Abstract
The vascular actions of insulin are complex, because it can stimulate both nitric oxide-mediated dilatation and endothelin (ET)-1-mediated constriction. We examined vasoreactivity to insulin in isolated feed arteries of the gastrocnemius (GFA) and soleus muscles (SFA) of 32-week-old Long–Evans Tokushima Otsuka (LETO) and Otsuka Long–Evans Tokushima fatty (OLETF) rats, a hyperphagic rodent model of obesity and insulin resistance. The insulin-induced vasoreactivity of SFA and GFA was similar in LETO (healthy) and OLETF (obese/insulin-resistant) rats. However, examination of between-vessel effects revealed a number of novel insights into the heterogeneous vascular effects of insulin. Soleus feed arteries dilated more than GFA in LETO at 100 and 1000 µIU ml−1 insulin (23 versus 6 and 28 versus 0%, respectively; P < 0.05 for between-vessel differences). Likewise, in OLETF rats there was significantly greater dilatation in SFA than GFA at 10, 100 and 1000 µIU ml−1 insulin (28 versus 3, 30 versus 0 and 34 versus 0%, respectively; all P < 0.05). In the presence of 3 µm tezosentan, a non-specific endothelin-1 receptor blocker, insulin-induced dilatation of the GFA was enhanced such that differences between vessels were largely abolished in both groups. Furthermore, acetylecholine-induced dilatation was significantly greater in SFA than GFA within each group, whereas sodium nitroprusside-induced dilatory responses were greater in the GFA compared with the SFA. Overall, our findings indicate that the insulin/endothelin-1 vasoconstrictor pathway is more active in GFA than in SFA, independent of obesity in the OLETF rat model.
Introduction
Defective insulin signalling in peripheral tissues is a hallmark characteristic of type 2 diabetes mellitus (Reaven, 1995). Impairments in skeletal muscle insulin signalling are commonly thought of as being largely responsible for the development of whole-body insulin resistance, and the mechanisms underlying such defects are fairly well understood (Petersen & Shulman, 2002; Samuel & Shulman, 2012). However, it is becoming increasingly apparent that impairments in microcirculatory vasoreactivity to insulin may also play an important role in the pathogenesis of skeletal muscle insulin resistance (Jansson, 2007). Indeed, it has been proposed that insulin-induced vasodilatation is the rate-limiting step in the regulation of the action of insulin in skeletal muscle (Barrett et al. 2009).
Insulin can induce vasodilatation via the nitric oxide synthase (NOS) pathway as well as vasoconstriction via activation of the endothelin-1 (ET-1) pathway (Steinberg et al. 1994; Muniyappa & Quon, 2007). Obesity and insulin resistance are associated with a downregulation of insulin-induced NOS activation (Baron, 1996). This phenomenon of ‘pathway selective insulin resistance’ in endothelial cells is thought to contribute to impairments in insulin-stimulated increases in blood flow and glucose uptake by target tissues, such as skeletal muscle (Laakso et al. 1992;Mather et al. 2004;Muniyappa & Quon, 2007). Importantly, recent in vivo experimental evidence from patients with type 2 diabetes supports the link between insulin resistance and impaired insulin-mediated capillary recruitment (Scheede-Bergdahl et al. 2009).
Therefore, using the obese, hyperphagic Otsuka Long–Evans Tokushima fatty (OLETF) rat, we investigated the influence of obesity/insulin resistance on insulin-induced vasoreactivity of skeletal muscle feed arteries. The OLETF rat, characterized by a mutated cholestykinin-1 receptor that results in a chronic hyperphagia-induced obesity, is an established model of insulin resistance and type 2 diabetes (Kawano et al. 1992). Our group and others have shown that this model is characterized by macro and microvascular complications (Matsumoto et al. 2008; Bunker et al. 2010; Mikus et al. 2010, 2012; Bender et al. 2011; Martin et al. 2012). Feed arteries were chosen for study because of their established regulatory role in the control of blood flow to skeletal muscle in rats (Williams & Segal, 1993). In particular, we focused on the soleus feed arteries (SFA) and gastrocnemius feed arteries (GFA), because these arteries perfuse skeletal muscle with differential fibre type composition and recruitment patterns. We hypothesized that hindlimb muscle feed arteries of OLETF rats would display reduced vasodilatory responses to insulin compared with those of Long–Evans Tokushima Otsuka (LETO; lean, normophagic control) rats via an upregulation of insulin-induced ET-1 activation.
Methods
Animals
All animal protocols were approved by the University of Missouri Institutional Animal Care and Use Committee. Male LETO and OLETF rats (age, 4 weeks; Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan) were housed on a 12 h–12 h light–dark cycle and provided with water and standard rodent chow (Formulab 5008; Purina Mills, St Louis, MO, USA) ad libitum. Body weights and food intakes were recorded on a weekly basis. At 32 weeks of age, rats were anaesthetized by intraperitoneal injection of pentobarbital sodium (100 mg kg−1) in the morning following a 12 h overnight fast. The soleus and gastrocnemius muscles were harvested for arteriole isolation. Rats were then killed by exsanguination, and blood was collected for determination of glycosylated haemoglobin (HbA1c) via boronate-affinity HPLC (Primus Diagnostics, Kansas City, MO, USA). It should be noted that some data from these animals appear in recently published reports from our laboratory addressing fundamentally different research questions from that of the present report, i.e. the functional adaptation of the skeletal muscle microvasculature to exercise training (Martin et al. 2012) and the effects of combined exercise training and metformin on adipose tissue inflammation (Jenkins et al. 2012b).
