Abstract
Nutrition and physical exercise can enhance cognitive function but the specific combinations of dietary bioactives that maximize pro-cognitive effects are not known nor are the contributing neurobiological mechanisms. Epigallocatechin-3-gallate (EGCG) is a flavonoid constituent of many plants with high levels found in green tea. EGCG has anti-inflammatory and anti-oxidant properties and is known to cross the blood brain barrier where it can affect brain chemistry and physiology. β-alanine (B-ALA) is a naturally occurring β–amino acid that could increase cognitive functioning by increasing levels of exercise via increased capacity of skeletal muscle, by crossing the blood brain barrier and acting as a neurotransmitter, or by free radical scavenging in muscle and brain after conversion into carnosine. The objective of this study was to determine the effects of EGCG (∼ 250 mg/kg/day), B-ALA (∼550 mg/kg/day), and their combination with voluntary wheel running exercise on the following outcome measures: body composition, time to fatigue, production of new cells in the granule layer of the dentate gyrus of the hippocampus as a marker for neuronal plasticity, and behavioral performance on the contextual and cued fear conditioning tasks, as measures of associative learning and memory. Young adult male BALB/cJ mice approximately 2 months old were randomized into 8 groups varying the nutritional supplement in their diet and access to running wheels over a 39 day study period. Running increased food intake, decreased fat mass, increased time to exhaustive fatigue, increased numbers of new cells in the granule layer of the hippocampus, and enhanced retrieval of both contextual and cued fear memories. The diets had no effect on their own or in combination with exercise on any of the fitness, plasticity, and behavioral outcome measures other than B-ALA decreased percent body fat whereas EGCG increased lean body mass slightly. Results suggest that, in young adult BALB/cJ mice, a 39 day treatment of exercise but not dietary supplementation with B-ALA or EGCG, enhances measures of fitness, neuroplasticity and cognition.
Keywords: EGCG, β-alanine, voluntary wheel running, exercise, flavonoid, fear conditioning, adult neurogenesis, hippocampus, green tea, catechin
1. Introduction
Exercise and diet can influence cognition in animal models and humans [1-3]. However, most studies have examined exercise and dietary factors independently. Few studies have examined the extent to which specific micronutrients or dietary bioactives can interact or add to the effects of exercise and improve function greater than exercise or dietary treatments alone [1,4-6]. Given that specific nutritional supplements can improve muscle strength, reduce fatigue, enhance exercise capacity and improve overall health [7], in addition to having direct effects in the brain [1,2], it is reasonable to hypothesize that specific dietary supplementation might enhance the effects of exercise on cognition [3].
One of the most dramatic changes in the brain resulting from exercise in young adult mice is increased hippocampal neurogenesis [8]. In particular, wheel running is well established to increase levels of neurogenesis from 2 to 5 fold relative to sedentary housed controls in various inbred strains of mice [9]. The new neurons contribute to a larger granule layer of the dentate gyrus in running mice [10]. Although the functional significance of exercise-induced hippocampal neurogenesis is not known, increased adult neurogenesis in young adult mice is widely considered a biomarker for neuroplasticity, learning and memory [11,12]. The new neurons are thought to represent highly plastic units that are not yet fully integrated into the circuit and therefore are more moldable from experience as compared to older integrated neurons [13].
Epigallocatechin-3-gallate (EGCG) is the major flavonoid constituent of green tea. EGCG has been reported to increase neurogenesis, both by increasing proliferation and survival of new neurons in the dentate gyrus [14-16], and to enhance cognitive performance [17-20]. Epicatechin, a related catechin flavonoid, enhances angiogenesis and hippocampal-dependent behavioral performance in mice [1]. Increased angiogenesis provides a neurovascular niche that is conducive for the proliferation and survival of new neurons and is associated with increased neurogenesis from exercise [10,21]. Moreover, exercise and EGCG both enhance performance on similar hippocampal-dependent tasks in rodent models [22,23].
While exercise improves behavioral performance across multiple domains of cognition, one of the most robust effects of exercise on any hippocampal-involved behavioral measure is contextual fear conditioning [22,24-26]. Therefore, we hypothesized that combining EGCG with exercise might enhance neurogenesis and contextual fear conditioning at a level greater than exercise alone. The mechanisms by which EGCG increases angiogenesis and enhances hippocampal behaviors are not clear. However, it is known that EGCG crosses the blood brain barrier, where it can act as an anti-oxidant [27,28]. Exercise produces robust activation of the granule layer of the dentate gyrus [29-32], which requires significant metabolic activity and results in the production of free radicals. Hence our leading hypothesis is that EGCG acts as an anti-oxidant in the brain facilitating increased metabolic activity in brain cells with minimal damage to tissues.
β-Alanine (B-ALA) is a naturally occurring β amino acid that serves as the rate-limiting precursor of carnosine, a compound found highly concentrated in muscle [7,33]. Although the physiological role of carnosine is not well understood, evidence suggests it can improve muscle function and exercise performance [7,34]. Carnosine is thought to act as an antioxidant and pH buffer to counteract free radical exposure and post-workout acidosis in the muscles [35,36]. Increasing muscle carnosine through an increase in dietary supplementation of B-ALA increases the intracellular buffering capacity, which in turn increases high-intensity exercise capacity and performance [37]. Hence, we hypothesized that B-ALA would enhance the pro-cognitive effects of exercise, in part, by reducing fatigue and facilitating increased exercise capacity, intensity or duration.
We also considered the possibility that dietary B-ALA might affect brain function directly, though we were less certain about the mechanisms as compared to muscle. Amino acid uptake by the brain is carefully regulated, and hence we were uncertain to what extent the B-ALA provided in the diet would alter B-ALA levels in the brain [38]. Glycine, B-ALA, and GABA are consecutive members of a structurally homologous series of amino acids [39]. Glycine and GABA are established inhibitory neurotransmitters in the brain. Structural similarities of B-ALA to GABA and Glycine enable it to bind to the receptors of both [39]. In addition there may be G-protein coupled receptors that function as a specific membrane receptor for B-ALA [40]. However, in contrast to the other two neurologically active amino acids, the concentration of B-ALA in brain is low [41].
A recent study found B-ALA levels in the hippocampus of rats increased after a Morris water maze probe trial [42], consistent with a role for B-ALA in spatial memory retrieval. In addition, carnosine supplementation was shown to improve cognitive function in schizophrenic patients [43]. The putative pro-cognitive effects of carnosine are thought to be mediated, in part, by its anti-oxidant effects [44,45]. However, as with B-ALA, the functional significance of carnosine levels in the brain are not well understood.
