Abstract
CD4 T cells are both necessary and sufficient to mediate acute cardiac allograft rejection in mice. This process requires “direct” engagement of donor MHC class II molecules. That is, acute rejection by CD4+ T cells requires target MHC class II expression by the donor and not by the host. However, it is unclear whether CD4+ T cell rejection requires MHC class II expression on donor hemopoietic cells, nonhemopoietic cells, or both. To address this issue, bone marrow transplantation in mice was used to generate chimeric heart donors in which MHC class II was expressed either on somatic or on hemopoietic cells. We report that direct recognition of hemopoietic and nonhemopoietic cells are individually rate limiting for CD4+ T cell-mediated rejection in vivo. Importantly, active immunization with MHC class II+ APCs triggered acute rejection of hearts expressing MHC class II only on the somatic compartment. Thus, donor somatic cells, including endothelial cells, are not sufficient to initiate acute rejection; but they are necessary as targets of direct alloreactive CD4 T cells. Taken together, results support a two-stage model in which donor passenger leukocytes are required to activate the CD4 response while direct interaction with the somatic compartment is necessary for the efferent phase of acute graft rejection.
Numerous studies indicate that CD4+ T cells are of paramount importance in initiating cardiac allograft rejection. Studies using CD4+ T cell depletion with anti-CD4 mAb therapy or transplantation into CD4-deficient hosts demonstrate long-term allograft survival, indicating that CD4+ T cells are necessary for acute cardiac rejection (1– 6). Additionally, CD4+ T cells are sufficient for cardiac allograft rejection, as demonstrated by the normal tempo of rejection after adoptive transfer of enriched CD4+ T cells into heart-engrafted immunodeficient recipients (7). Importantly, CD4+ T cell-mediated rejection appears to be dependent on the direct pathway of Ag presentation. That is, CD4-mediated rejection of cardiac allografts requires donor MHC class II and does not require host MHC class II expression (7). Thus, direct but not indirect MHC class II presentation is necessary for acute CD4-mediated rejection. However, on which cellular compartment (or compartments) MHC class II expression is required remains unclear. More specifically, whether the primary APC for CD4+ T cell-mediated rejection can be hemopoietic-derived, somatic cell-derived, or both is unknown. Additionally, whether or not somatic cells, such as vascular endothelial cells, can in fact function as primary APCs in vivo is unclear. Therefore, a key goal of this study was to assess the ability of CD4+ T cells to mediate acute allograft rejection in immune-deficient hosts after isolation of allograft MHC class II expression to the bone marrow-derived (hemopoietic) or somatic (parenchymal) compartments, respectively.
Traditionally, bone marrow-derived dendritic cells have been implicated as the major APC in allotransplantation (8, 9). However, substantial in vitro data have implicated the vascular endothelial cell (EC3) as a potential APC (10 –12) and, more recently, in vivo evidence suggests that vascular EC may function as primary APCs in CD8-mediated cardiac rejection (13). It is controversial whether or not mouse EC constitutively express MHC class II or can prime CD4 T cells. On one hand, MHC class II expression appears to be inducible in vitro in that EC-mediated CD4+ T cell proliferation requires MHC class II induction with proinflammatory mediators such as IFN-γ (11). Conversely, other in situ evidence exists demonstrating baseline microvascular EC MHC class II expression without inflammatory induction (14), and histological evidence exists showing ongoing cardiac donor endothelial cell MHC class II expression after cardiac transplantation (15). Whether or not EC have stimulatory capacity in vivo is unclear and whether or not they are required as direct molecular targets of CD4-mediated rejection is essentially unknown. Thus far, the cellular population responsible for Ag presentation in CD4-mediated cardiac rejection has been primarily hypothesized based on in vitro results. Consequently, acute rejection with MHC class II expression isolated to either the donor hemopoietic or donor somatic compartments, respectively, will have major implications in terms of identifying the cellular population responsible for in vivo Ag presentation. Results show that MHC class II expression is required on both donor hemopoietic and somatic cells and that chimeric heart allografts expressing MHC class II only on somatic cells are rejected only after immunization of the host with MHC class II+ APCs, suggesting that hemopoietic-derived cells function as APCs and graft somatic cells as targets of CD4+ T cell-mediated rejection.
Materials and Methods
Mice
Inbred female C57BL/6ByJ (B6, H-2b), BALB/cByJ (BALB/c H-2d), immune-deficient C57BL/6-Rag1tm1/Mom (B6 rag1−/−, H-2b), and immune-deficient BALB/c-Rag1tm/Mom (C.129S7-rag1−/−, H-2d) were purchased from The Jackson Laboratory (Bar Harbor, ME). Female C57BL/6 I-Ab-gene targeted MHC class II-deficient (C2D, H-2b) mice were obtained from Taconic Farms (Germantown, New York). UBI-GFP/BL6 (C57BL/6 gfp, H-2b) mice (16) were a generous gift from Dr. P. Marrack (National Jewish Medical and Research Center, Denver, CO). Animals were housed under pathogen-free conditions at the University of Colorado Barbara Davis Center Animal Facility according to National Institutes of Health guidelines.
Antibodies
The following conjugated Abs were used to characterize leukocyte populations from either peripheral blood (PB) or organ/tissue digest suspensions: CD4 T cell-enriched populations were assessed using PE-CD4 (clone RM4-5), PE-CD8α (clone Ly-2), and PE-CD19 (clone ID3; BD PharMingen, La Jolla, CA). Phenotyping of bone marrow chimeric animals (both PBL and heart or spleen digest preparations) utilized biotin-I-Ab (clone 25-9-17) plus streptavidin-allophycocyanin, PE-CD11c (clone HL3), PerCP/Cy5.5-CD11b (clone M1/70), PE-CD45-PE (clone 30-F11). Green fluorescent protein (GFP) expression by cells derived from gfp-transgenic mice was assessed by endogenous fluorescence.
Bone marrow transplantation (BMT) and generation of chimeric mice
Recipient mice received a total dose of 1200 rad total-body irradiation split between two doses of 600 rad of gamma irradiation (delivered via a contained Cs137 irradiator; CIS Biointernational IBL 437C, Bedford, MA) separated by 4 h (to minimize acute toxicity to the intestinal tract) according to Yan et al. (17). Under aseptic conditions, the femurs and tibias of donor mice were removed and placed in sterile HBSS with 2% FCS. Bone marrow cells were then flushed from the tibias and femurs using a 27-gauge needle and 5 ml of HBSS with 2% FCS per long bone. The cell suspension was then centrifuged at 1000 × g for 5 min, washed with HBSS with 2% FCS, repelleted, washed again, and counted. Bone marrow cells were kept on ice (4°C) at all times. A total of 5 × 106 bone marrow cells was then injected i.v. (retro-orbitally). For C2D and B6 recipients of B6 bone marrow, donor B6 mice were tagged with a ubiquitous GFP expressed in all cells via the ubiquitin promoter (16). MHC class II-deficient C2D bone marrow donors were not tagged with the GFP protein. Postprocedure, bone marrow-transplanted mice were housed under pathogen-free conditions and received trimethoprim-sulfamethoxizole prophylaxis in autoclaved/acidified drinking water for 14 days. Eight weeks after BMT, the animals were retro-orbitally bled and assessed for chimerism by flow cytometric analysis (as detailed below). Note bone marrow-transplanted chimeras are denoted as bone marrow donor → bone marrow recipient (e.g., B6 → C2D and C2D → B6). Using this nomenclature, the compartmentalization of MHC class II expression in chimeric heart allografts is detailed in Table I.