Body composition and blood parameters
On the day of the experiments, body mass was measured to the nearest 0.01 g and, following induction of general anaesthesia, body composition was determined using a dual-energy X-ray absorptiometry instrument (Hologic QDR-1000, Bedford, MA, USA) calibrated for rodents. All serum chemistry parameters were analysed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO, USA) on an Olympus AU680 automated chemistry analyser (Beckman-Coulter, Brea, CA, USA) using commercially available assays according to manufacturers’ guidelines. Plasma insulin concentrations were determined using a commercially available, rat-specific ELISA (Alpco Diagnostics, Salem, NH, USA). Samples were run in duplicate, and manufacturers’ controls and calibrators were used according to assay instructions.
Vascular casting
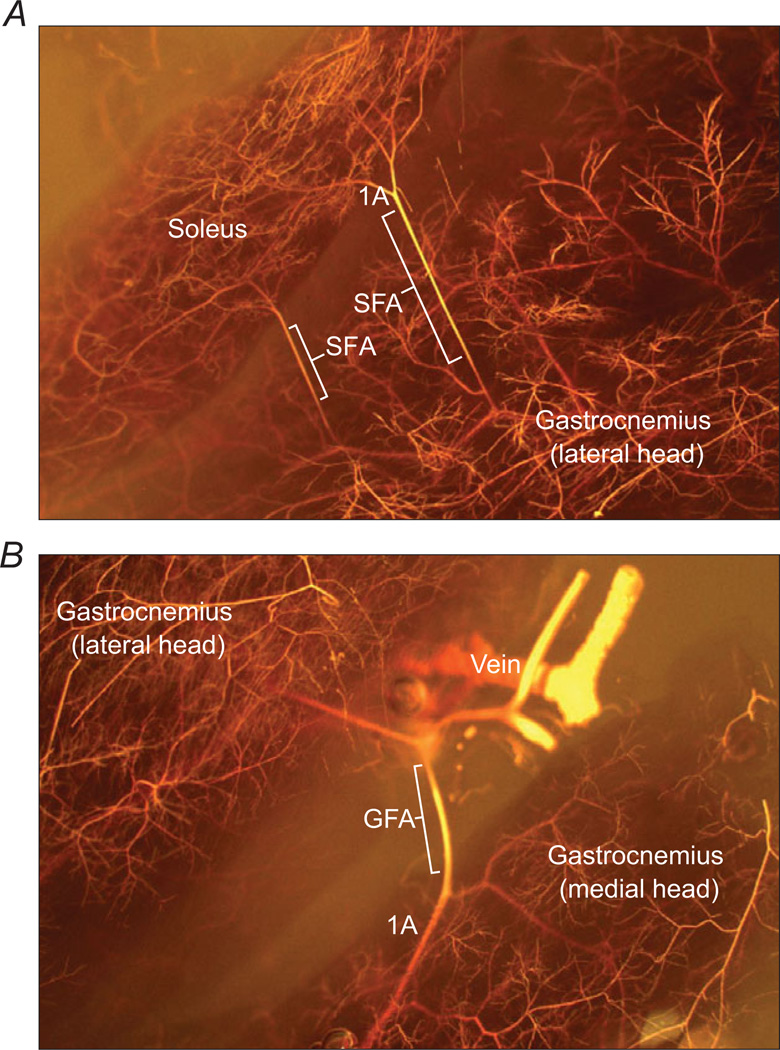
A visualization of the soleus/gastrocnemius muscle complex and the associated microvascular anatomical details, including the location and structure of the SFA and GFA, was obtained from the skeletal muscle microcirculation of an adult Wistar rat using the MICROFIL microvascular casting technique, as described previously (Binder et al. 2007). The SFA and GFA were visualized under a stereomicroscope, and the acquired photomicrographs are depicted in Fig. 1A (SFA) and B (GFA).
Figure 1. Microfilcasts of the vascular anatomy of the soleus (A) and gastrocnemius muscles (B).
The location and structure of soleus feed arteries (SFA) and gastrocnemius feed arteries (GFA) are shown, and first order (1A) branch arterioles shown for anatomical reference.
Isolated feed artery studies
The GFA and SFA were isolated, cannulated and pressurized at 90 cmH2O as described previously (Woodman et al. 2005; Bender et al. 2011; Martin et al. 2012). Following equilibration in physiological saline solution [composition (in mm): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 25.0 MOPS at pH 7.4] for 60 min at 37°C, arteries were preconstricted with phenylephrine to achieve >30% tone for examination of vasomotor responses. Phenylephrine was added to each bath prior to the first vasomotor response curve regardless of spontaneous tone. However, for subsequent curves, if sufficient tone (>30% of initial diameter) was present, then no further phenylephrine was added. All arteries achieved target preconstriction within a range of 10−7–10−5 m phenylephrine. Vasomotor responses to insulin (1, 10, 100 and 1000 µIU ml−1) were evaluated in 10 min increments (n =12 OLETF rats and n =10 LETO rats). It is important to note that in vivo serum insulin concentrations have been reported to range from ~3 to ~300 µIU ml−1 in rats (Mason et al. 1999; Chirieac et al. 2000). Thus, the highest dose of insulin used in our isolated vessel studies represents a supraphysiological concentration. Inner vessel diameters were recorded immediately prior to the addition of the next dose of insulin. To evaluate the role of ET-1 in the vasomotor response to insulin, vessels from a subset of animals (n = 8 OLETF rats and n = 7 LETO rats) were incubated for 20 min with tezosentan (3 µm), a non-selective ETA and ETB receptor antagonist, and vasoreactivity to insulin (1–1000 µIU ml−1) was re-assessed. Endothelium-dependent dilatation in response to ACh (10−9–10−4 m) and endothelium-independent dilatation in response to sodium nitroprusside (SNP) were determined on GFA and SFA also in a subset of animals (n = 6–10 per group). At the end of the experiment, the PSS bath was replaced with Ca2+-free PSS containing 100 µm SNP to determine maximal passive diameter. At maximal dilatation, the intima–media thickness (IMT) was measured as the width between the inner vessel wall (smooth muscle–endothelial border) and outer vessel wall (adventitia) using video callipers (as for vessel diameters).