The goal of this study was to examine the effects of a 39-day intervention with EGCG, B-ALA, and wheel running alone or in combination on several outcome measures related to physical fitness, neuronal plasticity and cognition. Specifically, after 39 days of treatment, we measured time to exhaustive fatigue, body composition, numbers of new cells in the dentate gyrus of the hippocampus, and associative learning and memory on the contextual and cued fear conditioning task in young adult BALB/cJ mice. To the best of our knowledge, this is the first study to investigate whether EGCG and B-ALA can augment the normal benefits of exercise on cognitive and neurological measures in young adult mice. It is also the first study we are aware of that tests the influence of these dietary bioactives on the contextual and cued fear conditioning tasks, widely used in the literature to measure associative learning in rodents. We hypothesized that B-ALA alone would reduce fatigue, enhance wheel running behavior and thereby enhance numbers of new cells in the hippocampus. The new cells we hypothesized would enhance performance on the contextual and cued fear conditioning tasks in the runners relative to the sedentary mice. We hypothesized that EGCG alone would enhance adult hippocampal neurogenesis and contextual fear conditioning. Finally we hypothesized that the combination of ECGC, B-ALA and wheel running would produce the greatest improvements in the outcome measures relative to the other groups.
2. Methods
2.1. Subjects and Husbandry
A total of 91 male BALB/cJ mice were used in this study. Five cohorts of 10 week old mice were obtained from Jackson Laboratories (Bar Harbor, ME). Upon arrival, mice were individually housed, and acclimated to the facility for 1 week before being assigned the experimental diets. During that first week, all mice were fed 8640 Teklad 22/5 rodent diet (Harlan Teklad, Indianapolis, IN). Mice were housed under a reverse 12h light-dark cycle with lights on at 10 PM and off and 10 AM. Autoclaved water was provided ad libitum at all times. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All measures were taken to minimize the number of mice used as well as the pain and suffering of the mice. The University of Illinois at Urbana-Champaign is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2. Diets
Control and experimental diets were purchased from Research Diets, Inc. (New Brunswick, NJ) and were based on AIN-93M maintenance rodent diet. Diets were made with 1.5 mg Teavigo® (>90% EGCG, DSM Nutritional Products, Basel, Switzerland) and/or 3.43 mg B-ALA (NutraBio, Middlesex, NJ) per gram of AIN-93M diet, which was then pelleted to match the consistency and appearance of the Control diet. The diets were independently assayed by Covance, Inc. (Princeton, NJ), and the experimental diet was found to contain 1.49 mg of EGCG per gram of diet (99.3% of expected) and 3.34 mg of B-ALA per gram of diet (97.4% of expected). The control diet AIN-93M diet was found to be free of both EGCG and B-ALA. The compounds were stable in the diet for at least 4 months. See Table 1 for a complete description of the nutritional components of the four diets: AIN-93M (control), B-ALA, EGCG, both B-ALA and EGCG.
Table 1. Nutritional composition of the 4 diets.
| Component | Control | EGCG | β-alanine | Combined | |
|---|---|---|---|---|---|
| Protein | (% by wt.) | 14 | 14 | 14 | 14 |
| Carbohydrate | " | 73 | 73 | 73 | 73 |
| Fat | " | 4 | 4 | 4 | 4 |
|
| |||||
| Energy | (kcal/g) | 3.8 | 3.8 | 3.8 | 3.8 |
|
| |||||
| Casein | (g/kg) | 140 | 140 | 140 | 140 |
|
| |||||
| L-Cystine | " | 1.8 | 1.8 | 1.8 | 1.8 |
|
| |||||
| Corn Starch | " | 496 | 496 | 496 | 496 |
|
| |||||
| Maltodextrin 10 | " | 125 | 125 | 125 | 125 |
|
| |||||
| Sucrose | " | 100 | 100 | 100 | 100 |
|
| |||||
| Cellulose, BW 200 | " | 50 | 50 | 50 | 50 |
|
| |||||
| Soybean Oil | " | 40 | 40 | 40 | 40 |
|
| |||||
| tButylhydroquinone | " | 0.008 | 0.008 | 0.008 | 0.008 |
|
| |||||
| Mineral Mix S10022M | " | 35 | 35 | 35 | 35 |
|
| |||||
| Vitamin Mix V10037 | " | 10 | 10 | 10 | 10 |
|
| |||||
| Choline Bitartrate | " | 2.5 | 2.5 | 2.5 | 2.5 |
|
| |||||
| Blue Dye #1 | " | — | 0.05 | ||
|
| |||||
| Red Dye #40 | " | — | — | 0.05 | — |
|
| |||||
| Yellow Dye #5 | " | — | 0.05 | — | — |
|
| |||||
| Teavigo (EGCG) | (mg/g) | 0 | 1.5 | — | 1.5 |
| β-alanine | " | 0 | — | 3.43 | 3.43 |
Based upon the rates of food disappearance and body masses of the mice, we estimated the average intake of EGCG and/or B-ALA per day for each experimental group (Table 2). The rationale for the EGCG dosage was based on previous studies demonstrating beneficial effects of EGCG on cognition in mice [46,47]. As there are few studies examining the effects of B-ALA supplementation on cognition or muscle function in mice, our B-ALA dosage was calculated from the effective dose in humans of 3-4 g/d that led to improved physical work capacity [48,49]. For a 70 kg person, this would equate to approximately 40-60 mg/kg/d. The dose was adjusted for species using the FDA-recommended conversion factor of approximately an order of magnitude (Food and Drug Administration, 2005) resulting in a target dose of 400-600 mg/kg/d.