Table I.
Compartmentalization of MHC class II expression in chimeric heart allografts
| Donor Heart | Somatic MHC Class II | Hematopoietic MHC Class II |
|---|---|---|
| C2D → B6 | Yes | No |
| B6 → C2D | No | Yes |
| B6 → B6 | Yes | Yes |
Flow cytometric analysis of PB for degree of hemopoietic chimerism in bone marrow-transplanted mice
At 8 wk after BMT, B6gfp → C2D and B6gfp → B6 chimeric mice PB was assessed for the degree of donor chimerism by flow cytometric analysis by the presence of CD45+ (leukocyte common Ag) GFP+ (bone marrow donor origin) cells. C2D → B6 chimeric mice were assessed for the presence of CD45+ MHC class II− (I-Ab)− cells (as C2D bone marrow donors were not tagged with the GFP). Additionally, all bone marrow recipient mice underwent flow cytometric analysis for MHC class II (I-Ab) before BMT and again 8 wk after BMT. Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). Briefly, 0.25–0.50 ml of heparinized PB was mixed 1:1 with HBSS and leukocytes were enriched over a Histopaque gradient (Lympholyte-M, CL5120; Cedarlane Laboratories, Hornby, Ontario, Canada) at room temperature. After centrifugation at 2000 rpm for 20 min, the leukocyte fraction was removed and washed twice in HBSS. Approximately 4 × 105 cells were labeled for 20 min on ice (4°C) with the indicated conjugated Abs. Frequency determinations were calculated from single-parameter and double-parameter fluorescence histograms on a BD Immunocytometry Systems FACSCalibur (San Jose, CA) after gating on viable lymphocytes. CellQuest software (BD Immunocytometry Systems) was used to analyze flow cytometry data
Cardiac and splenic digestion for flow cytometric analysis
Hearts from both control and chimeric mice were digested with collagenase A (C-7657; Sigma-Aldrich, St. Louis, MO) at a concentration 1 mg/ml with 0.1 mg/ml DNase (D-5025; Sigma-Aldrich). Three hearts from each group were harvested after euthanasia with CO2 asphyxiation. Hearts were bisected and then flushed with HBSS to remove blood. The bisected halves were then individually distended by direct injection of collagenase (1 mg/ml). Heart tissues were then minced with fine pointed scissors and placed in 15 ml of 1 mg/ml collagenase A in a 50-ml conical tube. Tubes were placed into a 37°C water bath for 90 min. After incubating for 90 min, the 50-ml conical tubes (with remaining heart fragments) were gently shaken to suspend the majority of the remaining cardiac tissue. The resultant cell suspension was run through a 70-µm nylon cell sieve (Falcon; BD Biosciences). Remaining debris was gently pressed through the sieve with the rubber end of a 5-ml syringe plunger. The digestion then was quenched with excess HBSS medium containing 10% FCS and this cellular suspension was pelleted, washed, and resuspended in medium with 10% FCS. The leukocyte fraction then was enriched using a Histopaque gradient (Lympholyte-M, CL5035; Cedarlane Laboratories). Splenic digests were performed identically to cardiac digests with the exception that the incubation time in 1 mg/ml collagenase A was 30 min.
Heterotopic heart transplantation
Cardiac allografts from B6, C2D, B6 → C2D, C2D → B6, and B6 → B6 donor mice were transplanted heterotopically into BALB/c rag1−/− recipient mice. Vascularized grafts were transplanted according to standard microsurgical techniques (18). Briefly, the harvested donor heart was placed in 4°C saline until transplantation. Under avertin-induced anesthesia, a 2-cm midline vertical incision was made and the abdominal cavity entered. The large and small intestines were reflected superiorly and wrapped in sterile saline-soaked gauze. The abdominal aorta and inferior vena cava were then dissected and isolated below the level of the renal vessels. A two-stage end-to-side running anastomosis was then made between the recipient abdominal aorta and donor aorta using 10-0 nylon suture (Ethicon, Somerville, NJ). A similar end-to-side anastomosis was then made from the donor pulmonary artery to the recipient inferior vena cava. The abdominal wall was closed in two layers using 5-0 silk suture in a running fashion. A 1-ml sterile saline fluid bolus was administered into the abdomen upon closure as fluid resuscitation. No other supportive measures were required during the procedure. Allograft survival was assessed by daily palpation with rejection defined as loss of palpable beating. Survival differences were determined using the nonparametric, unpaired Mann-Whitney U test.
CD4 T cell enrichment and adoptive transfer
Cervical, axillary, epitrochlear, para-aortic, and mesenteric lymph nodes (LNs) were harvested from BALB/c mice. Single-cell suspensions of LN cells were enriched for CD4+ T cells by negative selection of CD8+ T cells and B cells on an immunoaffinity column according to the manufacturer’s specifications (Cellect, CL111-2; Cedarlane Laboratories). Cellular phenotyping of freshly enriched CD4 T cell populations or of PBL of adoptive transfer recipients was determined by flow cytometry assessing staining of PE-labeled anti-CD4, anti-CD8, and anti-CD19 mAbs (BD PharMingen). CD4-enriched T cells contained <0.5% contaminating CD8+ T cells or CD19+ B cells. Ten million CD4-enriched T cells were injected i.p. into the indicated adoptive transfer recipients on day 0 relative to cardiac transplantation. As an added precaution, BALB/c rag−/− cardiac transplant recipients were treated with rat anti-mouse CD8 mAb 2.43 (IgG2b) (19) on post-CD4 transfer days 0 and 7 for depletion of any CD8+ T cells that may have been transferred with the heart allograft. Anti-CD8 mAb (2.43) was generated as ascites in BALB/c rag−/− mice and quantitated by isotype-specific ELISA.
Adoptive transfer of B6 rag−/− splenocytes as a source of MHC class II+ APCs
Spleens from B6 rag−/− female mice were homogenized to generate single-cell suspensions. Cells were washed and RBC were lysed by suspension in a RBC lysis buffer (R7757; Sigma-Aldrich) for 5 min per spleen. BALB/c rag−/− mice bearing established cardiac allografts were immunized with 2 × 106 viable B6 rag−/− splenocytes injected i.p. on day 3 post-CD4+ T cell transfer.
MLR of chimeric stimulators with reconstituting CD4+ T cell populations
MLR of adoptively transferred CD4+ T cells (and control LN cells) with control and chimeric stimulator splenocytes were performed to demonstrate cell viability and function of both the adoptively transferred cells and the chimeric stimulators (after BMT). Briefly, quadruplicate wells containing 2.0 × 105 responder cells were mixed with 3.0 × 105 irradiated (3000 rad) splenic stimulator cells in 96-well flat-bottom plates. Cells cultured in Eagle’s MEM supplemented with 10% FCS, 10−5 M 2-ME, and antibiotics were incubated at 37°C in 10% CO2. Cultures were then pulsed with 1.0 µCi [3H]thymidine for 6 h on the indicated day of cell culture. Plates were harvested and counted on a Trilux 1450 micro-beta scintillation counter (Wallac, Gaithersburg, MD).