We also examined the effects of insulin in the presence of 100 µm l-NAME (n = 10 OLETF rats and n = 8 LETO rats). Consistent with our group’s recent studies of OLETF second-order muscle arterioles (Mikus et al. 2012), these experiments were problematic. The addition of l-NAME initially (within ~20 min) produced the expected vasoconstriction resulting from the elimination of basal NO production. However, as before (Mikus et al. 2012), we observed gradual dilatation until ~40 min later, when the vessel returned to its initial diameter, making the assessment of insulin-induced NO activation using l-NAME impossible given the 40 min insulin treatment protocol (i.e. four sequential 10 min doses). Thus, although we would have liked to have assessed the NO component of vascular insulin signalling directly using l-NAME in our study, it appears that alternative strategies to this traditional approach are needed in OLETF and LETO rats, because the skeletal muscle microvasculature is only transiently affected by l-NAME administration. For the purposes of the present study, the reader is provided with an estimate of the NO component of the insulin effects from the data obtained during the ET-1 blockade condition. That is, if we assume that insulin can only activate either ET-1 or NOS pathways (Muniyappa & Quon, 2007), then NOS must be the insulin-responsive factor when ET-1 receptors are blocked by tezosentan. We acknowledge the inherent limitations of this assumption.
Data analysis and statistics
Data are presented as means ± SEM. Dilator responses are presented as the percentage possible dilatation, calculated as follows: ([Dd − Db]/[Dmax − Db]) × 100, where Dd is the diameter after insulin treatment, Db the baseline diameter andDmax the maximal passive diameter. Statistical analysis was performed using IBM SPSS Statistics 19 for Windows (Chicago, IL, USA). Differences in animal and vessel characteristics were evaluated using a one-way ANOVA with Fisher’s LSD post hoc analysis. Vasomotor responses were examined using ANOVA, with group and vessel as between-subjects factors and insulin as a repeated factor. A P value of ≤0.05 was considered statistically significant.
Results
Animal and vessel characteristics
The OLETF rats had substantially greater body weights, percentage body fat and fat pad masses, and displayed elevated standard blood markers of cardiovascular and metabolic disease compared with LETO rats (Table 1). Between-group comparisons of passive diameter and magnitude of preconstriction indicated no significant differences in either GFA or SFA (Table 2). Maximal diameters were different between GFA and SFA regardless of group. The dilatory response to 20 min incubation with 3 µm tezosentan was significantly greater for OLETF GFA than for LETO GFA (P < 0.05), but the difference between groups in tezosentan-induced dilatation in the SFA was not statistically significant.
Table 1.
Animal characteristics
| Characteristic | LETO rats | OLETF rats |
|---|---|---|
| Body weight (g) | 478 ± 10 | 640 ± 39* |
| Body fat (%) | 16 ±1 | 33 ± 2* |
| Total fat pad mass (g) | 17 ±1 | 64 ± 5* |
| Blood measures | ||
| Glucose (mg dl−1) | 152 ± 4 | 283 ± 21* |
| Insulin (µIU ml−1) | 3 ± 0.4 | 7 ± 1* |
| HbA1c (%) | 4.8 ± 0.1 | 7.3 ± 0.3* |
| Total cholesterol (mg dl−1) | 94 ± 3 | 146 ± 8* |
| LDL-C (mg dl−1) | 58 ±3 | 44 ± 6 |
| HDL-C (mg dl−1) | 27 ±1 | 35 ± 2* |
| Triglycerides (mg dl−1) | 44 ± 3 | 337 ± 46* |
Abbreviations: HbA1c, glycosylated haemoglobin; HDL-C, high-density lipoprotein lipid cholesterol; LDL-C, low-density lipoprotein lipid cholesterol; LETO, Long–Evans Tokushima Otsuka; and OLETF, Otsuka Long–Evans Tokushima fatty. Data are means ± SEM.
P < 0.05 versus LETO.
Table 2.
Vessel characteristics
| Vessel characteristic | LETO group | OLETF group |
|---|---|---|
| Gastrocnemius feed artery | ||
| Passive diameter (µm) | 289 ± 12 | 292 ± 13 |
| Preconstriction (%) | ||
| Insulin | 41 ±2 | 43 ± 3 |
| Insulin + tezosentan | 41 ±4 | 42 ± 3 |
| Dilatation in response to tezosentan (% possible) | 12 ±4 | 28 ± 5* |
| Soleus feed artery | ||
| Passive diameter (µm) | 185 ± 12 | 209 ± 6† |
| Preconstriction (%) | ||
| Insulin | 52 ±4 | 51 ± 3 |
| Insulin + tezosentan | 45 ±3 | 48 ± 4 |
| Dilatation in response to tezosentan (% possible) | 11 ± 13 | 31 ± 8 |
Data are means ± SEM.
P < 0.05 versus LETO.
P = 0.06 versus LETO.
