Abstract
While safe and effective treatments for glaucoma exist, their effectiveness is compromised by poor compliance. Patients who have problems with their topical glaucoma medication are acknowledged to be at higher risk for poor compliance, frequent medication switching, and surgery. Patient satisfaction with therapy and its associated benefits have until recently taken second place to efficacy. The present study is a transverse cross-sectional epidemiological survey among glaucoma patients receiving therapy with prostaglandin analogs. The primary objective was to determine and characterize patient satisfaction with glaucoma therapy, and the secondary objective was to identify factors that may contribute to poor patient satisfaction. Ophthalmologists in the Netherlands included 199 patients and 164 were analyzed. Patients were predominantly elderly with early, primary, open angle glaucoma. Eighty-nine percent of them stated they were satisfied or very satisfied with their treatment. However, signs of ocular surface disorder on ophthalmological examination were evident in 44% of patients, corneal fluorescein staining was positive in 28% of patients, and 38% of patients were using tear substitutes. The prevalence of blepharitis/meibomian gland dysfunction and dry eye was more than twice as high after the commencement of therapy compared with before therapy. Univariate analysis revealed that patient dissatisfaction with their glaucoma therapy was statistically significantly (P<0.001) associated with the presence of ocular surface disease, hyperemia, ocular signs, symptoms upon and between instillation, and the use of tear substitutes. Apparently, patients in the present study are satisfied with their treatment; 89% expressed satisfaction compared with only 11% who professed dissatisfaction. The results suggest that even if local adverse events and ocular surface disease, in particular, contribute to glaucoma patient dissatisfaction, only a minority of patients expressed such dissatisfaction. At the time of the study, most (94%) of the patients included were receiving preserved preparations. Further studies should evaluate the influence of preservative on patient satisfaction.
Keywords: prostaglandin analogs, cross-sectional study, compliance, adherence, ocular surface disease, dry eye, meibomian gland dysfunction
Introduction
Chronic and potentially sight-threatening damage to the optic nerve can be prevented in glaucoma patients by the effective reduction in intraocular pressure. A number of studies have revealed the extent of poor compliance with glaucoma medication1–4 and the degree to which such poor compliance may contribute to progression of the illness,5 despite the availability of a range of topical medications that are effective and without significant systemic adverse effects.
As with other insidious but symptomless conditions such as hypertension and type 2 diabetes, patients need sufficient encouragement and information from their practitioner as well as effective, convenient, and acceptable medications. However, studies to date on interventions designed to improve compliance do not appear to be encouraging.6 Given the serious sequelae of glaucoma and the acknowledged imperfect compliance with therapy, providing patients with satisfactory medication would seem to be a worthwhile objective.
Patient satisfaction with their treatment is acknowledged to be an important factor in ensuring adherence with treatment regimes and cooperation with medical practitioners.7 Patient satisfaction, particularly from the tolerability standpoint, has not been a priority, although methodologies have been developed for assessing patient satisfaction,8,9 patient-reported outcomes from glaucoma treatment, and measurement of the degree of adherence, sometimes using electronic devices.10,11 A prospective cohort study among 2,541 subjects suggested that 80% of patients were satisfied or very satisfied with their treatment.12 Another prospective observational study identified a number of factors that were predictive of patient satisfaction with glaucoma treatment.13 Nevertheless, problems with glaucoma medication are common, and patients who report problems are less likely to be adherent to their medication regime.14–16
The most recent generations of glaucoma medications are generally without systemic adverse events, and local tolerability is the factor most likely to compromise compliance. Various manifestations of ocular surface disease and hyperemia are the most common adverse events of topical medication, the latter very often associated not only with the active ingredient in the eye drops but also with preservative frequently added to prevent bacterial contamination.17 The objective of the present study is to quantify the degree of patient satisfaction with glaucoma treatment and identify factors that may influence it with particular regard to ocular surface disease.