Tissue histological examination
Transplanted and native hearts were removed and divided in half in the long axis perpendicular to the intraventricular septum. Halves were then placed in 4% paraformaldehyde for 4–6 h at 4°C. Cardiac tissue sections were then placed in 30% sucrose for 2 h at 4°C, then 1/1 30% sucrose/OCT for 2 h at 4°C, and finally OCT for 1 h at 4°C. Cardiac halves were then snap frozen in OCT on dry ice and placed in −80°C for storage. Sections were cut and stained with H&E. These were examined in a blinded fashion to determine the extent of myocardial damage, mononuclear and granulocyte cell infiltration, and vasculitis. Parallel sections were analyzed by immunohistochemistry on frozen tissue by air drying overnight and fixing with acetone. Sections were rehydrated in TBS, washed, and then blocked with 1/5 normal rabbit serum in TBS containing Vector avidin DH (Vector Laboratories, Burlingame, CA). KT6 supernatant (rat anti-mouse CD4) or YTS105 supernatant (rat anti-mouse CD8) was applied and incubated for 45 min at room temperature. Biotinylated rabbit anti-rat Ig (1/200) was applied and incubated for 30 min. Vectastain Elite ABC Reagent (Vector Laboratories) was applied and then counterstained with Mayer’s hematoxylin. Tissue sections were examined for immunoperoxidase staining by light microscopy for the presence of CD4+ and/or CD8+ T cells.
Results
Generation and characterization of chimeric heart donors with MHC class II restricted to hemopoietic or somatic compartments
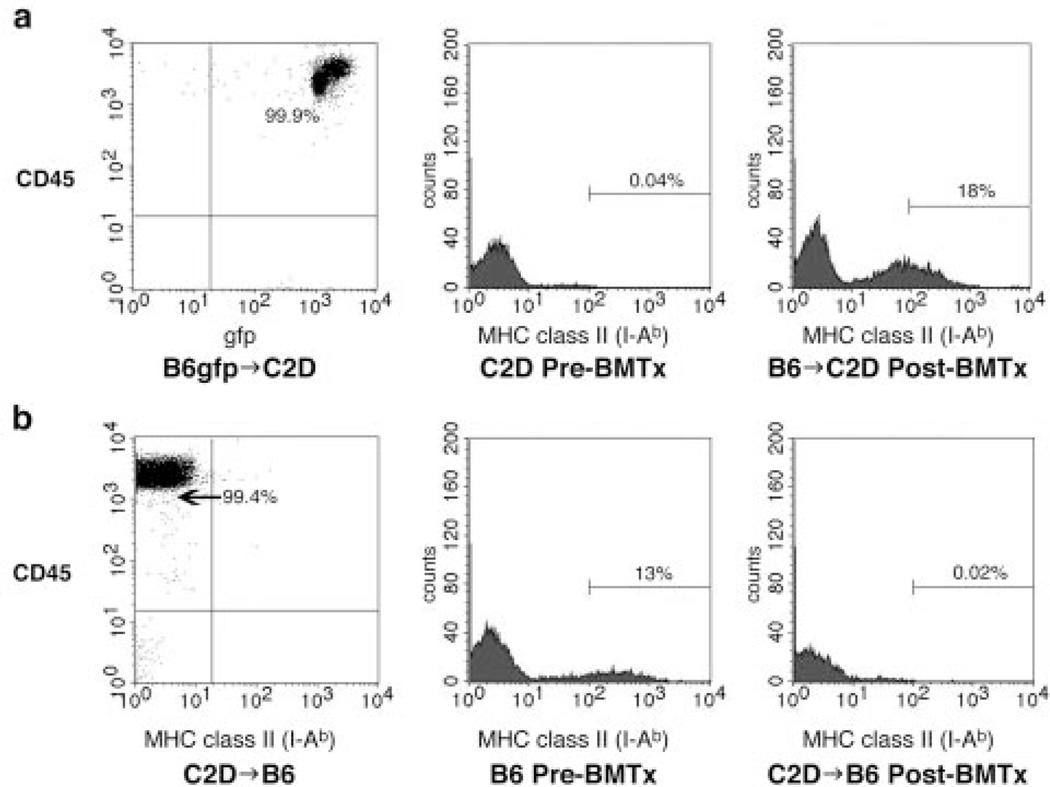
To effectively isolate MHC class II expression in donor hearts to either the bone marrow-derived or somatic compartments respectively, complete chimerism of bone marrow-engrafted mice must be achieved before heart transplantation. To assess the degree of hemopoietic chimerism, PB was analyzed for the presence of CD45+ (leukocyte common Ag) GFP+ (bone marrow donor origin) cells. Flow cytometric analysis of C2D mice (MHC class II−) after myeloid ablation (1200 rad) and reconstitution with wild-type C57BL/6 (B6) bone marrow tagged with the ubiquitous GFP marker demonstrated complete donor peripheral chimerism (>99% of CD45+ cells being GFP+; Fig. 1a). Results were similar when B6gfp → B6 control chimeras were analyzed by flow cytometry (data not shown). Additionally, PB flow cytometric analysis of C2D mice demonstrated undetectable MHC class II+ cells preirradiation and positive staining for MHC class II 8 wk after bone marrow rescue with wild-type B6 bone marrow (Fig. 1a). Conversely, PB flow cytometric analysis of B6 mice after lethal conditioning and rescue with C2D bone marrow demonstrated complete donor chimerism (>99% of CD45+ cells being devoid of MHC class II). C57BL/6 mice demonstrated PB MHC class II positivity by flow cytometric analysis preirradiation and complete loss of MHC class II expression after engraftment (8 wk after BMT) with C2D bone marrow (Fig. 1b).
FIGURE 1.
Bone marrow-engrafted mice demonstrate complete peripheral donor chimerism. a, C2D mice demonstrate complete chimerism after lethal ablation (1200 rad) and rescue with 5 × 106 GFP+ C57BL/6 bone marrow cells with 99.9% of PB CD45+ lymphocytes also staining positive for GFP by flow cytometric analysis (first histogram). This is further demonstrated by PB flow cytometry of C2D mice preradioablation showing a complete lack of MHC class II (second histogram) and significant reconstitution of MHC class II 8 wk postengraftment with wild-type C57BL/6 bone marrow (third histogram). b, MHC class II-positive wild-type C57BL/6 mice demonstrate complete chimerism after ablation and engraftment with 5 × 106 C2D bone marrow cells with 99.4% of PB CD45+ lymphocytes being MHC class II-negative by flow cytometric analysis (first histogram). Wild-type C57BL/6 mice preradioablation are positive by flow cytometric analysis for MHC class II (second histogram) and demonstrate a complete loss of MHC class II postengraftment with C2D bone marrow (third histogram).