Insulin-, ACh- and SNP-induced vasoreactivity
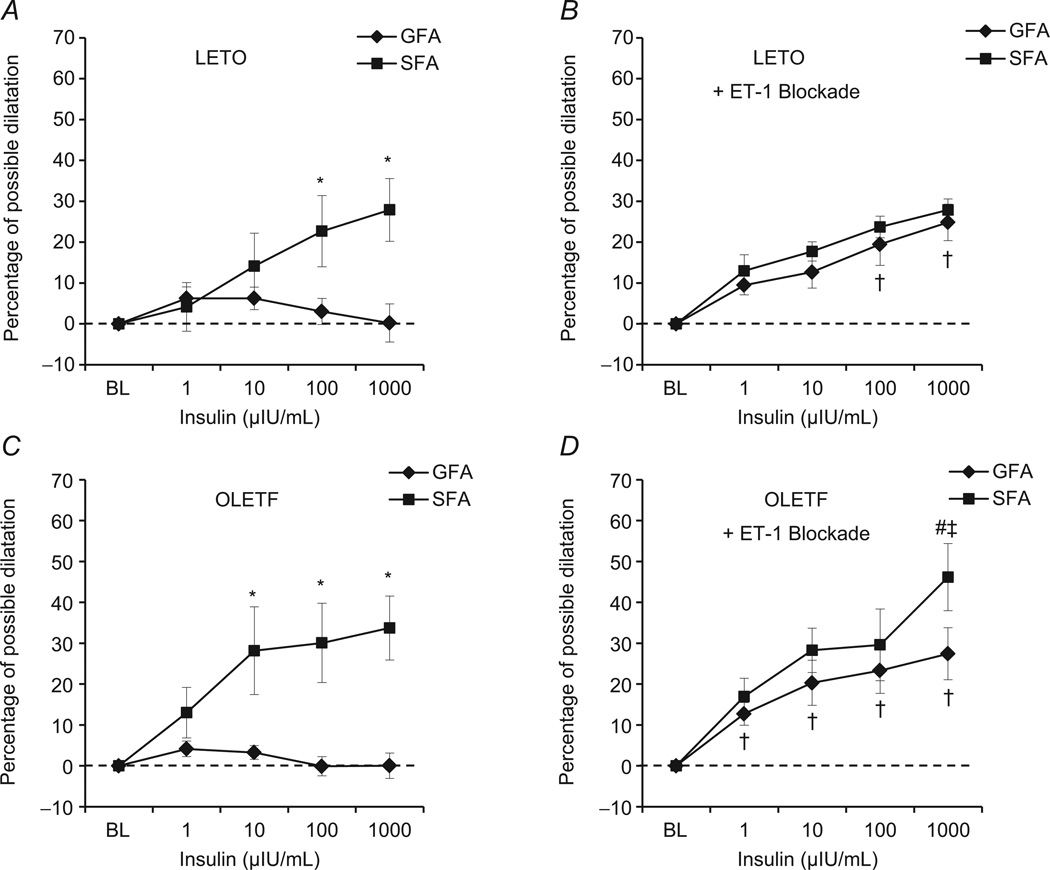
The insulin-induced vasoreactivity of SFA and GFA was similar in LETO and OLETF rats in the absence of tezosentan (Fig. 2A and C). However, examination of between-vessel effects revealed a number of novel insights into the heterogeneous vascular effects of insulin. Soleus feed arteries dilated more than GFA in LETO rats at 100 and 1000 µIU ml−1 insulin (23 versus 6 and 28 versus 0% possible dilatation, respectively; P < 0.05 for between-vessel differences; Fig. 2A). Likewise, in OLETF rats there was significantly greater dilatation in SFA than GFA at 10, 100 and 1000 µIU ml−1 insulin (28 versus 3, 30 versus 0 and 34 versus 0% possible dilatation, respectively; all P < 0.05; Fig. 2C).The change in diameter in the GFA was statistically significantly different from zero at only 1 and 10 µIU ml−1 insulin in both groups. In contrast, changes in SFA diameter were significantly different from zero at all insulin doses in OLETF rats and at all but the lowest insulin dose in LETO rats. In the presence of 3 µm tezosentan (Fig. 2B and D), a non-specific ET-1 receptor blocker, insulin-induced dilatation of the GFA was enhanced such that differences between vessels were largely abolished in both groups. Dilatation was significantly different from zero in both vessels of both groups in the presence of tezosentan. Examination of group × vessel × insulin dose interaction effects indicated the presence of the following two notable findings at the highest (supraphysiological) dose of insulin in the presence of tezosentan: (i) the SFA of OLETF rats dilated to a greater extent in the presence of tezosentan compared with the insulin-alone control conditions; and (ii) the SFA of OLETF rats dilated to a greater extent than that of LETO rats. Otherwise, there were no differences between groups with or without tezosentan exposure.
Figure 2. Insulin-induced vascular reactivity in SFA and GFA of Long–Evans Tokushima Otsuka (LETO; A and B) and Otsuka Long–Evans Tokushima fatty (OLETF) rats (C and D).
Left panels (A and C) present data from insulin dose–response curves performed in control condtions (i.e. insulin alone), and right panels (B and D) present data from insulin dose–response curves in the presence of 3 µm tezosentan, a non-specific inhibitor of endothelin-1 (ET-1) receptors. *Significant difference between vessels, within group (P < 0.05). †Significant effect of ET-1 blockade within vessel, within group (P < 0.05). #Significant difference between groups, within vessel in ET-1 blockade condition (P < 0.05). ‡Significant difference between vessels, within group in ET-1 blockade condition (P < 0.05).