Methods
The study comprised a multi-center, international (Belgium, the Netherlands, UK), cross-sectional epidemiological survey in patients suffering from glaucoma treated with prostaglandins. The surveys in the Netherlands were conducted between January 2013 and December 2013, although the study continues in other countries. The preliminary results presented here are from the 21 sites across the Netherlands in which investigators recruited their next 10 consecutive glaucoma patients. Adult male or female outpatients suffering from glaucoma or ocular hypertension, currently being treated with prostaglandins (regardless of which specific prostaglandin was being used, the duration of treatment, or if they were concurrently receiving another glaucoma medication), were eligible for inclusion. There were no specific exclusion criteria.
Ethical approval
Because no treatment interventions were required, this observational survey lies beyond the scope of the International Conference of Harmonization directives. However, the study was undertaken according to the International Epidemiological Association Good Epidemiological Practice guidelines.18
The local ethical committees of the participating investigators approved the study.
Demographic information/medical history
Data were collected on sex, age, ophthalmological and other medical history, duration, type, and the stage (ocular hypertension, early glaucoma [<6 dB], moderate glaucoma [6–12 dB], severe glaucoma [>12 dB]) of glaucoma. Intraocular pressure was measured. Patient records were used in combination with direct questioning of patients to obtain answers to the questionnaire.
Primary parameter
The primary objective was to determine the degree of satisfaction among glaucoma patients currently being treated by prostaglandin analogs, in general ophthalmological practice. Patients were questioned by their ophthalmologist regarding their degree of satisfaction and tolerability with their current medication (very satisfied, satisfied, unsatisfied, very unsatisfied).
Secondary parameters
Visual analog scale
Patients evaluated the tolerability of their current glaucoma medication on a 0–100 visual analog scale (0= very poor tolerability, 100= very good tolerability).
Current treatment and treatment switches
The nature of the patient’s current treatment and its duration were collected. In addition, the treating ophthalmologist collected information on the patient’s previous treatments and the reasons for switching (insufficient efficacy, patient request, local tolerability, insufficient compliance, systemic tolerability, other). In addition, current and previous treatments were identified as preserved or preservative free.
Symptoms upon and between instillation
Patients were asked about local symptoms experienced upon instillation of their current topical glaucoma medication (no symptoms, pain or discomfort, blurred vision).
Patients were asked to identify any local symptoms between instillations of their glaucoma medication (no symptoms, watering, crusts on eyelashes, tingling, photophobia, dry eye sensation, itching, foreign body sensation, red eye, burning).
Ophthalmological examination and tear film break-up time
Investigators performed a slit-lamp ophthalmological examination and recorded the severity of ocular signs (lid redness, lid swelling, lid scale or crusts, conjunctival hyperemia, chemosis, positive corneal fluorescein staining, positive conjunctival fluorescein staining) on a 0–3 scale (0= absent, 1= mild, 2= moderate, 3= severe).
Tear film break-up time was measured by the ophthalmologist using their usual technique.
Ocular surface disease
The investigator recorded whether the patient had experienced ocular surface disease during their glaucoma treatment, and if so, what disease (blepharitis/meibomian gland dysfunction, dry eye, eczema, rosacea, allergic conjunctivitis, or other) and at what degree of severity (mild, moderate, severe). Information was obtained from patients records where possible or from direct questioning.
Use of topical ocular therapy
The investigator recorded whether the patient was currently using any other topical ocular treatment (tear substitutes, anti-allergic eye drops, other).
Statistical analysis
Quantitative variables will be described in terms of mean, standard deviation and median, and range where appropriate. Univariate analysis was used to identify relationships between patient satisfaction and other study parameters. The χ2 test, Fischer’s exact test, Student’s t-test, and Wilcoxon signed-rank test were used for group comparisons as appropriate.
The present report describes the results from the Netherlands.
Results
Patient disposition
A total of 199 patients were recruited of whom 35 were excluded because they were not currently receiving treatment with a prostaglandin. The analysis set included all those patients entered after January 2, 2013 who provided primary endpoint data and who took a prostaglandin analog.