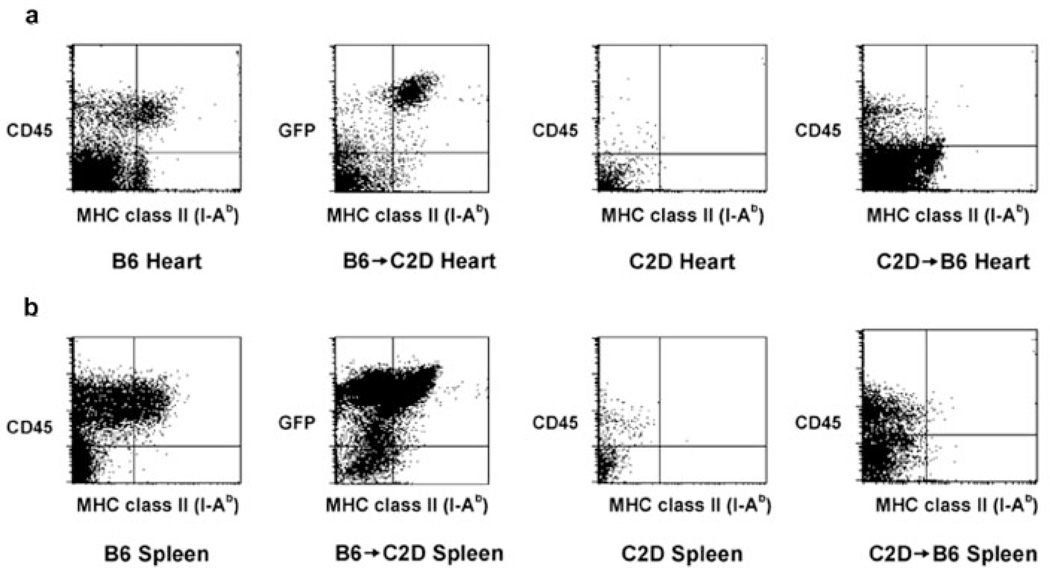
Because CD4+ T cell-mediated acute cardiac rejection is primarily dependent on the direct pathway of Ag presentation (7), complete donor chimerism must also be demonstrated within the allograft itself before transplantation. To further analyze the degree of chimerism, chimeric hearts (and spleens for comparison; Fig. 2b) were digested with collagenase 8–10 wk after BMT with consequent flow cytometric analysis of the lymphoid compartment (after separation using a Ficoll gradient). C57BL/6 control hearts (n = 3) demonstrated CD45+ MHC class II+ (I-Ab) cells, whereas C2D control hearts (n = 3) demonstrated a complete lack of MHC class II+ cells (Fig. 2a). B6 → C2D chimeric hearts (n = 3) demonstrated significant numbers of MHC class II+ cells. Virtually all of these MHC class II+ cells were of bone marrow donor origin (GFP+), thus demonstrating complete donor chimerism in the heart at the time these hearts would be transplanted into BALB/c rag−/− recipients. Conversely, C2D → B6 chimeric hearts demonstrated a paucity of CD45+ MHC class II+ cells, with <1% of CD45+ cells being MHC class II+ (Fig. 2a). Additional markers of myeloid lineage cells were used to determine the phenotype of donor hemopoietic cells. In chimeric B6 → C2D hearts, additional staining for CD11b+ cells, (pan-macrophage marker) and CD11c+ cells, (dendritic cell marker) demonstrated that virtually all such cells were of bone marrow donor origin (GFP+) (data not shown). Essentially, complete replacement of hemopoietic-derived cells by bone marrow donor cells was achieved. Taken together, these results indicate that both the periphery and the donor heart demonstrated virtually complete donor chimerism. Importantly, a scarcity of residual host CD45+ MHC class II+ cells was found within the heart of C2D → B6 chimeras. Thus, these results demonstrate that radioresistant bone marrow-derived cells did not persist in the donor hearts at the time of transplantation.
FIGURE 2.
Evidence for complete organ-specific chimerism in B6 → C2D chimeric hearts and spleens. a, Control C57 BL/6, B6 → C2D, and C2D → B6 chimeric hearts were digested into single-cell suspensions (n = 3 hearts digested/group). C57BL/6 control hearts demonstrated significant numbers of CD45+ MHC class II+(I-Ab) cells, whereas C2D control hearts demonstrated undetectable MHC class II+ cells. B6 → C2D chimeric hearts demonstrated significant numbers of MHC class II+ cells with virtually all of these being GFP+ (of bone marrow donor origin). Additionally, C2D → B6 chimeric hearts demonstrated <1% CD45+ MHC class II+ cells, with the vast majority of MHC class II+ cells being CD45− (presumably graft somatic cells). b, Control B6, B6 → C2D, and C2D → B6 chimeric spleens were also digested with collagenase A and DNase to control for the heart digest procedure (n = 3 spleens digested/group). B6 control spleens demonstrated nearly 100% of MHC class II+ cells being CD45+. C2D control spleens demonstrated undetectable MHC class II+ cells. B6 → C2D chimeric spleens demonstrated >99% of MHC class II+ cells being GFP+ (of donor origin) and C2D → B6 chimeric spleens demonstrated a near complete lack of MHC class II+ cells (0.02% of the total number of cells), with <0.03% of CD45+ cells being MHC class II+.
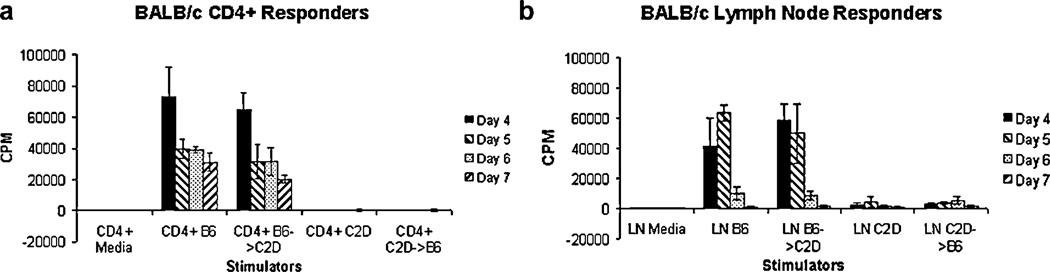
Phenotypic analysis (described above) indicated complete hemopoietic chimerism. We further demonstrated the functional impact of chimerism by assessing the capacity of CD4+ T cells to directly respond to splenocytes from different chimeric donors. MLRs with B6 → C2D (MHC class II expression isolated to the hemopoietic compartment) stimulator cells resulted in CD4+ T cell proliferation comparable to that of B6 control stimulator cells (Fig. 3a). Conversely, MLR with C2D → B6 (MHC class II expression isolated to the somatic compartment) chimeric stimulator cells did not lead to appreciable CD4+ T cell proliferation, similar to that seen with MHC class II-deficient C2D control stimulator cells (Fig. 3a). As a final control, MLR responses were obtained using nonpurified BALB/c LN cells (as a source of CD4+ and CD8+ T cells). The response of LN cells to control B6 and B6 → C2D stimulator cells was robust and similar, whereas the response of C2D control and C2D → B6 chimeric stimulators reflected typical BALB/c CD8+ T cell responses to B6 controls (reflecting a lack of MHC class II expression in both the C2D control and C2D → B6 chimeric splenocytes; Fig. 3b).