Table 2. Average (± SE) dose of EGCG and β-alanine ingested by each group.
| ` | Sedentary | Runner | ||
|---|---|---|---|---|
| Diets | EGCG (mg/kg/d) | β-alanine (mg/kg/d) | EGCG (mg/kg/d) | β-alanine (mg/kg/d) |
| Control | — | — | — | — |
| EGCG | 230.38 ± 4.57 | — | 269.53 ± 3.72 | — |
| β-alanine | — | 533.54 ± 9.13 | — | 625.53 ± 10.29 |
| Combined | 236.01 ± 4.77 | 529.05 ± 10.70 | 272.86 ± 5.27 | 611.64 ± 11.81 |
2.3. Experimental Design
Mice were randomized into either sedentary or exercise groups for a total of 39 days. Each sedentary and exercise group was further randomized into four diet groups either receiving Control (n=11 sedentary and 11 runner), B-ALA (n=11 sedentary and 12 runner), EGCG (n=12 sedentary and 11 runner), or combined EGCG and B-ALA (n=12 sedentary and 11 runner). Sedentary mice were individually housed in standard polypropylene shoebox cages (29 cm L × 19 cm W × 13 cm H). Mice in the exercise condition were individually house in cages (36 cm L × 20 cm W × 14 cm H) with a 23 cm diameter running wheel (Respironics, Bend, OR). Wheel rotations were monitored continuously in 1 hr increments throughout the experiment via magnetic switches interfaced to the computer. During the first 10 days, all mice received daily injections of 5-bromo-deoxyuridine (BrdU; 50 mg/kg) to label dividing cells. Body weight and food intake was measured multiple times throughout the experiment (Day 1, 5, 8, 12, 15, 19, 22, and 26).
2.4. Body Composition
Body composition was measured for each mouse by MRI (EchoMRI™, Houston, TX). Measurements of body fat and lean mass were recorded at two different time points. Body composition was measured before and exactly one month after each mouse was placed on the experimental diets. Body fat and lean mass composition data were expressed in grams for each group.
2.5. Time to Fatigue
An exhaustive treadmill test was performed to measure fatigability on day 36 approximately 4 hr into the dark phase of the light-dark cycle. As previous described in Martin et al. (2013), mice were run on an inclined (5%), motorized treadmill (Jog-a-Dog, Ottawa Lake, MI) using an incremental running velocity. Mice ran to fatigue as defined by an inability to continue running despite gentle prodding for at least 10 sec. The test was truncated at 120 min if mice had not reached fatigue. No electric shock was used. Data were expressed as time-to-exhaustion (min) for each group.
2.6. Fear Conditioning
A fear conditioning procedure was performed on day 37 and 38. All training and testing phases for the fear conditioning task occurred approximately 4 hr into the dark phase of the light-dark cycle. For the training phase, the mice were individually placed into a rectangular chamber (32 cm L × 28 cm W × 30 cm H, dark grey walls) with metal grid floor connected to a shock scrambler controlled by a digital timer (Med Associates, St. Albans, VT). The mice were allowed to acclimate to the chamber for two min. After the acclimation period, the mice were presented with a tone that co-terminated with a shock for most mice (N=68 total; n=8-9 per group). A small number of mice in each group (N=23 total; 2-3 per group) received the tone but no shock (un-shocked controls). The tone/shock delivery occurred with the presentation of a tone (86 dB) for 20 sec with the administration of a 0.75 mA foot shock delivered during the last two sec of the tone. The tone and shock delivery occurred at 120 sec and 200 sec. The mouse remained in the chamber for an additional 30 sec after the last tone/shock delivery before being returned to their home cage.
On the second day, mice were tested for both contextual and cued fear conditioning in counterbalanced order, i.e., half the mice were tested for cued first and then contextual, and the other half vice versa. For contextual conditioning, mice were placed into the original square chamber and left undisturbed for a total of 250 sec in absence of shock or tone. For cued conditioning, the mice were placed into a novel octagon-shaped chamber with white and black striped walls and a smooth floor and were presented a tone at 120 and 200 sec, but no shock. Freezing was recorded by TopScan video tracking software (CleverSystems, Reston, VA) as the total number of sec when the mouse's center of mass did not register horizontal movement (± 1 mm). Freezing data were converted into percent time spent freezing by dividing the total number of sec a mouse spent freezing by the total number of sec of testing and multiplied by 100.
2.7. Tissue Collection
On day 40 (when mice were 117 days old), mice were euthanized by CO2 asphyxiation and then immediately transcardially perfused with ice-cold saline to remove the blood. Brains were removed and longitudinally cut into hemispheric sections. One half was snap-frozen by emersion in liquid nitrogen then stored at -80 C for a different project. The other half was dedicated to immunohistochemistry and was immediately placed in 4% paraformaldehyde to fix overnight. After overnight fixation, the half brains were transferred into a 30% sucrose solution and stored at 4 C until sectioning. Brains were sectioned into 40 μM thick sections in the coronal plane using a cryostat. Sections were stored in tissue cryoprotectant (50% 0.1 M Phosphate buffer solution, 25% glycerol, and 25% ethylene glycol) at -20°C until processed for immunohistochemical detection of BrdU.
2.8. Immunohistochemistry
After overnight fixation in 4% paraformaldehyde, the tissue was transferred into 30% sucrose solution. Brains were sectioned at 40 μM using a cryostat. A one-in-six series was stained for BrdU to identify newly divided cells. Free-floating sections were washed in tissue buffing solution (TBS) and then treated with 0.6 % hydrogen peroxide in TBS for 30 min at room temperature. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 10% 20× saline sodium citrate buffer, rinsed in 2 M hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (pH 8.5) for 10 min at room temperature. Sections were then treated (blocked) with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat (1:100; AbD Serotec, Raleigh, NC) in TBS-X plus for 72 hr at 4 °C. Sections were then washed in TBS, treated with TBS-X plus for 30 min and then incubated in secondary antibody against rat made in goat (1:250; Vector Laboratories, Burlingame, CA) in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector Laboratories, Burlingame, CA; cat# PK-6100) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO; cat# D4418-50SET).
2.9. Image analysis
Following Clark et al. [22], the entire granule layer represented in the one-in-six series was photographed by advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs under 10× magnification. From the photographs, cells positively labeled for BrdU were automatically counted with ImageJ software using a validated threshold. A minimum of 20 images for each cohort of mice were also counted manually and a threshold was considered valid if the Pearson's correlation between the automated and manual counts was greater than or equal to 0.95. To obtain unbiased estimates of total number of BrdU cells, total counts were adjusted by removing the fraction of cells predicted to cross the boundary of the section on one side based on the average size of the particles and the section thickness.