Demographics
Patients were predominantly elderly with 76.9% being 60 years of age or older and only 0.6% being younger than 40 years of age. They were approximately evenly distributed between sexes. Primary glaucoma was by far the most frequent diagnosis (greater than 90%). The great majority of patients with primary glaucoma had the open angle type (greater than 95%), while those with secondary glaucoma were distributed approximately equally between pigmentary, exfoliative, and other types. The mean time since diagnosis was 8.6 years with a large range from recently diagnosed patients to those having been treated for more than 40 years. The mean intraocular pressure was 17.6 and 17.2 in the right and left eyes, respectively, reflecting effective treatment of glaucoma, although some patients had intraocular pressures well outside the normal range. Most patients suffered from early glaucoma (<6 dB). More than half of the patients reported at least one medical history event, most commonly cataract surgery (39.6%); 9.1% had undergone surgery for glaucoma and 17.1% were diabetic (Table 1).
Table 1.
Demographics and baseline characteristics
| Parameter | Value | |
|---|---|---|
| N (entered/analyzed) | 164/133 | |
| Age (years) | ||
| 18–39 | 0.60% | |
| 40–49 | 10% | |
| 50–59 | 12.50% | |
| 60–69 | 27.50% | |
| 70–79 | 34.40% | |
| >80 | 15% | |
| Sex (M/F) | 46%/54% | |
| Time since diagnosis of glaucoma or ocular hypertension (years) (N=133) | ||
| Mean ± SD | 8.6±8.4 | |
| Median | 6 | |
| Range | 0–42 | |
| Intraocular pressure (mmHg) | Right eye | Left eye |
| N | 152 | 152 |
| Mean ± SD | 17.6±5.6 | 17.2±5.5 |
| Median | 16 | 16 |
| Range | 8–46 | 8–42 |
Note: N=164 for each parameter unless otherwise stated.
Current treatment
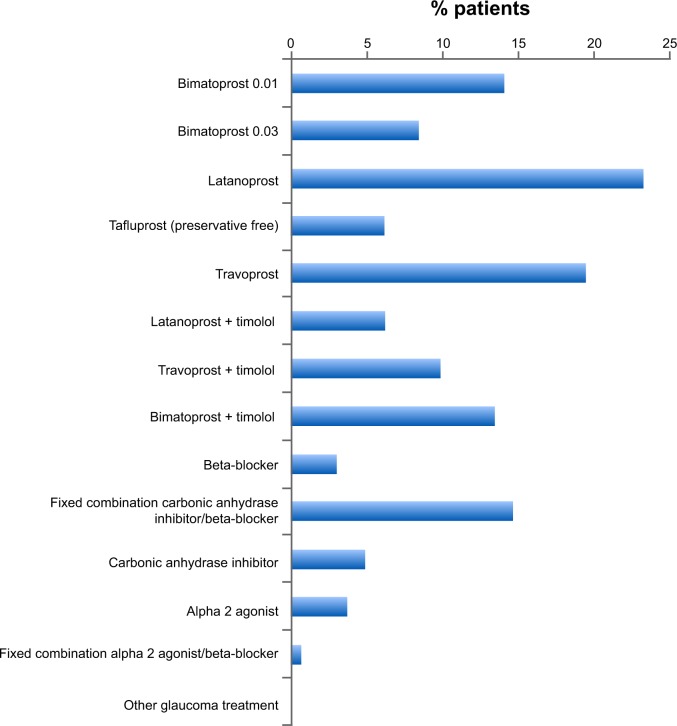
Patients’ ongoing treatment at the time of the study was with prostaglandin analogs as monotherapy or as a component of multi-therapy, with the largest proportion using latanoprost (Figure 1). The duration of the current treatment was 2.2±2.9 years, though some patients had been using their current therapy for up to 15 years. Monotherapy accounted for more than half of all ongoing treatment.
Figure 1.
Patients’ current glaucoma treatment (N=164).
Primary efficacy variable
Overall 89% of patients were either satisfied (67.7%) or very satisfied (21.3%) with their treatment. Only 11% were unsatisfied, and none were very unsatisfied.
Secondary efficacy variables
Visual analog scale
The mean score on the 0–100 visual analog scale that was used to assess tolerability was 76.4±17.5 mm.