FIGURE 3.
MLR of CD4+ T-enriched lymphocytes. a, Proliferative response of BALB/c CD4+ T cells to B6 control and B6 → C2D chimeric stimulators and lack of proliferation to C2D control and C2D → B6 chimeric stimulators (splenocytes). b, Proliferative response of BALB/c unseparated LN cells to B6, B6 → C2D, C2D, and C2D → B6 stimulator splenocytes. Note the typical proliferative response to C2D control and C2D → B6 chimeric splenocytes, reflecting proliferation of CD8+ responders. Data are representative of three experiments testing CD4 T cell-dependent reactivity to donor splenocytes derived from chimeric animals.
MHC class II expression by hemopoietic and somatic cells are individually rate limiting for CD4+ T cell-mediated acute cardiac allograft rejection
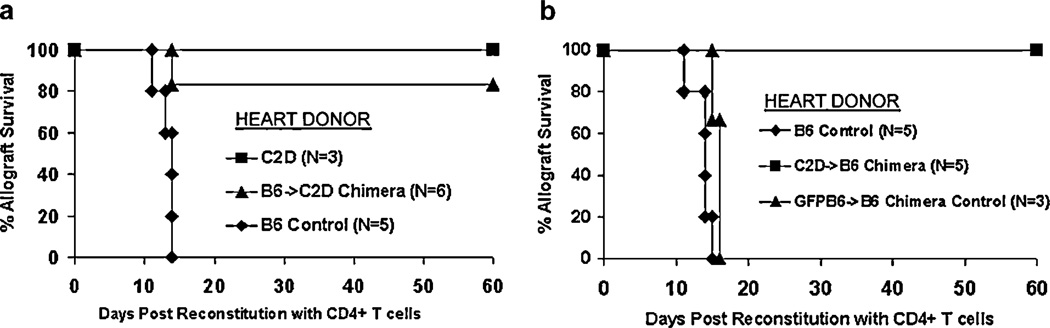
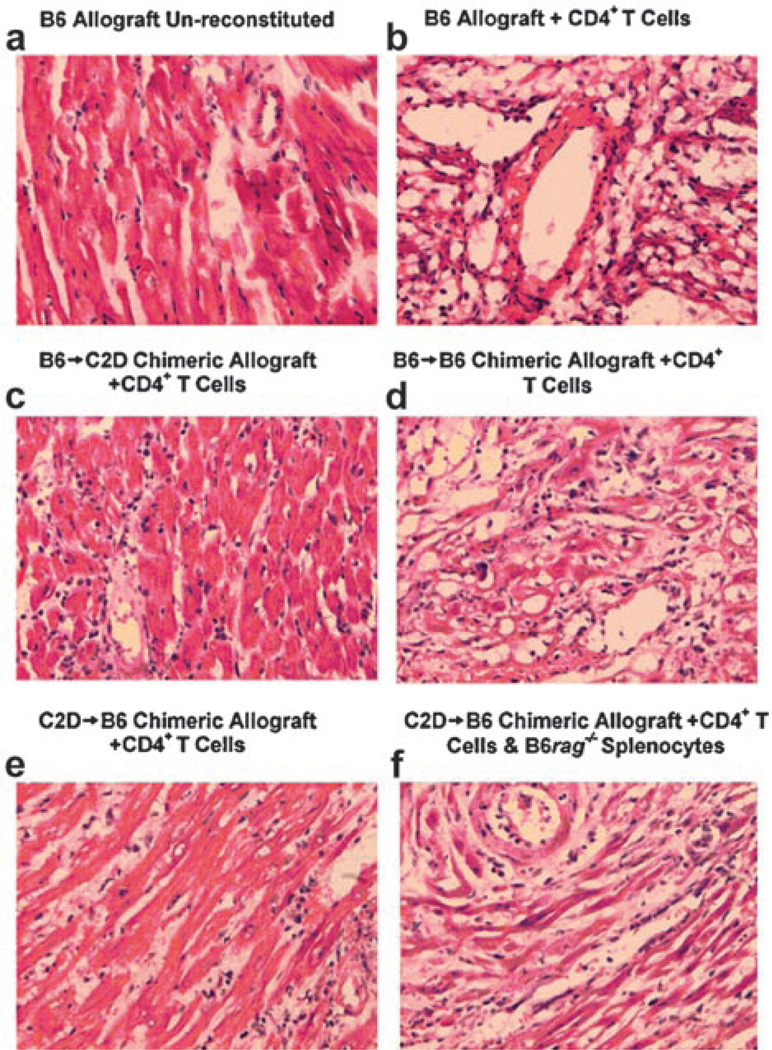
Having demonstrated the degree of chimerism in donor hearts, we then determined whether B6 → C2D or C2D → B6 chimeric heart allografts could be acutely rejected by alloreactive CD4+ T cells. To determine this, chimeric or control hearts were established in immune-deficient BALB/c rag−/− recipients that were then reconstituted with enriched CD4+ T cells and monitored for graft survival. Of note, all groups demonstrated survival >60 days without CD4+ T cell reconstitution (data not shown). Wild-type MHC class II-bearing control C57BL/6 heart allografts (n = 5) were all rejected by 15 days after CD4+ T cell transfer. Conversely, MHC class II-deficient (C2D) allografts (n = 3) were not rejected by CD4+ T cells within the 60-day observation period (p = 0.04 vs positive B6 controls), consistent with our previous findings (7). Such results illustrate the requirement of donor MHC class II expression in CD4-mediated cardiac rejection. Interestingly, CD4+ T cells fail to reject the majority of B6 → C2D chimeric hearts in which MHC class II expression was isolated to the hemopoietic compartment, with five of six allografts surviving >60 days (Fig. 4a, p = 0.02 vs positive B6 controls). Histological examination of these B6 → C2D allografts demonstrated significant numbers of graft-infiltrating CD4+ lymphocytes, without evidence of graft injury (Fig. 5c). This is in contrast to the B6 control allografts, which demonstrated significant mononuclear cell infiltration, cardiomyocyte damage, necrosis (Fig. 5b), and overt graft failure. Parallel immunostaining of such grafts revealed pronounced anti-CD4 staining and undetectable anti-CD8 staining (data not shown). Reciprocal experiments determined whether CD4+ T cells could reject C2D → B6 chimeric allografts (MHC class II isolated to the somatic compartment). Results show that five of five C2D → B6 chimeric allografts survived >60 days following CD4+ T cell transfer (Fig. 4b, p = 0.008 vs positive B6 controls). Such C2D → B6 allografts demonstrated moderate graft-infiltrating CD4+ lymphocytes (relative to the B6 → C2D allografts) and minimal cardiomyocyte/tissue cell damage (Fig. 5e). Thus, neither MHC class II expression on the hemopoietic compartment nor the somatic compartment was sufficient to elicit acute CD4 T cell-mediated rejection. Finally, as a control for the irradiation protocol itself, B6 → B6 control chimeric allografts were acutely rejected, with three of three allografts rejecting within 16 days after CD4+ T cell transfer (Fig. 4b), demonstrating that the chimeric allograft APCs are functional in this model in vivo. Histological assessment of these B6 → B6 control chimeric allografts (Fig. 5d) was comparable to that found in nonchimeric control B6 allografts (Fig. 5b).
