med. 1987 Sep 1;166(3):816–821. doi: 10.1084/jem.166.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Mudd J. B. Malaria infection (Plasmodium iophurae): changes in free amino acids. Science. 1966 Oct 14;154(3746):287–289. doi: 10.1126/science.154.3746.287. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Tanigoshi L. Purification of Plasmodium lophurae cathepsin D and its effects on erythrocyte membrane proteins. Mol Biochem Parasitol. 1983 Jul;8(3):207–226. doi: 10.1016/0166-6851(83)90044-0. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Fletcher K. A., Maegraith B. G. The use of electron microscope autoradiography for examining the uptake and degradation of haemoglobin by Plasmodium berghei. Ann Trop Med Parasitol. 1970 Mar;64(1):63–71. doi: 10.1080/00034983.1970.11686664. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. The effect of leupeptin on intracellular digestion of asialofetuin in rat hepatocytes. Exp Cell Res. 1981 Jul;134(1):207–217. doi: 10.1016/0014-4827(81)90478-x. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Campos N. M. Characterization of a hemoglobin-degrading, low molecular weight protease from Plasmodium falciparum. Mol Biochem Parasitol. 1986 Mar;18(3):389–400. doi: 10.1016/0166-6851(86)90095-2. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., Sherman I. W. Plasmodium lophurae: composition and properties of hemozoin, the malarial pigment. Exp Parasitol. 1979 Aug;48(1):61–74. doi: 10.1016/0014-4894(79)90055-9. [DOI] [PubMed] [Google Scholar]
- Yayon A., Cabantchik Z. I., Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984 Nov;3(11):2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Timberg R., Friedman S., Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984 Aug;31(3):367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Zarchin S., Krugliak M., Ginsburg H. Digestion of the host erythrocyte by malaria parasites is the primary target for quinoline-containing antimalarials. Biochem Pharmacol. 1986 Jul 15;35(14):2435–2442. doi: 10.1016/0006-2952(86)90473-9. [DOI] [PubMed] [Google Scholar]