2.10. Statistical Analysis
Data were analyzed using SAS software version 9.2. P<0.05 was considered statistically significant. Body mass and food intake were analyzed using a repeated measures ANOVA with day as the within subjects factor and EGCG (present in the diet or not), B-ALA (present in the diet or not), Exercise (Runner or Sedentary), and all interactions as between-subjects factors. Wheel running data were averaged across the study for individuals and average distance traveled was analyzed by 2-way ANOVA with EGCG, B-ALA and the interaction as factors. The body composition data (fat mass and lean mass) were analyzed similar to body mass and food intake except with time (pre or post intervention) as the within subjects factor instead of day. The following variables were measured by 3-way ANOVA with EGCG, B-ALA, Exercise and all interactions as factors: time to exhaustive fatigue, number of BrdU+ cells in the granule layer of the dentate gyrus, gene expression values. Fear conditioning data were analyzed as follows. First an analysis of duration freezing in the original context was conducted using 4-way ANOVA with shock status (shocked on day 1 or not-shocked on day 1), EGCG, B-ALA, Exercise and all interactions as factors. This was followed by a 3-way ANOVA of only the shocked mice. Duration of freezing behavior in the novel environment in response to the tone cue was analyzed using repeated measures ANOVA with time (pre or post tone) as the within-subjects factor, and EGCG, B-ALA, Exercise and all interactions entered as factors.
3. Results
3.1. Body mass
Body mass increased over the days of the experiment (Fig. 1A; F7,588=57.7, P<0.0001). Mice gained approximately 1 gram from the beginning to end of the experiment. No effects of diet, exercise, or interactions with time were detected.
Figure 1. Body mass, food intake and wheel running behavior.
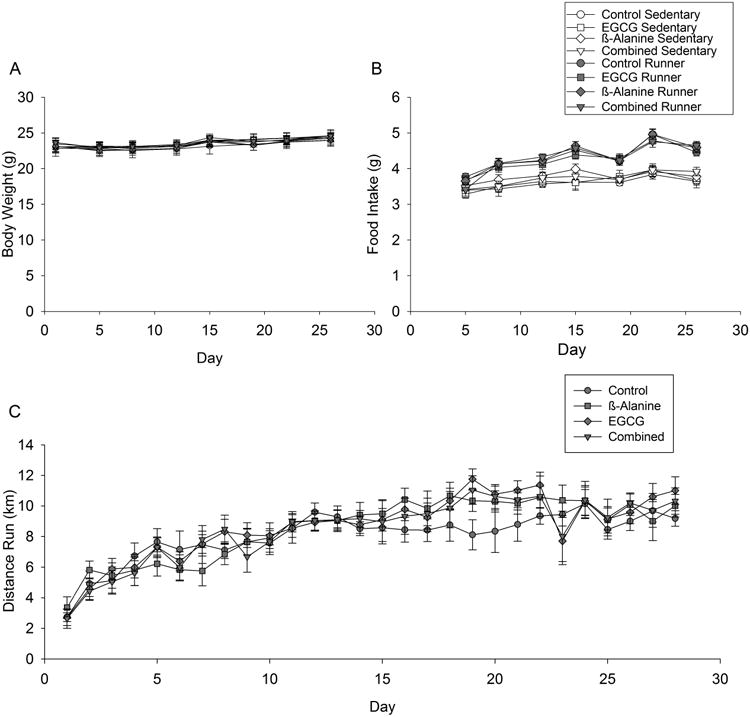
A) Average body mass is shown every 3 or 4 days for the first 28 days of the study before behavioral testing began (e.g., treadmill running to exhaustion, contextual fear conditioning) separately by treatment group. B) Same as A for food intake. C) Average distance traveled in km per day for runners only by diet group. Standard error bars shown (n=11 or 12 per group).
3.2. Food intake
Food intake increased over the days of the experiment (Fig. 1B; F6,498=51.2, P<0.0001). Runners ate more food than sedentary mice (F1,79=175.4, P<0.0001). The difference in food intake between runners and sedentary mice became greater as the experiment progressed as indicated by a significant interaction between day and exercise treatment (F6,498=10.1, P<0.0001). No effect of the diets or interactions between diets and the other factors were detected.
Table 2 shows the average dose of EGCG and B-ALA consumed by each of the groups in the study based on food intake, body mass, and concentration of the micronutrients in the diets.
3.3. Wheel running
No differences in wheel running were observed between the 4 diet groups. As typically observed for mice, wheel running steadily increased over the first 20 days and thereafter maintained a plateau (Fig. 1C).
3.4. Body composition
Both B-ALA and exercise reduced fat mass, as indicated by significant main effect of exercise (Fig. 2A; F1,85=5.3, P=0.02), main effect of time (F1,53=58.4, P<0.0001), time-by-B-ALA interaction (F1,53=5.9, P=0.02) and time-by-exercise interaction (F1,53=11.0, P=0.002). However, the combination of B-ALA and exercise did not reduce fat mass any further than either one alone. No other main effects or interactions were significant.
Figure 2. Body composition.
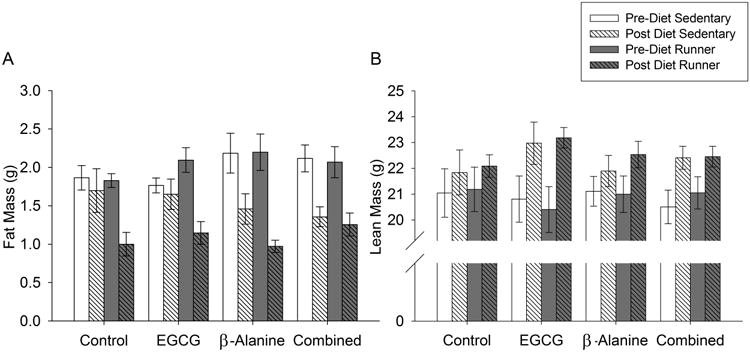
Average A) fat mass and B) lean mass as measured by echo-MRI. Pre-Diet measures were taken at the start of the experiment and Post-Diet measures were taken after one month of exposure to the diets. Standard error bars shown (n=11 or 12 per group).
EGCG increased lean mass as indicated by a significant EGCG-by-time interaction (Fig 2B; F1,53=4.6, P=0.04). No other main effects or interactions were significant.
3.5. Time to fatigue
Wheel running increased time to fatigue in the treadmill test (Fig. 3; F1,67=8.9, P=0.004). No effect of the diets or interactions between the diets and exercise were significant.
Figure 3. Time to fatigue.