FIGURE 4.
MHC class II expression is required on both graft bone marrow-derived and somatic cells for direct CD4+ T cell-mediated acute cardiac allograft rejection to occur. a, C57BL/6 allografts are rejected in BALB/c rag−/− recipients reconstituted with enriched CD4+ T cells (n = 5), whereas C2D allografts are not rejected (n = 3, p = 0.04 vs controls). B6 → C2D chimeric allografts (MHC class II isolated to the bone marrow-derived compartment) do not vigorously reject (five of six allografts functioning >60 days after CD4+ T cell reconstitution in BALB/c rag−/− recipients; p = 0.02 vs controls). b, C57BL/6 cardiac allografts are rejected in BALB/c rag−/− recipients reconstituted with enriched CD4+ T cells (n = 5). C57BL/6 → C57BL/6 (B6 → B6) chimeric control allografts are also rejected in BALB/c rag−/− recipients reconstituted with enriched CD4+ T cells (n = 3, p = NS vs controls). C2D → B6 chimeric allografts are not rejected in BALB/c rag−/− recipients with five of five allografts functioning beyond 60 days postreconstitution with column-enriched CD4+ T cells (p = 0.008 vs controls).
FIGURE 5.
Histological assessment of cardiac allografts. a, C57BL/6 donor allograft harvested from a BALB/c rag−/− recipient left unreconstituted at >60 days demonstrating normal cardiomyocytes and vasculature without evidence of rejection. b, C57BL/6 control donor allograft rejected in BALB/c rag−/− recipient reconstituted with enriched CD4+ T cells demonstrating a moderate to severe lymphocytic infiltration and cardiomyocyte necrosis. c, B6 → C2D chimeric donor allograft (MHC class II expression isolated to the bone marrow-derived compartment) harvested 60 days after CD4+ T cell transfer demonstrating mild lymphocytic infiltration, relatively intact cardiomyocytes, and no obvious evidence of necrosis. d, B6 → B6 control chimeric donor allograft demonstrating moderate to severe lymphocytic infiltration and cardiomyocyte necrosis similar to that of C57BL/6 control allografts. e, C2D → B6 (MHC class II expression isolated to the somatic compartment) harvested 60 days after CD4+ T cell transfer demonstrating mild lymphocytic infiltration and mild cardiomyocyte damage (some minimal early necrosis). f, C2D → B6 donor allograft rejected 18 days after CD4+ T cell transfer and 16 days after transfer of C57BL/6 rag−/− splenocytes (source of MHC class II+ APCs) demonstrating moderate to severe lymphocytic infiltration and cardiomyocyte necrosis. Immunoperoxidase staining for CD4 and CD8 demonstrated CD4+ staining and a lack of CD8+ staining in all allografts (data not shown).
MHC class II expression on graft somatic cells is essential for rejection after priming CD4 T cells in vivo
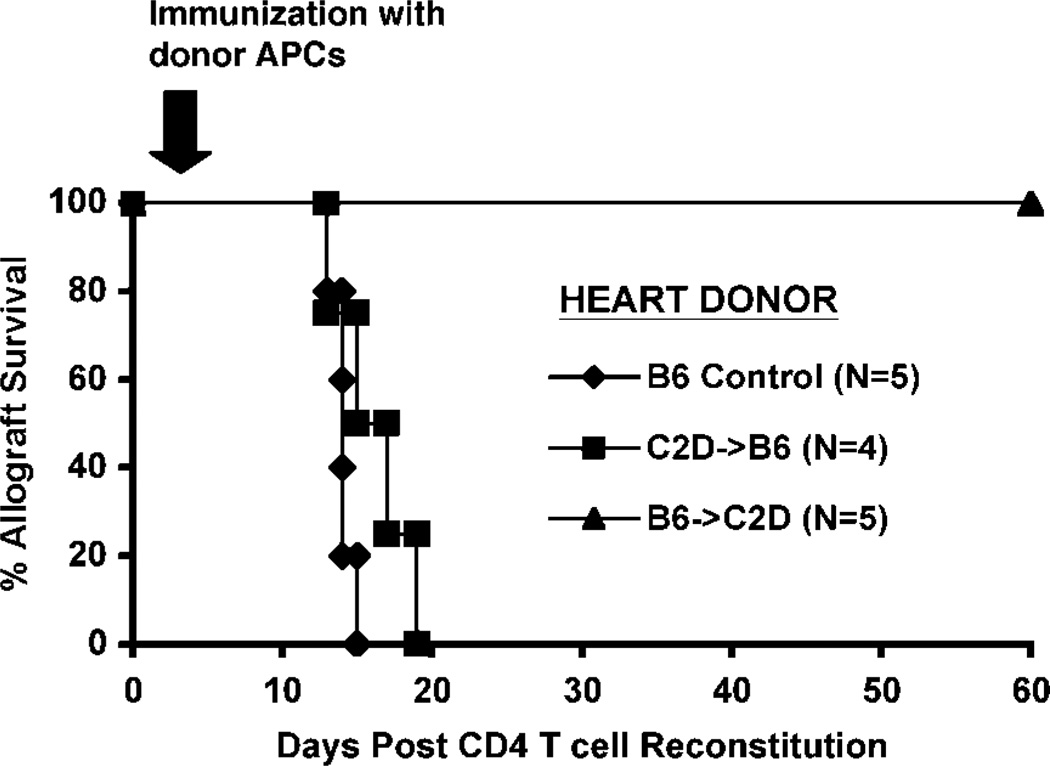
Since neither B6 → C2D nor C2D → B6 chimeric allografts were able to elicit acute CD4+ T cell-mediated rejection, it became apparent that MHC class II expression was required on both hemopoietic and somatic cells for graft rejection to occur. We hypothesized that the hemopoietic cells might in fact be functioning as primary APCs resulting in CD4+ T cell activation and proliferation, but that MHC class II-bearing somatic cells (such as ECs) are necessary as targets of the effector pathway. To test this hypothesis, we transplanted wild-type hearts, C2D → B6 or B6 → C2D chimeric heart allografts into BALB/c rag−/− recipients and adoptively transferred purified CD4+ T cells as in the previous experiments. In addition, this group was actively immunized with C57BL/6 rag−/− (B6 rag−/−) splenocytes as a source of donor hemopoietic-derived, MHC class II-bearing APCs. Importantly, active immunization with MHC class II-bearing APCs triggered acute rejection of CD2 → B6 cardiac allografts in recipients reconstituted with CD4+ T cells (Fig. 6). Histological examination of these allografts demonstrated CD4+ T cell infiltration, cardiomyocyte damage, and necrosis similar to that seen in both B6 and B6 → B6 control allografts (Fig. 5f). These results suggest that MHC class II expression by the somatic compartment of the heart allograft is sufficient to be rejected by CD4+ T cells provided that an appropriate APC source is provided. The fact that MHC class II expression on allograft somatic cells is required for rejection following host immunization is illustrated by the finding that B6 → C2D hearts, those expressing MHC class II on donor hemopoietic cells but not on somatic cells, failed to reject despite donor APC challenge (five of five grafts surviving for >60 days postimmunization). Thus, graft somatic cell MHC class II expression was required for this rejection induced by host immunization, while donor hemopoietic cell MHC class II expression was neither necessary nor sufficient.