Treatment switch
More than 70% of patients had switched medication at some point during their glaucoma therapy. Treatment switches occurred at a mean of 1.6 times per patient, though some reported much more frequent switches (up to 10 times). The reason for switching treatment was most commonly lack of efficacy (56%), although almost one-quarter of patients reported switching treatment due to poor local tolerance. Most patients had previously received therapy with prostaglandin analogs (mono- or bitherapy), most commonly latanoprost, although a substantial number received a beta-blocker (22%); no other treatment had been used by more than 10% of patients (Table 2).
Table 2.
Duration of previous treatment and number of treatment changes experienced by subjects
| Total (N=164) | |
|---|---|
| Duration of previous treatment (years) | |
| N | 137 |
| Mean (SD) | 8.6±8.0 |
| Median | 7 |
| Range | 0–42 |
| Number for treatment changes | |
| N | 159 |
| Mean (SD) | 1.6±1.7 |
| Median | 1 |
| Range | 0–10 |
| At least one reason for treatment change reported (N=121) | 73.80% |
| Insufficient efficacy | 55.50% |
| Local intolerance | 23.80% |
| Systemic intolerance | 3.00% |
| Patient’s request | 4.90% |
| Insufficient compliance | 3.70% |
| Other | 6.10% |
| Previous therapy | |
| Monotherapy | 66.90% |
| Bitherapy | 33.10% |
| Tritherapy | 0% |
| Quadtherapy | 0% |
| Ongoing therapy | |
| Monotherapy | 51.80% |
| Bitherapy | 28% |
| Tritherapy | 16.50% |
| Quadtherapy | 5.70% |
Note: N=164 for each parameter unless otherwise stated.
Symptoms upon and between instillation
More than one-quarter (27.4%) of patients suffered pain or discomfort upon instillation of their eye drops, and a further 5.5% had blurred vision. In all, 47.4% of patients reported a variety of symptoms, including tingling, watering, photophobia, and burning (Figure 2), but almost half had no symptoms between instillations.
Figure 2.
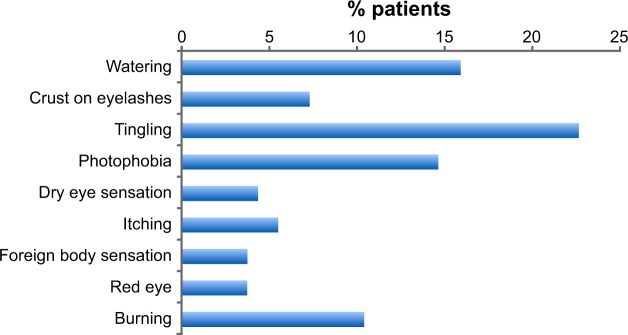
Proportion of patients experiencing symptoms between eye drop instillations (N=164).
Ophthalmological examination and tear film break-up time
Ophthalmological examination revealed a high frequency of ocular signs, including conjunctival hyperemia (47%), lid redness encrustation, and swelling and chemosis (Figure 3).
Figure 3.
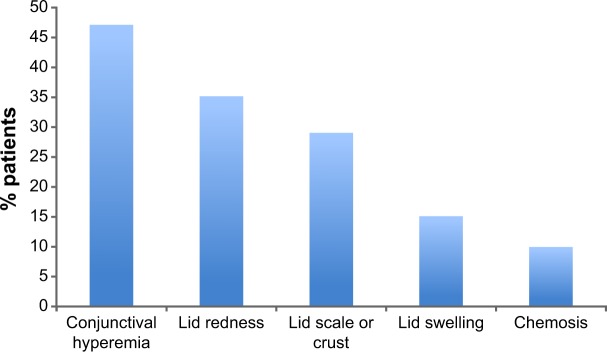
Proportion of patients exhibiting ocular signs at ophthalmological examination (N=164).
Tear break-up time was most frequently in the range of 5–10 seconds (60.7%), but was less than 5 seconds in more than 10% of patients. However, this parameter was measured only in a limited number (31%) of patients.
Fluorescein staining
More than one-quarter of eyes tested were positive for corneal fluorescein staining (28.3%). Positive tests for conjunctiva were less common (14.5%).