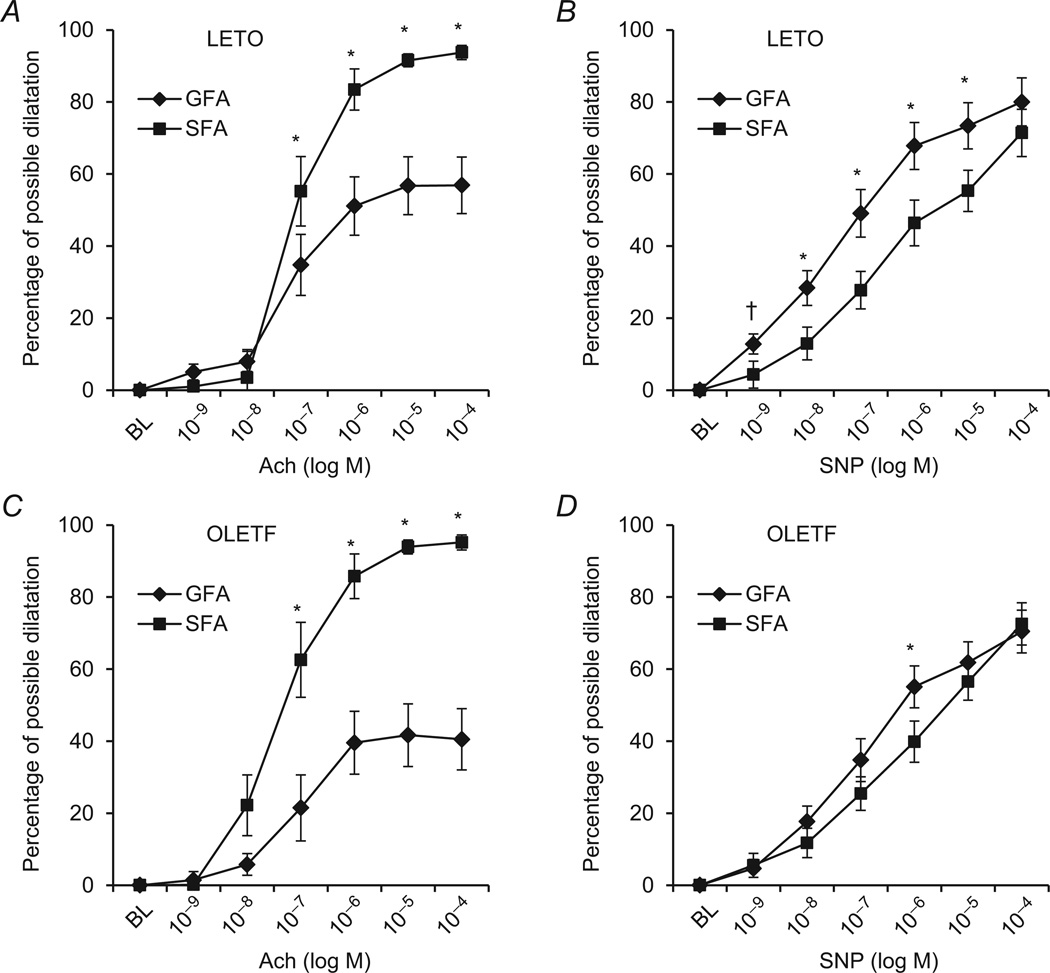
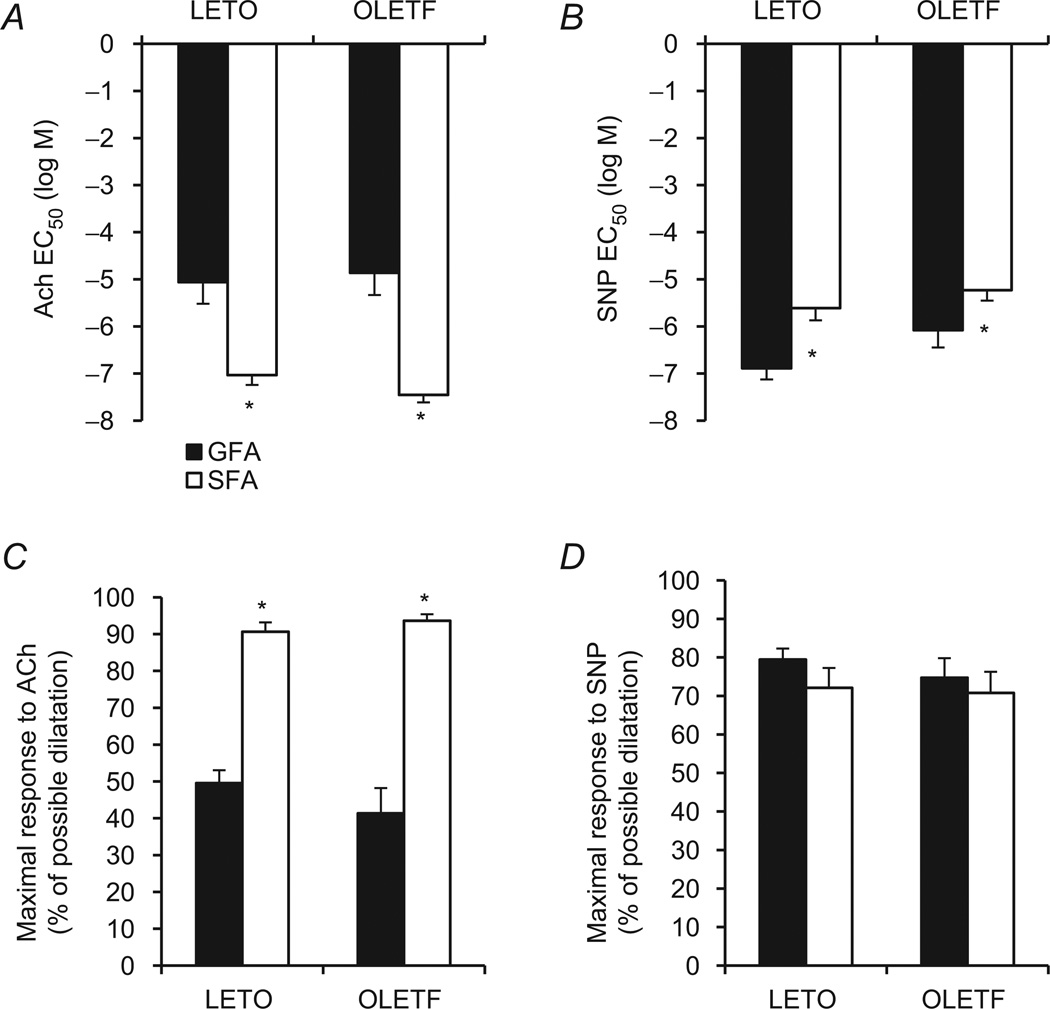
Acetylcholine-induced endothelium-dependent dilatation was similar in SFA and GFA of LETO and OLETF rats. However, the SFA and GFA exhibited substantially different vasodilatory responses to ACh within both groups (Fig. 3A and C). Soleus feed arteries dilated more in response to ACh than GFA at 10−7, 10−6, 10−5 and 10−4 m in LETO rats (55 versus 34, 83 versus 51, 92 versus 56 and 94 versus 57% possible dilatation, respectively; all P < 0.05; Fig 3A). Likewise, SFA dilated more in response to ACh than GFA at 10−7, 10−6, 10−5 and 10−4 m in OLETF rats (63 versus 21, 86 versus 40, 94 versus 42 and 95 versus 41% possible dilatation, respectively; all P < 0.05; Fig. 3C). There were no differences between LETO and OLETF rats in endothelium-independent vasodilator responses to SNP in the SFA and only at the lowest SNP dose in the GFA (Fig. 3A). Within LETO animals, the GFA dilated in response to SNP to a greater extent than the SFA at all doses (P < 0.05; Fig. 3B) except at the highest SNP concentration (P > 0.05). Within OLETF animals, the magnitude of SNP-induced dilatation was similar between vessels at all doses except for 10−6 m, where the GFA dilated to a greater extent than the SFA (Fig. 3D). Analysis of EC50 values and maximal dilatory responses indicated that SFA were more sensitive and demonstrated greater maximal responses to ACh compared with GFA within both groups (P < 0.05; Fig. 4A and C), whereas the SFA were significantly less sensitive (P < 0.05; Fig. 4B) and displayed similar maximal responsiveness to SNP (P > 0.05; Fig. 4D).