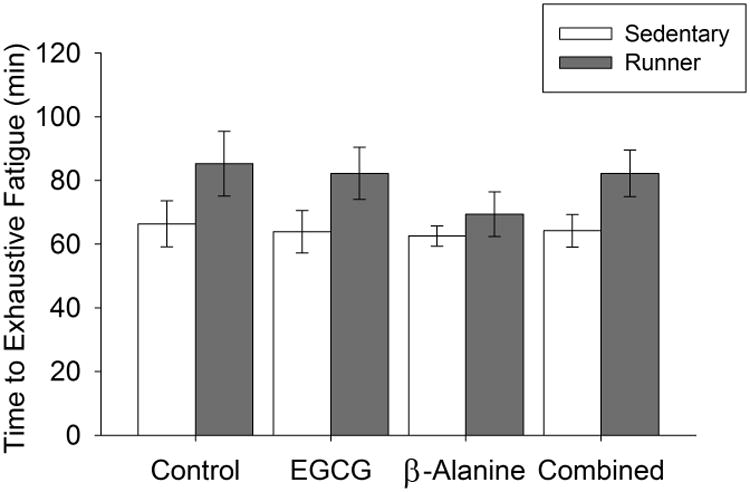
Average time to fatigue on an accelerating inclined treadmill. Standard error bars are shown (n=11 or 12 per group).
3.6. Fear conditioning
Contextual fear conditioning
Mice learned the contextual fear conditioning task, as indicated by a greater duration of freezing on day 2 in the original context in groups that received a shock as compared to no-shock on day 1 (Fig. 4A; F1,71=5.9, P=0.02). Running increased duration of freezing in the original context on day 2 in the shocked group (F1,56=28.6, P<0.0001). No effect of diet or interaction between diet and exercise were detected for contextual fear conditioning in the original context.
Figure 4. Fear conditioning.
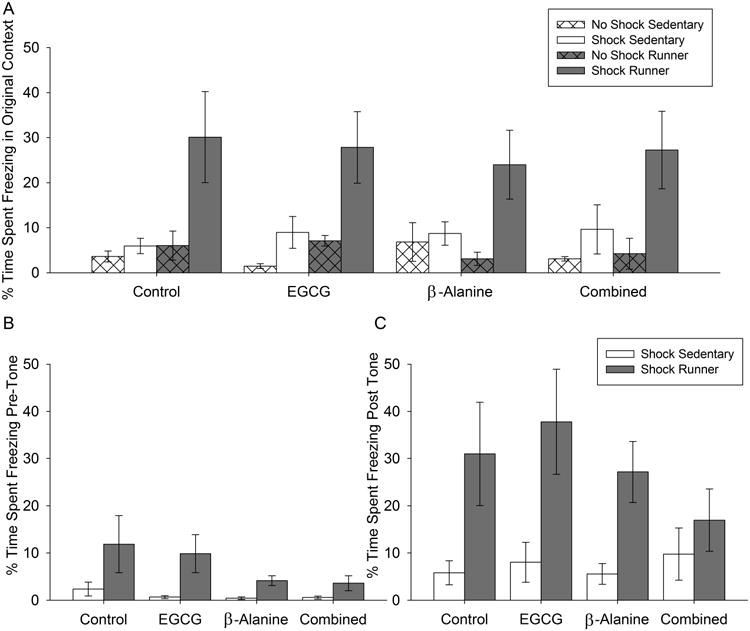
A) Average duration freezing in the original context on day 2 of the procedure expressed as percentage of total duration of the test, with separate bars for each treatment group. B) Average duration freezing in the novel context on day 2 before the tone was presented. C) Same as B but during the period after the tone was presented. Standard error bars are shown (n=2-3 per group for no-shock controls; n=8-9 for shock groups).
Cued fear conditioning
Mice learned the association between the cue (tone) and the shock as indicated by a greater duration of freezing on day 2 in the novel context after the tone was presented as compared to day 2 before the tone was presented (Fig. 4B and C; F1,31=43.3, P<0.0001). Runners displayed approximately a 4-fold greater duration of freezing behavior than sedentary mice during all phases of the cued fear conditioning task on day 2 as indicated by a significant effect of exercise (F1,50=14.5, P=0.0004) but no interactions with phase (i.e., pre or post tone). No effect of diet or interactions between diet and the other factors were detected.
3.7. Adult hippocampal neurogenesis
Exercise increased the total number of BrdU+ cells in the granule layer of the dentate gyrus by approximately 4-fold over sedentary mice (Fig. 5A and B; F1,69=73.0, P<0.0001). No effect of diet or interactions between diet and exercise were detected.
Figure 5. New cells in the dentate gyrus of the hippocampus.
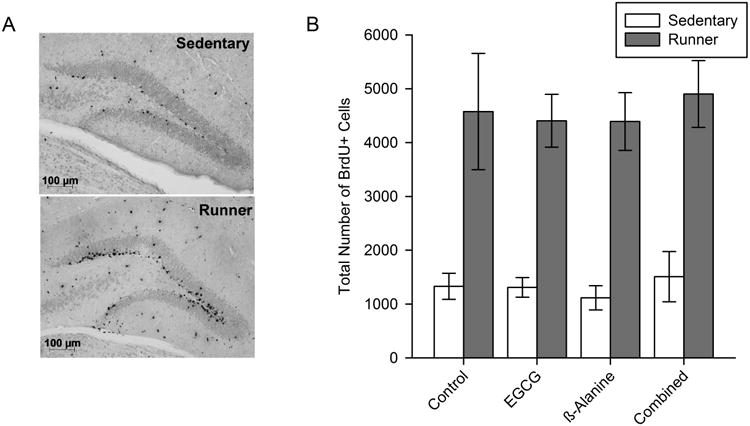
A) Representative microscopic image of a brain section stained for BrdU revealing new cells (born during the first 10 days of the study and still survived 1 month later) in the granule layer of the dentate gyrus. B) Average total number of BrdU+ cells in the granule layer of the dentate gyrus. Standard error bars are shown (n=9-12 per group).
4. Discussion
The main discovery from this study is that dietary supplementation with EGCG and/or B-ALA over 39 days had no significant influence on physical fitness, adult hippocampal neurogenesis, or learning and memory measures whereas exercise had robust effects on all three outcomes. We found similar negative results for EGCG and B-ALA in an aged population of BALB/cJ mice housed with and without voluntary running wheels [49]. Our negative findings for EGCG on cognition are consistent with a recent report using young adult and aged male Wistar rats fed green tea via the drinking water (with EGCG at an approximately dose of 30 mg/kg/day) for 3 months in combination with treadmill exercise [6]. Exercise significantly enhanced object recognition and inhibitory avoidance learning and memory in the rats whereas the green tea had no effect. It is possible that exercise produces the maximal enhancement in performance which cannot be modified further. However, we know, at least for neurogenesis, that different levels occur with different levels of exercise, implying that a ceiling was unlikely for this variable. For contextual fear conditioning or other forms of learning improved from exercise, it is more difficult to say. Regardless of a ceiling or not, results of our work in combination with the recent finding in rats suggest it may not be possible to enhance contextual fear learning or memory with EGCG or B-ALA either when administered alone or in combination with exercise.