Ocular surface disease
Ocular surface disease was identified in 44% of patients; although it was mild in around two-thirds of patients (67.3%), moderate and even severe ocular surface disease was diagnosed in 28.8% and 3.8% of patients, respectively. The prevalence of ocular surface disease before the commencement of glaucoma treatment and after treatment is illustrated in Figure 4, which shows that the incidence of blepharitis/meibomian gland disorder and dry eye was uncommon before glaucoma treatment and more than doubled after the commencement of therapy.
Figure 4.
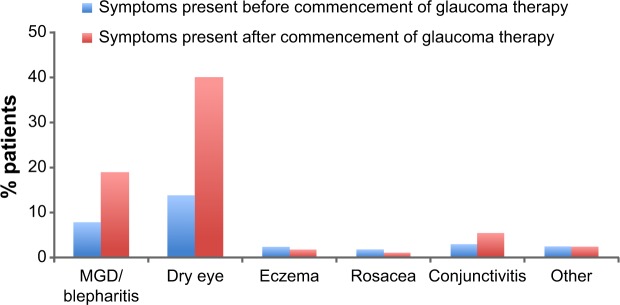
Proportion of patients reporting symptoms before and after the commencement of glaucoma therapy (N=164).
Abbreviation: MGD, meibomian gland dysfunction.
Use of topical ocular therapy
More than one-third (37.7%) of patients were using a mean of 3.3±1.1 drops of tear substitutes per day, and the majority of them were using preserved eye drops (55.8%). Use of anti-allergic eye drops and other ocular topical treatments was reported in only small numbers of patients (Table 3).
Table 3.
Use of additional topical ocular therapy
| Parameter | Value |
|---|---|
| Tear substitutes (N=154) | |
| Not used | 62.30% |
| Used | 37.70% |
| Preserved | 55.80% |
| Unpreserved | 44.20% |
| Drops/day (mean) | 3.3±1.1 |
| Anti-allergic eye drops (N=127) | |
| Not used | 95.30% |
| Used | 4.70% |
| Other topical treatments (N=126) | |
| Not used | 98.40% |
| Used | 1.60% |
Note: N=164; multiple responses are possible.
Ocular surface disease analyzed by patient subgroup
Univariate analysis of a number of parameters according to whether patients were satisfied with treatment or not identified several parameters associated with patients who were dissatisfied with their glaucoma treatment. The factors significantly associated with dissatisfaction with glaucoma treatment included presence of ocular surface disease, hyperemia, ocular signs, symptoms on or between instillations of glaucoma treatment, and use of tear substitutes. Factors not associated with patient dissatisfaction included age, sex, stage of glaucoma, ocular hypertension, duration of first treatment, duration of last treatment, number of previous treatments, and presence of positive corneal or conjunctival fluorescein staining (Table 4).
Table 4.
Parameters with significant association with patient satisfaction (univariate analysis)
| Parameter | Subgroup | Patient satisfied (N=146), n/N (%) | Patient unsatisfied (N=18) | P-value, χ2 test |
|---|---|---|---|---|
| Presence or not of ocular surface disease | No Yes |
87/89 (97.8%) 54/70 (77.1%) |
2/89 (2.2%) 16/70 (22.9%) |
<0.001 |
| Presence or not of hyperemia | No Yes |
82/84 (97.6%) 59/75 (78.7%) |
2/84 (2.4%) 16/75 (21.3%) |
<0.001 |
| Presence or not of ocular signs | No Yes |
55/55 (100.0%) 84/102 (82.4%) |
18/102 (17.6%) | <0.001 |
| Presence of symptoms upon glaucoma medication instillation | No Yes |
107/108 (99.1%) 33/50 (66.0%) |
1/108 (0.9%) 17/50 (34.0%) |
<0.001 |
| Presence of symptoms between glaucoma medication instillations | No Yes |
81/81 (100.0%) 55/73 (75.3%) |
18/73 (24.7%) | <0.001 |
| Use of tear substitutes | No Yes |
86/89 (96.6%) 46/59 (78.0%) |
3/89 (3.4%) 13/59 (22.0%) |
<0.001 |
Notes: n=patients satisfied or unsatisfied, N=number of patients analyzed.