FIGURE 6.
Allograft MHC class II+ somatic cells are targets for direct CD4+ T cell-mediated rejection. BALB/c rag-1−/− recipients were grafted with chimeric C2D → B6 (■), B6 → C2D (▲), or control B6 (◆) cardiac allografts. Allograft recipients also received the adoptive transfer of 10 × 106 CD4+ T cells on day 0 relative to transplant and a subsequent immunization with 2 × 106 C57BL/6 rag−/− splenocytes (a source of donor-type MHC class II+ APCs) on day 3. C2D → B6 chimeric donor allografts were rejected postchallenge with C57BL/6 class II+ APCs (n = 4, p = NS vs controls). B6 → C2D grafts survived >60 days after host challenge (zero of five rejected, p < 0.02 relative to both B6 and C2D → B6 grafted groups).
Discussion
CD4+ T cells are central to the triggering of acute cardiac allograft rejection. However, the role of the CD4+ T cell in this process has been somewhat ambiguous. CD4+ T cells have long been known as classical helper cells (20–23), facilitating the activation and function of other cells. However, it is clear that CD4+ T cells can also function as formidable effector cells (7, 24, 25) and can mediate graft rejection in the absence of other lymphocyte subpopulations (7). Enriched CD4+ effector T cells appear to require IFN-γ interaction with the target in that IFN-γ R-deficient cardiac allografts are not rejected by CD4+ T cells (26). Additionally, acute CD4-mediated cardiac allograft rejection is known to be dependent on the direct pathway of Ag presentation, as MHC class II-deficient heart grafts are not rejected by CD4+ T cells (7). Finally, CD4-mediated rejection does not require the indirect pathway in that MHC class II-deficient recipients reject MHC class II-positive hearts when reconstituted with CD4+ T cells (7). In summary, it is clear that CD4-mediated cardiac rejection requires donor MHC class II expression, but does not require host MHC class II expression. This being the case, a major secondary issue is what compartment of the heart is actually responsible for donor MHC class II recognition.
It has been controversial for many years whether or not the hemopoietic compartment and/or the somatic compartment (including ECs and/or cardiomyocytes) are necessary or sufficient to stimulate primary acute cardiac rejection. The purpose of this study was therefore to determine whether or not graft hemopoietic cells, somatic cells, or both are necessary for CD4-mediated acute cardiac rejection. To determine this, BMT was used to generate chimeric mice in which MHC class II was expressed only on somatic or hemopoietic cells. Chimeric donor hearts were transplanted into immune-deficient recipients that were then reconstituted with adoptively transferred CD4+ T cells. Surprisingly, results indicated that MHC class II expression by each compartment was individually rate limiting for triggering acute CD4-mediated rejection. This finding suggested that a two-step process was involved in acute CD4+ T cell-mediated rejection. Consistent with this notion was the finding that immunizing recipients with donor-specific MHC class II+ APC populations triggered acute rejection of established allografts with somatic cell-restricted MHC class II expression. From this data, a straightforward model emerges for CD4+ T cell-mediated rejection: 1) allograft-derived MHC class II+ hemopoietic cells function as essential primary APCs, leading to activation and proliferation of CD4+ effector T cells and 2) MHC class II+ graft somatic cells serve as obligate direct targets for activated CD4+ T cells, leading to ultimate destruction of the allograft. Therefore, once appropriate CD4 T cell activation occurs, the somatic (parenchymal) cells are sufficient to serve as targets of acute CD4+ T cell-mediated rejection. An important caveat to this model is the implication that these “steps” are discrete, compartmentalized stages of CD4 T cell activation and ensuing graft rejection. That is, as presented above, this model implies a temporal progression beginning with CD4 T cell activation by hemopoietic APCs followed by recognition of MHC class II expressing somatic cells for rejection. Although hemopoietic APCs are well appreciated for their role in initiating T cell activation, the present study does not exclude a potential role for the recognition of somatic cell-derived MHC class II during the initial response to the cardiac allograft. However, once CD4 T cell activation occurs in this model, MHC class II expression by somatic cells is necessary for rejection, while MHC class II expression on graft-derived hemopoietic cells is neither necessary nor sufficient for this response (Fig. 6).
An important implication of this study is that the somatic cells, including the vascular endothelium, are not sufficient to trigger primary CD4-mediated acute rejection. There has been tremendous controversy over the role of the EC as an APC for many years, based largely on in vitro studies. Recent in vivo data have suggested that ECs might function as primary APCs in a CD8-isolated system (13). Our data, however, demonstrate that for CD4-mediated acute rejection, the vascular endothelium is not sufficient to trigger acute rejection, but can potentially serve as a target for effector CD4+ T cells. Of course, a significant question left unanswered is which graft somatic cellular population(s) is(are) targeted by CD4+ effector T cells. Previous in situ studies have demonstrated a lack of MHC class II expression on cardiomyocytes (15, 27) but varying degrees of MHC class II expression on microvascular endothelial and smooth muscle cells (14, 15). Given that endothelial MHC class II expression has been shown to be at least partially dependent on inflammatory mediators (including IFN-γ) (11) and is up-regulated on donor EC after cardiac transplantation (15), it is conceivable that IFN-γ may be necessary for the maintenance of MHC class II expression on donor EC for efficient effector CD4+ T cell targeting. The fact that CD4+ T cell-mediated cardiac rejection is IFN-γ dependent (26) would suggest that IFN-γ plays a major role in enhancing MHC class II expression on the vascular endothelium or other parenchymal/somatic cellular targets (28–30).
Another important ancillary issue raised by these results is the implication regarding the contribution from the “indirect” (host MHC-restricted) pathway in CD4+ T cell-mediated rejection. In the model system presented, the recipient BALB/c rag−/− mice were MHC class II+ and therefore possessed an intact indirect pathway. Since CD4-mediated acute cardiac rejection is dependent on the direct pathway and occurs independent of the indirect pathway (7), it is unlikely that the indirect pathway contributed significantly to acute rejection. Importantly, in these experiments whereby the recipient mice possessed an intact indirect pathway (MHC class II+), chimeric heart grafts with MHC class II expression isolated to the somatic compartment (intact targets) were not rejected by CD4+ T cells unless a source of donor APCs was provided. This demonstrates that the indirect pathway was not sufficient to drive a primary acute alloresponse sufficiently robust to elicit rejection. However, histology of both B6 → C2D and C2D → B6 chimeric allografts 60 days after CD4+ T cell transfer did demonstrate some mild tissue damage and moderate CD4+ T cell infiltration (despite allograft function). These histological changes may suggest Ag presentation in the absence of MHC class II expression on the target cells (in the case of B6 → C2D allografts) or some low-level somatic cell (e.g., EC) Ag presentation not sufficiently robust to cause a decrease in graft function (C2D → B6 allografts). As multiple studies implicate both the CD4+ T cell and the indirect pathway of Ag presentation in chronic cardiac allograft rejection (7, 31–33), it is probable that a CD4-dependent contribution from the indirect pathway of Ag presentation occurred in both subsets of chimeric allografts, leading to the relatively minor histological changes discussed above. Although beyond the scope of this study, future histological evaluation of chimeric allografts for evidence of chronic cardiac rejection (vasculopathy) are warranted.