There are several alternative explanations for why we did not find any significant effects of the EGCG and/or B-ALA on numbers of new cells in the hippocampus, and memory performance even though other studies reported positive results for similar measures. All the studies inevitably differ from each other in methodology, so methodological differences could explain the discrepancies. On the other hand, we chose the parameters because they were within the range expected to have an influence, and at least some of the studies used a similar duration [15] and dose [14,46,47] as we did, and found positive results for related outcome measures. Hence, an alternative explanation is that the actual effect size for the influence of EGCG and B-ALA on neuroplasticity and cognitive measures is small. We believe our results, along with the recent report in rats [6], are an important addition to the literature because they emphasize the weakness of the effects of EGCG and B-ALA in comparison to exercise on physical fitness and neurological measures. The observation of robust effects of exercise on time to fatigue (Fig. 3), contextual and cued fear conditioning (Fig. 4), and number of new cells in the dentate gyrus (Fig. 5) demonstrates that our outcome measures were sensitive enough to detect effects of the dietary interventions in our study if they existed and were of reasonable magnitude.
Despite not observing EGCG and B-ALA influences on hippocampal cell survival or behavioral performance on the fear conditioning tasks, we did observe some interesting effects of the dietary treatments on body composition. Specifically, B-ALA reduced body fat by approximately 30%, and EGCG increased lean mass by approximately 10% relative to the control diets (Fig. 2). The functional significance of these differences is unclear. In a clinical trial with 46 men assigned to either a placebo or B-ALA (B-ALA, 3-6 g/day) in combination with high intensity exercise training on a stationary bicycle, only the B-ALA group displayed increased lean mass in response to the exercise intervention [49]. In a study of 22 college wrestlers and 15 college football players, B-ALA supplemented at a dose of (4 g/day), significantly increased lean mass [48]. In our study, B-ALA reduced body fat rather than increased lean mass (though there was a trend for increased lean mass in response to B-ALA as well). However, similar to the clinical trial, runners with B-ALA had the greatest change in body composition relative to the other groups. This was because effects of B-ALA and exercise were additive (see Fig. 2A, B-ALA group). Many studies have reported that EGCG reduces adiposity [50-54], though fewer studies have reported increased lean mass. In aged rats, EGCG has been shown to reverse plantaris muscle atrophy resulting from disuse [55], perhaps by activating myogenic progenitor cells. In addition to generally supporting the literature on the positive effects of B-ALA and EGCG on body composition outcomes, the results are important because they demonstrate that the compounds entered the body and were biologically active. Hence, the negative results for the cognitive and neurogenesis outcomes are probably not trivially related to the compounds not being absorbed or being rapidly eliminated.
Although we observed a small effect of B-ALA on increasing lean body mass, we did not observe the expected increase in time to exhaustive fatigue or wheel running behavior. Although the effect of B-ALA in humans is well-established [56], the influence of B-ALA on muscle function in rodents is less well-understood. A recent study demonstrated enhanced fatigue resistance in mice given B-ALA orally for 8-12 weeks [57], a significantly longer period and a significantly higher daily dose (∼3.5-fold) than in our study. It is possible that a longer duration of supplementation and/or a higher dosage is required to improve muscle function in this model.
In our study, we observed no significant influences of any of the dietary treatments on number of new cells in the granule layer of the hippocampus as measured using the BrdU labeling method (Fig. 5). In young adult mice, it is established that a majority of newly divided cells in the subgranule layer of the dentate gyrus die within the first few days [58,59]. After 30 days, only a small number of the proliferating cells remain. However, 80-90 percent of the surviving cells display NeuN neuronal nuclear marker [9,13,60]. Hence, the differences in BrdU cell numbers we observed in our study likely reflect differential survival of new neurons in the hippocampus from exercise.
Our negative result for EGCG on the neurogenesis outcome measure is inconsistent with at least three other published reports we are aware of suggesting EGCG enhances neurogenesis. In the first study [16], three month old male C57BL/6J mice were injected i.p with EGCG (20 mg/kg) once daily for 60 days. Methodological differences could explain the discrepancies between studies as the mode for delivery of EGCG was different (i.p., versus via chow) and the dose was much different. In our study, mice ate approximately 4 g of chow per day, at a dose of 1.5 mg/g chow equates to approximately 240 mg/kg/day (see Table 2), about an order of magnitude higher dose than Wang et al. [16]. Another difference is that in our study the dose was delivered via the food, over the course of a feeding day, as opposed to in a bolus in the peritoneal cavity.
It is also important to recognize that in the Wang et al. [16] study, mice were evaluated for proliferation of new cells 2 hr after BrdU injections, which is very different from what was done in our study, where mice were injected with BrdU multiple times over the course of the first 10 days of intervention, and evaluated for numbers of surviving cells approximately 1 month later. The authors reported increases in proliferation using the 2-hr BrdU method, which they emphasized in their paper. However, the authors also included a treatment where after one month the mice received 4 consecutive daily injections of BrdU and then were euthanized for immunohistochemical detection of BrdU after an additional month. The BrdU measurement of neurogenesis in this 1-month BrdU group was very similar to ours, and the results were also similar, as the authors found no difference in survival of BrdU positive cells in the granule layer in response to chronic EGCG administration. This is an extremely important result because it suggests the proliferation differences the authors reported in the paper likely did not translate to more total numbers of neurons or new neurons integrated into the hippocampal circuitry. In other words, higher proliferation rates observed in the mice treated with EGCG may be countered by higher rates of apoptosis [61] resulting in no net increases in neurogenesis.