Figure 3. Vasodilatation induced by ACh and sodium nitroprusside (SNP) in SFA and GFA of LETO (A and B) and OLETF rats (C and D).
Left panels (A and C) present data from ACh dose–response curves, and right panels (B and D) present data from SNP dose–response curves. *Significant difference between vessels, within group (P < 0.05). †Significant difference between groups, within vessel (GFA) (P < 0.05).
Figure 4. Values of EC50 (A and B) and maximal responses (C and D) to ACh and SNP in SFA and GFA of LETO and OLETF rats.
*Significant difference between vessels, within group (P < 0.05).
Intima–media thickness
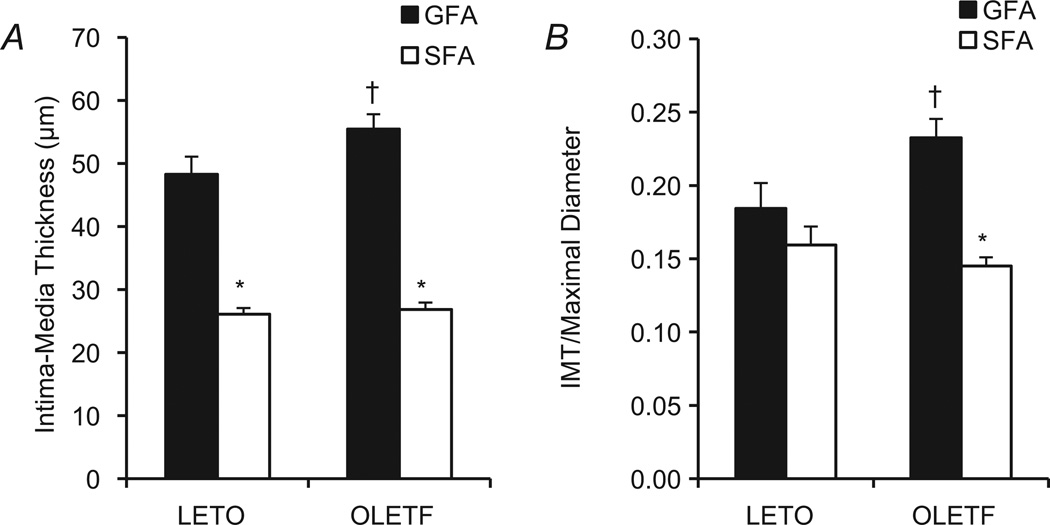
There was a significant vessel × group interaction effect on IMT in both absolute terms (Fig. 5A) and after normalizing the IMT to maximal passive diameter (Fig. 5B). The IMT of GFA was greater than that of SFA in both groups (P < 0.05), and the GFA IMT of OLETF rats was significantly greater than that of LETO rats (P < 0.05). As wall thickness is generally related to vessel diameter, IMT was also expressed relative to maximal passive diameter (Fig 5B). Significant between-vessel differences in normalized IMT were present only within the OLETF group (P < 0.05).