In summary, these studies support a straightforward two-step model of CD4-mediated acute cardiac rejection in which the donor graft hemopoietic cells must directly stimulate host CD4 T cells (step 1) which in turn activate graft-destructive CD4 T cells that must directly engage MHC class II on the parenchymal compartment of the graft to mediate acute rejection (step 2). Clinical strategies to limit rejection should not only consider graft hemopoietic cells as primary APCs, but also the role of graft parenchymal cells (such as ECs) as required immune targets of the alloresponse.
Acknowledgments
We thank Susan Cushing, Philip Pratt, Leslie Bloomquist, and An Doan for technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants NRSA F32 HL074694 (to T.J.G.), RO1 HL67976 (to B.A.P.), RO1 DK 33470 (to R.G.G.), and DERC P30 DK57516.
Abbreviations used in this paper: EC, endothelial cell, C2D, MHC class II deficient, BMT, bone marrow transplantation, GFP, green fluorescent protein; LN, lymph node; PB, peripheral blood.
References
- 1.Shizuru JA, Seydel KB, Flavin TF, Wu AP, Kong CC, Hoyt EG, Fujimoto N, Billingham ME, Starnes VA, Fathman CG. Induction of donor-specific unresponsiveness to cardiac allografts in rats by pretransplant anti-CD4 monoclonal antibody therapy. Transplantation. 1990;50:366. doi: 10.1097/00007890-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Onodera K, Lehmann M, Akalin E, Volk HD, Sayegh MH, Kupiec-Weglinski JW. Induction of “infectious” tolerance to MHC-incompatible cardiac allografts in CD4 monoclonal antibody-treated sensitized rat recipients. J. Immunol. 1996;157:1944. [PubMed] [Google Scholar]
- 3.Pearson TC, Hamano K, Morris PJ, Wood KJ. Anti-CD4 monoclonal antibody-induced allograft survival is associated with a defect in interleukin-2-dependent T-cell activation. Transplant. Proc. 1993;25:786. [PubMed] [Google Scholar]
- 4.Pearson TC, Darby CR, Wood KJ. Successful secondary heterotopic cardiac transplantation in the mouse. Transplantation. 1992;53:701. [PubMed] [Google Scholar]
- 5.Orosz CG, Wakely E, Bergese SD, VanBuskirk AM, Ferguson RM, Mullet D, Apseloff G, Gerber N. Prevention of murine cardiac allograft rejection with gallium nitrate: comparison with anti-CD4 monoclonal antibody. Transplantation. 1996;61:783. doi: 10.1097/00007890-199603150-00019. [DOI] [PubMed] [Google Scholar]
- 6.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 1996;184:2013. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J. Clin. Invest. 2000;106:1003. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens: a novel pathway for initiation of rejection. J. Exp. Med. 1990;171:307. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brent L, Medawar PB. Cellular immunity and the homograft reaction. Br. Med. Bull. 1967;23:55. doi: 10.1093/oxfordjournals.bmb.a070517. [DOI] [PubMed] [Google Scholar]
- 10.Burger DR, Ford D, Vetto RM, Hamblin A, Goldstein A, Hubbard M, Dumonde DC. Endothelial cell presentation of antigen to human T cells. Hum. Immunol. 1981;3:209. doi: 10.1016/0198-8859(81)90019-7. [DOI] [PubMed] [Google Scholar]
- 11.Geppert TD, Lipsky PE. Antigen presentation by interferon-γ-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J. Immunol. 1985;135:3750. [PubMed] [Google Scholar]
- 12.Wagner CR, Vetto RM, Burger DR. The mechanism of antigen presentation by endothelial cells. Immunobiology. 1984;168:453. doi: 10.1016/S0171-2985(84)80130-8. [DOI] [PubMed] [Google Scholar]
- 13.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat. Med. 2002;8:233. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 14.Momburg F, Koch N, Moller P, Moldenhauer G, Butcher GW, Hammerling GJ. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-γ. J. Immunol. 1986;136:940. [PubMed] [Google Scholar]
- 15.Hasegawa S, Becker G, Nagano H, Libby P, Mitchell RN. Pattern of graft- and host-specific MHC class II expression in long-term murine cardiac allografts: origin of inflammatory and vascular wall cells. Am. J. Pathol. 1998;153:69. doi: 10.1016/S0002-9440(10)65547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 2001;214:110. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 17.Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402. [PubMed] [Google Scholar]
- 18.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice: the role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 1980;125:2665. [PubMed] [Google Scholar]
- 20.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc. Natl. Acad. Sci. USA. 1993;90:3373. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 24.Gill RG, Wolf L, Daniel D, Coulombe M. CD4+ T cells are both necessary and sufficient for islet xenograft rejection. Transplant. Proc. 1994;26:1203. [PubMed] [Google Scholar]
- 25.VanBuskirk AM, Wakely ME, Orosz CG. Acute rejection of cardiac allografts by noncytolytic CD4+ T cell populations. Transplantation. 1996;62:300. doi: 10.1097/00007890-199607270-00026. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman AC, Pietra BA, Kelly BP, Rayat GR, Rizeq M, Gill RG. Donor IFN-γ receptors are critical for acute CD4+ T cell-mediated cardiac allograft rejection. J. Immunol. 2001;167:5457. doi: 10.4049/jimmunol.167.9.5457. [DOI] [PubMed] [Google Scholar]
- 27.Mirzaie M, Meyer T, Berger D, Saalmuller A, Dalichau H. Expression of porcine major histocompatibility antigens in cardiac tissue. APMIS. 1998;106:935. doi: 10.1111/j.1699-0463.1998.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 28.Orosz CG. Endothelial activation and chronic allograft rejection. Clin. Transplant. 1994;8:299. [PubMed] [Google Scholar]
- 29.Takei Y, Sims TN, Urmson J, Halloran PF. Central role for interferon-γ receptor in the regulation of renal MHC expression. J. Am. Soc. Nephrol. 2000;11:250. doi: 10.1681/ASN.V112250. [DOI] [PubMed] [Google Scholar]
- 30.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-γ deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J. Clin. Invest. 1997;100:550. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mhoyan A, Wu GD, Kakoulidis TP, Que X, Yolcu ES, Cramer DV, Shirwan H. Predominant expression of the Th2 response in chronic cardiac allograft rejection. Transplant. Int. 2003;16:464. doi: 10.1007/s00147-003-0590-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamada A, Laufer TM, Gerth AJ, Chase CM, Colvin RB, Russell PS, Sayegh MH, Auchincloss H., Jr Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am. J. Transplant. 2003;3:23. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- 33.Szeto WY, Krasinskas AM, Kreisel D, Krupnick AS, Popma SH, Rosengard BR. Depletion of recipient CD4+ but not CD8+ T lymphocytes prevents the development of cardiac allograft vasculopathy. Transplantation. 2002;73:1116. doi: 10.1097/00007890-200204150-00019. [DOI] [PubMed] [Google Scholar]