Multivariate analysis showed that having an ocular surface disease increases the proportion of dissatisfaction (OR=6.47).
Discussion
An association between glaucoma and ocular surface disease is particularly unfortunate since both increase in prevalence with age. However, a number of studies have shown that ocular surface disease is more common in patients taking glaucoma medication than in the population in general.19–22 In particular, multivariate analysis in a recent study in 516 glaucoma patients showed that factors associated with the severity of ocular surface disease included age, number of daily eye drops, switches in treatment caused by local intolerance, and the severity of the underlying disease.23
Apparently, patients in the present study are satisfied with their treatment; 89% expressed satisfaction compared with only 11% who professed dissatisfaction. The results from the visual analog scale support this contention. On the other hand, more than 80% had switched therapy at some point, some up to 10 times, and while lack of efficacy was the most common reason, nearly one-quarter switched because of poor local tolerance.
The study presents significant evidence that ocular surface disorder may be widespread in this patient population; more than 25% of the group had pain or discomfort on instillation and around half also reported symptoms between instillations. Ocular signs on clinical examination were present in significant numbers of patients with 47% having hyperemia and more than one-quarter having positive corneal staining. Ocular surface disease was observed at clinical examination in 44% of patients. Perhaps most tellingly, the incidence of blepharitis/meibomian gland dysfunction and dry eye was twice as high after the commencement of glaucoma treatment as before. At the time of the study, the great majority (more than 90%) of topical glaucoma treatments contain preservatives suspected to be the cause of ocular surface disorder.17 More than one-third of the patients were using tear substitutes, presumably to treat symptoms of ocular surface disease (ironically, such tear substitutes themselves contain preservatives). Finally, although only a minority of patients expressed dissatisfaction with their treatment, univariate analysis showed that dissatisfaction was significantly associated with hyperemia and, in particular, ocular surface disease. The presence of ocular surface disease, ocular signs, and the use of tear substitutes were all associated with patient dissatisfaction.
The results of the present study are generally in accord with those of two other significant studies of patient satisfaction with glaucoma treatment. Kerr et al12 found a similar proportion of glaucoma patients were satisfied or very satisfied with their treatment (80%), but the factors that were most strongly associated with satisfaction were frequency of drop use, subjective convenience, and ease of administration – factors that were not assessed in the present study. Regression models in a study by Day et al13 indicated that the factors most associated with patient satisfaction were effectiveness, ocular irritation, conjunctival hyperemia, and ease/convenience of use.
The question may therefore be asked as to why patients profess satisfaction with their therapy in the face of a burden of local toxicity? Part of the answer may lie in the commendable educative efforts made by ophthalmologists in stressing the importance of reducing intraocular pressure to their patients. Perhaps also to the fortitude of patients who appear ready to tolerate a degree of discomfort in avoiding risks to their sight from glaucoma. Against this, however, has to be set the well-known poor compliance and persistence with glaucoma therapy. The population of patients considered in this study was currently in contact with their ophthalmologist and had recently consulted. They may not, therefore, be fully representative of a more general population of patients with raised intraocular pressure that includes recidivist non-compliers and patients who had stopped using their eye drops. In addition, the great majority (94%) of the treatments used by patients in the current study contained preservatives that, as well as being responsible for much of their toxicity, may cause a degree of local corneal anesthesia that masks symptoms of ocular surface disease.24,25 Questioning patients regarding adverse effects of glaucoma treatment may, therefore, be an unreliable means of establishing the presence of ocular surface disease associated with glaucoma treatment. Such occult ocular surface disease may have serious consequences if allowed to develop over the extended time scales required in glaucoma treatment.
Treatment switching is a common feature of long-term glaucoma therapy; in the present study, 23.8% of patients reported switching therapy. Of the 74% who reported at least one reason for switching, local intolerance was responsible in 32% of cases, suggesting that this is an important generator of treatment switches.