The best explanation we can offer for the discrepancy between our results as compared to the other two studies is methodology and small effect sizes for the nutritional interventions. One of the studies used male Wistar rats [14]. EGCG was administered with other green tea catechins at a concentration of approximately 0.5% w/v for 26 weeks in the drinking water which equated to approximately 270 mg/kg/day. The rats were injected with BrdU for 5 consecutive days then euthanized 5 weeks later. Hence, the method for measuring neurogenesis and the dose was similar to our study. However, the species was different (rat not mouse), and mode for delivery was different (via drinking water versus via food). The third study [15] used male C57BL/6J mice that received 25 mg/kg/day EGCG via oral gavage for one month and were treated with a single i.p injection of 50 mg/kg BrdU on day 21. The difference here is the route of administration (gavage versus food), strain of mouse (C57BL/6J versus BALB/cJ) and the lower dose of EGCG (25 versus 240 mg/kg/day), as compared to our study. Additional research is needed to further evaluate the influence of EGCG and B-ALA on adult hippocampal neurogenesis to identify the parameters that make the difference. We hypothesize that these nutritional compounds regardless of their dose or duration will have weak influence on adult hippocampal neurogenesis. Our hypothesis is based on the negative results we obtained in the current study and the idea that levels of adult hippocampal neurogenesis are heavily influenced by levels of physical activity [8,23,31], and subsequent activation of the dentate gyrus of the hippocampus [29,30,62], something we think is unlikely to happen with mere addition of single micronutrients into the diet.
We are not aware of any previous study that evaluated contextual or cued fear conditioning in response to EGCG or B-ALA. Most of the studies have measured spatial learning and memory using the Morris water maze or 8-arm maze [18-20]. Some studies also included the Y-maze and passive avoidance tests [17,63]. The passive avoidance test is similar to the contextual fear conditioning task in the sense that both tasks measure associative memory of a context previously paired with an aversive foot shock. The authors administered EGCG via oral gavage at a dose of 20 or 40 mg/kg/day for 7 weeks [17]. They found a benefit in diabetic mice that displayed impaired baseline performance, but no effect was found in the normal control mice, consistent with our results. In fact, most of the studies that found a positive influence of EGCG on cognitive performance found the effect in an animal that was impaired to begin with [17-19,63]. Few studies found benefits in normal mice but see [20]. It is possible that the effect of EGCG is specific to the domain of spatial learning and memory, or that the effect is only observable in the background of a deficit.
In our study, exercise enhanced both contextual and cued fear conditioning (Fig. 4). The literature suggests that the contextual fear conditioning task critically involves the hippocampus whereas the cued conditioning task does not involve the hippocampus as much [64,65]. Hence our results are consistent with the idea that effects of exercise in male BALB/cJ mice are broad, involving changes in both the hippocampus and other areas of the brain. These results are consistent with a growing literature suggesting that the effects of exercise on the brain are substantial, affecting multiple domains of cognitive performance in both humans and animal models [22,66].
In summary, our data suggest that dietary interventions with B-ALA and EGCG have little if any influence on physical fitness, adult hippocampal neurogenesis, and cognitive performance measures. While our study did not completely replicate the parameters of any specific previous study reporting positive results, the parameters we used were well within the ranges expected to have an influence from the literature. Hence, although we cannot rule out the possibility that methodological differences explain the discrepancy, we favor the conclusion that these micronutrients have limited influences on fitness and cognitive outcomes, at least relative to exercise. Our negative results were not likely due to excessive individual variation, low repeatability, or ceiling effects in our outcome measures because we demonstrated highly statistically significant benefits of voluntary wheel running administered for the same duration as the dietary interventions. Therefore, the question remains as to whether the putative/neurological benefits of EGCG and B-ALA are real and what the true effect sizes are. The compounds are safe. Therefore, in addition to additional animal work, we strongly encourage large clinical trials to help establish the legitimacy of the cognitive and neurological claims for these micronutrients.
Acknowledgments
The work was funded by the Center for Nutrition, Learning and Memory, a partnership between the University of Illinois at Urbana-Champaign and Abbott Nutrition, and also NIH RO1 grants MH083807 (J.S.R), DA027487 (J.S.R.) and AG016710 (R.W.J.). We would like to thank and acknowledge Drs. Neile Edens and Jeffrey Baxter (Abbott) for their assistance with determining dietary dosages of EGCG and β-alanine and helping edit the manuscript.
References
- 1.van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rendeiro C, Foley A, Lau VC, Ring R, Rodriguez-Mateos A, Vauzour D, Williams CM, Regan C, Spencer JP. A role for hippocampal PSA-NCAM and NMDA-NR2B receptor function in flavonoid-induced spatial memory improvements in young rats. Neuropharmacology. 2014;79:335–344. doi: 10.1016/j.neuropharm.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinilla FG. The impact of diet and exercise on brain plasticity and disease. Nutr Health. 2006;18:277–284. doi: 10.1177/026010600601800310. [DOI] [PubMed] [Google Scholar]
- 4.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2010;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holguin S, Huang Y, Liu J, Wurtman R. Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats. Behav Brain Res. 2008;191:11–16. doi: 10.1016/j.bbr.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flôres MF, Martins A, Schimidt HL, Santos FW, Izquierdo I, Mello-Carpes PB, Carpes FP. Effects of green tea and physical exercise on memory impairments associated with aging. Neurochemistry International. 2014;78:53–60. doi: 10.1016/j.neuint.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Artioli GG, Gualano B, Smith A, Stout J, Lancha AH., Jr Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc. 2010;42:1162–1173. doi: 10.1249/MSS.0b013e3181c74e38. [DOI] [PubMed] [Google Scholar]
- 8.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 9.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011;10:345–353. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 13.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himi KB, Hashimoto M, Katakura M, Haque AM, Hara Y, Shido O. Long-term administration of green tea catechins increases antioxidative actions and enhances neurogenesis in the hippocampus of rats. Current Topics in Nutraceutical Research. 2009;7:131–140. [Google Scholar]
- 15.Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM, Shin HC, Kang IJ, Won MH. (-)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res. 2010;24:1065–1070. doi: 10.1002/ptr.3083. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res. 2012;56:1292–1303. doi: 10.1002/mnfr.201200035. [DOI] [PubMed] [Google Scholar]