Figure 5. Intima–media thickness of SFA and GFA in LETO and OLETF rats, expressed as absolute thickness (in micrometres; A) and normalized to vessel diameter (B).
*Significant difference between vessels, within group (P < 0.05). †Significant difference between groups, within vessel (P < 0.05).
Discussion
The major finding of this study is that there are substantial differences between the GFA and SFA with respect to the vascular actions of insulin in the OLETF rat model, independent of obesity status. Namely, insulin induces substantial vasodilatation in the SFA, while the GFA is minimally responsive to insulin. This difference between vessels is due to a lack of insulin-induced ET-1 activation in the SFA, whereas in the GFA there is substantial activation of ET-1 upon exposure to insulin.
Despite our observation that obesity does not appear to influence the vasomotor actions of insulin in the GFA or SFA in the OLETF rat model, the present study provides novel evidence that the vascular actions of insulin are distinct between these feed arteries. The mechanisms responsible for the differences between GFA and SFA dilatory responses to insulin are not fully understood. One possible explanation relates to differences between the haemodynamics of the arteries owing to differences in muscle fibre type and local muscle recruitment patterns between the gastrocnemius and soleus muscles. The SFA is characterized by chronically high levels of flow and, presumably, high shear stress relative to the GFA, owing to continuous recruitment of the soleus muscle for postural maintenance and normal cage activity (Laughlin & Armstrong, 1982). In contrast, the gastrocnemius muscle is not substantially recruited unless the animal engages in vigorous contraction of the hindlimb, in which case the GFA can be exposed to substantial elevations in flow (Laughlin & Armstrong, 1982). Due to methodological limitations, we were unable to measure in vivo shear rates directly in the GFA and SFA in the present study. However, given the mathematical relationship between flow, vessel diameter and shear stress, it is reasonable to speculate that the SFA is exposed to chronically elevated shear as a result of the habitual recruitment of the soleus muscle for postural support and normal cage activity. Previous data from our laboratory provide quantitative support for this speculation. Using an in vitro isolated artery preparation, Woodman et al. (2003) demonstrated that flow rates analogous to maximal in vivo physiological soleus muscle blood flows induced SFA dilatation by ~40%. Thus, approximately fourfold greater resting flow in the SFA (Laughlin & Armstrong, 1982) is likely to produce relatively minor changes in diameter and is therefore not sufficient to offset the induction of a chronic shear stimulus.
Along these lines, the findings from the present study are consistent with the hypothesis we recently put forth that one beneficial effect of increased shear might be to enhance the sensitivity of the endothelium to the vasodilator effects of insulin (Padilla et al. 2011; Jenkins et al. 2012a). Specifically, the SFA of both OLETF and LETO rats displayed marked (~30–40%) dilatation in response to insulin, whereas the GFA was largely unresponsive to insulin alone in both groups (Fig. 2A and C). Furthermore, our experiments revealed a role for ET-1 in the differential effects of insulin between the vessels, as indicated by the GFA and SFA dilating to a similar extent in the presence of tezosentan (Fig. 2B and D). Additionally, the lack of further insulin-induced dilatation in the SFA with ET-1 blockade over insulin alone in either group (with the exception of the highest, supraphysiological dose of insulin in the OLETF rats; Fig. 2D) indicates that insulin does not appreciably stimulate the ET-1 pathway in the SFA.
A surprising finding of our study was that vasodilatory effects of insulin were similar between SFAs of LETO and OLETF rats (Fig. 2). It is interesting to note that the finding of greater serum insulin concentrations in the OLETF compared with LETO rats (Table 1) would be postulated to be an indication of insulin resistance. However, in our in vitro preparation, the SFA did not appear to become resistant to the vasodilatory effect of insulin despite greater chronic exposure to insulin in the OLETF rat. These findings are generally consistent with previous data from our laboratory indicating that the SFA of the OLETF rat is less vulnerable to obesity-associated endothelial dysfunction and enhanced ET-1-induced vasoconstriction (Bender et al. 2011).
Consistent with our observations in the insulin-induced vasoreactivity experiments, we also observed markedly lower dilatation in response to ACh, a classical endothelium-dependent dilator of rat skeletal muscle microvessels, in the GFA compared with the SFA in both LETO and OLETF groups (Figs 3A and C and 4A and C). These results suggest that the differences between GFA and SFA are not specific to insulin; rather, these vessels exhibit intrinsic differences in their endothelium-dependent dilatory capacity. Of note, we and others have determined that the vasodilatory response to insulin is an endothelium-dependent response, i.e. the dilatory response is largely abolished by endothelial denudation (Misurski et al. 2001; Martin et al. 2012). Additionally, it is important to mention that the diameters of SFA and GFA were significantly different. However, our observed differences in vasomotor responses were unlikely to be attributable to differences in diameter because if they were the result of differences in diameter, the differential vasomotor responses would have remained upon stimulation of any dilator, including SNP. Clearly, this was not the case in the present study as, overall, the GFA displayed greater responsiveness to SNP than the SFA (Figs 3 and 4). In addition, our finding that GFA treated with an ET-1 receptor blocker exhibited an insulin-induced dilatory response similar to that exhibited by the SFA further supports the idea that our findings are not simply attributable to diameter differences between arteries. Nevertheless, it remains a possibility that differences observed between the GFA and the SFA could be attributed to differences in vessel size and may not necessarily or solely be due to differences in the vascular beds studied. Future studies incorporating distal branch orders of the gastrocnemius and soleus muscle vascular beds are needed to clarify this issue.