Ninety-four percent of glaucoma treatments used by patients in this study contained preservatives, and such preservatives have well-documented ocular toxicity.17 Now that preservative-free prostaglandin eye drops are available and have been shown to improve tolerability,26–28 it will be of interest to review patient satisfaction in future studies when such preservative-free preparations are more widely deployed.
More than one-third of patients used tear substitutes, presumably for symptoms of dry eye. However, more than half of these tear substitutes containing preservatives similar or identical to those in the patient’s glaucoma medication could have been responsible for the dry eye symptoms in the first place. Given the wide range of preservative-free glaucoma medications and tear substitutes available at the modest cost, this seems to be an unnecessary risk to the health of patients’ ocular surface.
The significant incidence of dry eye and meibomian gland dysfunction in older subjects suggests that consideration of the use of tear substitutes is also appropriate. Artificial tears, used by more than one-third of patients in the present study, frequently contain preservatives, and more than half of the patients using tear substitutes in this study were using preserved products, despite International Dry Eye WorkShop (DEWS) 2007 suggesting that preservative-free preparations were the “single most critical advance in the treatment of dry eye.”29
Although a number of guidelines for glaucoma make reference to the possible deleterious effects of preservatives in glaucoma medication, and suggest that preservative-free alternatives be considered, particularly where patients experience tolerability problems, none yet make specific recommendations that preservative-free preparations be used more generally.30–32 The European Medicines Agency advises that preserved eye drops should be avoided in patients undergoing long-term treatment, such as glaucoma.33
Overall, it seems remarkable that ocular surface disease is widespread among patients, the great majority of whom profess to be satisfied with their treatment. Ophthalmologists are clearly being effective in stressing the importance of the medication to their patients; perhaps, a degree of corneal anesthesia induced by preservatives also contributes to this. Despite the patients’ apparent satisfaction with their glaucoma treatment, closer inspection reveals significant levels of ocular surface disease and other tolerability issues. This suggests that merely enquiring about adverse effects offers the risk of missing a diagnosis of ocular surface disease with possible serious consequences.
Several lines of evidence now suggest that at least some of this poor tolerability is likely to be associated with the use of preserved medication that could be readily avoided by a switch to one of the preservative-free medications now available. Given the common acceptance that preservatives should be avoided, as far as possible, in food and cosmetics, persuading patients to use preservative-free eye drops would not appear to be overly challenging.
Acknowledgments
The GOAL (glaucoma patients treated with prostaglandins satisfaction evaluation) Study Netherlands investigators comprised the following:
NAM Bemelmans, St Elisabeth Ziekenhuis, Tilburg, the Netherlands; PSJR Crobach, Laurentius Ziekenhuis, Roermond, the Netherlands; LP Cruysbergh, Heerlen, the Netherlands; PS Edelbroek-Hoogendoorn, Ziekenhuis Rivierenland, Tiel, the Netherlands; F Hageman, OPSIS Oogziekenhuis Amstelveen, Amstelveen, the Netherlands; JGMM Hoevenaars, VieCuri – Medisch Centrum, Venlo, the Netherlands; CF Hommersom, Máxima Medisch Centrum, Veldhoven, the Netherlands; LS Koetsier, Leids Universitair Medisch Centrum, Leiden, the Netherlands; H Lemij, Rotterdam Eye Hospital, Rotterdam, the Netherlands; ILA Liempt, Amphia Ziekenhuis, Breda, the Netherlands; W Maat, Maasstad Ziekenhuis, Rotterdam, the Netherlands; RCM Maatman, Maasstad Ziekenhuis, Leiderdorp, the Netherlands; K van der Maesen, Oogziekenhuis Zonnestraal OMC Haarlem, Haarlem, the Netherlands; JAW Metzelaar-Blok, Albert Schweitzer ziekenhuis, Zwijndrecht, the Netherlands; LJ Noordzij, VieCuri – Medisch Centrum, Venlo, the Netherlands; H van Santbrink-Bakker, Rijnland Ziekenhuis, Leiderdorp, the Netherlands; TL van der Shaft, Maasziekenhuis Pantein, Beugen, the Netherlands; W Swart, Leids Universitair Medisch Centrum, Leiden, the Netherlands; S Tecim, Tergooi, Blaricum, the Netherlands; UF Tegelberg, Admiraal De Ruyter Ziekenhuis, Goes, the Netherlands; CJG Van Tilburg, Vlietland Ziekenhuis, Schiedam, the Netherlands; and C van der Windt, Ziekenhuis Rivierenland, Tiel, the Netherlands.