- 17.Baluchnejadmojarad T, Roghani M. Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behav Brain Res. 2011;224:305–310. doi: 10.1016/j.bbr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Wang MH, Chang WJ, Soung HS, Chang KC. (-)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behav Pharmacol. 2012;23:771–780. doi: 10.1097/FBP.0b013e32835a3bc8. [DOI] [PubMed] [Google Scholar]
- 19.Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid Based Complement Alternat Med. 2012;2012:163106. doi: 10.1155/2012/163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 21.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 23.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohman RA, Clark PJ, Deyoung EK, Bhattacharya TK, Venghaus CE, Rhodes JS. Voluntary wheel running enhances contextual but not trace fear conditioning. Behav Brain Res. 2012;226:1–7. doi: 10.1016/j.bbr.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- 26.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 27.Faria A, Pestana D, Teixeira D, Couraud PO, Romero I, Weksler B, de Freitas V, Mateus N, Calhau C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2:39–44. doi: 10.1039/c0fo00100g. [DOI] [PubMed] [Google Scholar]
- 28.Faria A, Pestana D, Teixeira D, Azevedo J, De Freitas V, Mateus N, Calhau C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell Mol Biol Lett. 2010;15:234–241. doi: 10.2478/s11658-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- 32.Ferri P, Cecchini T, Ciaroni S, Ambrogini P, Cuppini R, Santi S, Benedetti S, Pagliarani S, Del Grande P, Papa S. Vitamin E affects cell death in adult rat dentate gyrus. J Neurocytol. 2003;32:1155–1164. doi: 10.1023/B:NEUR.0000021909.84327.e8. [DOI] [PubMed] [Google Scholar]
- 33.Tiedje KE, Stevens K, Barnes S, Weaver DF. Beta-alanine as a small molecule neurotransmitter. Neurochem Int. 2010;57:177–188. doi: 10.1016/j.neuint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 34.del Favero S, Roschel H, Solis MY, Hayashi AP, Artioli GG, Otaduy MC, Benatti FB, Harris RC, Wise JA, Leite CC, Pereira RM, de Sa-Pinto AL, Lancha-Junior AH, Gualano B. Beta-alanine (Carnosyn) supplementation in elderly subjects (60-80 years): effects on muscle carnosine content and physical capacity. Amino Acids. 2012;43:49–56. doi: 10.1007/s00726-011-1190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellia F, Vecchio G, Cuzzocrea S, Calabrese V, Rizzarelli E. Neuroprotective features of carnosine in oxidative driven diseases. Mol Aspects Med. 2011;32:258–266. doi: 10.1016/j.mam.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris RC, Sale C. Beta-alanine supplementation in high-intensity exercise. Med Sport Sci. 2012;59:1–17. doi: 10.1159/000342372. [DOI] [PubMed] [Google Scholar]
- 38.Betz AL, Goldstein GW. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978;202:225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- 39.McGlone SJ, Godfrey PD. Rotational spectrum of a neurohormone: Beta-Alanine. J Am Chem Soc. 1995;117:1043–1048. [Google Scholar]
- 40.Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- 41.Toggenburger G, Felix D, Cuenod M, Henke H. In vitro release of endogenous beta-alanine, GABA, and glutamate, and electrophysiological effect of beta-alanine in pigeon optic tectum. J Neurochem. 1982;39:176–183. doi: 10.1111/j.1471-4159.1982.tb04716.x. [DOI] [PubMed] [Google Scholar]
- 42.Sase A, Dahanayaka S, Hoger H, Wu G, Lubec G. Changes of hippocampal beta-alanine and citrulline levels are paralleling early and late phase of retrieval in the Morris Water Maze. Behav Brain Res. 2013;249:104–108. doi: 10.1016/j.bbr.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Chengappa KN, Turkin SR, DeSanti S, Bowie CR, Brar JS, Schlicht PJ, Murphy SL, Hetrick ML, Bilder R, Fleet D. A preliminary, randomized, double-blind, placebo-controlled trial of L-carnosine to improve cognition in schizophrenia. Schizophr Res. 2012;142:145–152. doi: 10.1016/j.schres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Boldyrev AA, Stvolinsky SL, Tyulina OV, Koshelev VB, Hori N, Carpenter DO. Biochemical and physiological evidence that carnosine is an endogenous neuroprotector against free radicals. Cell Mol Neurobiol. 1997;17:259–271. doi: 10.1023/A:1026374114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hipkiss AR, Preston JE, Himsworth DT, Worthington VC, Keown M, Michaelis J, Lawrence J, Mateen A, Allende L, Eagles PA, Abbott NJ. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann N Y Acad Sci. 1998;854:37–53. doi: 10.1111/j.1749-6632.1998.tb09890.x. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Zhao H, Zhao M, Zhang Z, Li Y. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6J mice. Brain Res. 2010;1353:28–35. doi: 10.1016/j.brainres.2010.07.074. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Kern BD, Robinson TL. Effects of beta-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J Strength Cond Res. 2011;25:1804–1815. doi: 10.1519/JSC.0b013e3181e741cf. [DOI] [PubMed] [Google Scholar]
- 49.Smith AE, Walter AA, Graef JL, Kendall KL, Moon JR, Lockwood CM, Fukuda DH, Beck TW, Cramer JT, Stout JR. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J Int Soc Sports Nutr. 2009;6:5. doi: 10.1186/1550-2783-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan CY, Wei L, Castro-Munozledo F, Koo WL. (-)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011;89:779–785. doi: 10.1016/j.lfs.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes (Lond) 2012;36:735–743. doi: 10.1038/ijo.2011.136. [DOI] [PubMed] [Google Scholar]
- 53.Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (-)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012;20:2311–2313. doi: 10.1038/oby.2011.139. [DOI] [PubMed] [Google Scholar]
- 54.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 55.Alway SE, Bennett BT, Wilson JC, Edens NK, Pereira SL. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp Gerontol. 2014;50:82–94. doi: 10.1016/j.exger.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobson RM, Saunders B, Ball G, Harris RC, Sale C. Effects of beta-alanine supplementation on exercise performance: a meta-analysis. Amino Acids. 2012;43:25–37. doi: 10.1007/s00726-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Everaert I, Stegen S, Vanheel B, Taes Y, Derave W. Effect of beta-alanine and carnosine supplementation on muscle contractility in mice. Med Sci Sports Exerc. 2013;45:43–51. doi: 10.1249/MSS.0b013e31826cdb68. [DOI] [PubMed] [Google Scholar]
- 58.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 59.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 60.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 62.Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, DeYoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012;22:1860–1867. doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu M, Chen F, Sha L, Wang S, Tao L, Yao L, He M, Yao Z, Liu H, Zhu Z, Zhang Z, Zheng Z, Sha X, Wei M. (-)-Epigallocatechin-3-Gallate Ameliorates Learning and Memory Deficits by Adjusting the Balance of TrkA/p75(NTR) Signaling in APP/PS1 Transgenic Mice. Mol Neurobiol. 2014;49:1350–1363. doi: 10.1007/s12035-013-8608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 65.Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 66.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]