While the SFA was protected from impaired insulin-induced dilatation in OLETF rats, it seems that systemic risk factors specific to the obese OLETF group may have adversely impacted the GFA phenotype, as indicated by our finding of greater IMT in the OLETF GFA compared with OLETF SFA and both vessels of the LETO group (Fig. 5). Likewsie, our observation that the OLETF GFA exhibited a greater dilatory response during the 20 min pretreatment with tezosentan compared with the LETO GFA suggests a greater contribution of ET-1 to basal tone in the GFA of the OLETF rat (Table 2), consistent with the existing evidence that ET-1 tone is elevated in inactive and obese individuals (Mather et al. 2002; Weil et al. 2011). This finding was specific to the GFA, and there were no differences between groups in basal ET-1 tone of the SFA, providing further support for the idea that the SFA is protected from adverse influences of the obese/insulin-resistant circulating environment.
Limitations and future directions
Although we previously reported that vasoconstrictor responses toET-1wereenhanced in GFA but not SFA of 20-week-old OLETF rats compared with LETO rats (Bender et al. 2011), we did not assess the constrictor responses to ET-1 in the present study. Furthermore, we did not determine whether the mechanisms responsible for ET-1-mediated inhibition of insulin-induced dilatation involved direct vasoconstrictor effects or indirect effects, such as generation of reactive oxygen species. We studied only male rats in the present investigation; experiments examining sex differences in insulin-induced dilatation of the skeletal muscle vasculature are warranted. Finally, although our data support a mechanistic role for ET-1 in the differential responses to insulin between SFA and GFA, future studies are required to evaluate experimentally our proposal that in vivo haemodynamic factors might be responsible for the heterogeneity between the vessels.
In summary, our data suggest that compared with the GFA, the SFA is sensitized to the vasodilatory actions of insulin, independent of obesity, in the OLETF rat model. The mechanisms underlying the apparent upregulation of the insulin/ET-1 pathway in GFA compared with SFA should be elucidated in future studies.
New findings.
What is the central question of this study?
This study investigated the influence of obesity on insulin-induced vasomotor reactivity in rat skeletal muscle feed arteries.
What is the main finding and its importance?
Irrespective of obesity, the gastrocnemius feed artery displayed diminished insulin-induced vasodilatory response compared to the soleus feed artery. This difference between the arteries was abolished in the presence of an endothelin-1 receptor antagonist. Therefore, our findings demonstrate that insulin-induced endothelin-1 production is not uniform among all skeletal muscle resistance vessels.
Acknowledgements
Pam Thorne and Grace M. Meers are thanked for expert technical assistance. We thank Dr Mike Davis for helpful discussion of the data.
Funding
Funding was provided by NIH RO1-HL036088 (M.H.L.), Department of Veterans’ Affairs VA-CDA2 IK2 BX001299-01 (R.S.R.), NIH T32-AR48523 (N.T.J. and J.S.M.) and American Heart Association 11POST5080002 (J.P.). This work was supported in part with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO, USA.
Footnotes
Competing interests
None declared.
References
- Baron AD. The coupling of glucose metabolism and perfusion in human skeletal muscle. The potential role of endothelium-derived nitric oxide. Diabetes. 1996;45(Suppl 1):S105–S109. doi: 10.2337/diab.45.1.s105. [DOI] [PubMed] [Google Scholar]
- Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender SB, Newcomer SC, Laughlin MH. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol. 2011;300:H1434–H1441. doi: 10.1152/ajpheart.01093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder KW, Murfee WL, Song J, Laughlin MH, Price RJ. Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle. Microcirculation. 2007;14:181–192. doi: 10.1080/10739680601139294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol. 2010;298:H1889–H1901. doi: 10.1152/ajpheart.01252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab. 2000;279:E1003–E1011. doi: 10.1152/ajpendo.2000.279.5.E1003. [DOI] [PubMed] [Google Scholar]
- Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262:173–183. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: implications for cardiovascular disease. Curr Cardiovasc Risk Rep. 2012a;6:331–346. doi: 10.1007/s12170-012-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Padilla J, Arce-Esquivel AA, Bayless DS, Martin JS, Leidy HJ, Booth FW, Rector RS, Laughlin MH. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. J Appl Physiol. 2012b;113:1873–1883. doi: 10.1152/japplphysiol.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–1083. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Martin JS, Padilla J, Jenkins NT, Crissey JM, Bender SB, Rector RS, Thyfault JP, Laughlin MH. Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol. 2012;113:1223–1232. doi: 10.1152/japplphysiol.00823.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524–530. doi: 10.2337/diabetes.48.3.524. [DOI] [PubMed] [Google Scholar]
- Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;295:H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]
- Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol. 2010;109:1203–1210. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus CR, Roseguini BT, Uptergrove GM, Morris EM, Rector RS, Libla JL, Oberlin DJ, Borengasser SJ, Taylor AM, Ibdah JA, Laughlin MH, Thyfault JP. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin-resistant rats. Microcirculation. 2012;19:729–738. doi: 10.1111/j.1549-8719.2012.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misurski DA, Wu SQ, McNeill JR, Wilson TW, Gopalakrishnan V. Insulin-induced biphasic responses in rat mesenteric vascular bed: role of endothelin. Hypertension. 2001;37:1298–1302. doi: 10.1161/01.hyp.37.5.1298. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care. 2007;10:523–530. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheede-Bergdahl C, Olsen DB, Reving D, Boushel R, Dela F. Insulin and non-insulin mediated vasodilation and glucose uptake in patients with type 2 diabetes. Diabetes Res Clin Pract. 2009;85:243–251. doi: 10.1016/j.diabres.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol. 2011;301:H689–H695. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]