The study was funded by Laboratoires Théa. Dr JF Stolz assisted with the preparation of the manuscript. This assistance was funded by Laboratoires Théa.
Footnotes
Disclosure
Dr Baudoin reports consultancies and research grants with and from Alcon, Allergan, Santen and Thea. Dr Hoevenaars once received a research grant from Thea. The authors report no other conflicts of interest in this work.
References
- 1.Quigley HA. Improving eye drop treatment for glaucoma through better adherence. Optom Vis Sci. 2008;85(6):374–375. doi: 10.1097/OPX.0b013e318177ec17. [DOI] [PubMed] [Google Scholar]
- 2.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441–463. doi: 10.2147/PPA.S23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha A, Fernandes S, Bunce C, Franks WA. Avoidable sight loss from glaucoma: is it unavoidable? Br J Ophthalmol. 2012;96(6):816–820. doi: 10.1136/bjophthalmol-2012-301499. [DOI] [PubMed] [Google Scholar]
- 6.Waterman H, Evans JR, Gray TA, Henson D, Harper R. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev. 2013;4:CD006132. doi: 10.1002/14651858.CD006132.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware JE, Jr, Snyder MK, Wright WR, Davies AR. Defining and measuring patient satisfaction with medical care. Eval Program Plann. 1983;6(3–4):247–263. doi: 10.1016/0149-7189(83)90005-8. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann JP, Denis P, Vigneux M, Trudeau E, Guillemin I, Berdeaux G. Development of the conceptual framework for the eye-drop satisfaction questionnaire (EDSQ) in glaucoma using a qualitative study. BMC Health Serv Res. 2007;7:124. doi: 10.1186/1472-6963-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann JP, Baudouin C, Renard JP, et al. Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a survey. Clin Ophthalmol. 2010;4:731–739. doi: 10.2147/opth.s11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Cate H, Bhattacharya D, Clark A, Holland R, Broadway DC. Patterns of adherence behaviour for patients with glaucoma. Eye (Lond) 2013;27(4):545–553. doi: 10.1038/eye.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr NM, Patel HY, Chew SS, Ali NQ, Eady EK, Danesh-Meyer HV. Patient satisfaction with topical ocular hypotensives. Clin Experiment Ophthalmol. 2013;41(1):27–35. doi: 10.1111/j.1442-9071.2012.02823.x. [DOI] [PubMed] [Google Scholar]
- 13.Day DG, Sharpe ED, Atkinson MJ, Stewart JA, Stewart WC. The clinical validity of the treatment satisfaction survey for intraocular pressure in ocular hypertensive and glaucoma patients. Eye (Lond) 2006;20(5):583–590. doi: 10.1038/sj.eye.6701932. [DOI] [PubMed] [Google Scholar]
- 14.Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. doi: 10.1016/j.ophtha.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman TJ, Hahn SR, Gelb L, Tan H, Kim EE. The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: changes in prescription patterns and patient persistence. J Ocul Pharmacol Ther. 2009;25(2):145–152. doi: 10.1089/jop.2008.0072. [DOI] [PubMed] [Google Scholar]
- 16.Sleath BL, Blalock SJ, Muir KW, et al. Determinants of self-reported barriers to glaucoma medicine administration and adherence: a multisite study. Ann Pharmacother. 2014;48(7):856–862. doi: 10.1177/1060028014529413. [DOI] [PubMed] [Google Scholar]
- 17.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Good Epidemiological Practice (GEP) International Epidemiological Association. 2007. Available from: http://ieaweb.org/good-epidemiological-practice-gep/
- 19.Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–446. doi: 10.2147/OPTH.S29158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 21.Van Went C, Brasnu E, Hamard P, Baudouin C, Labbé A. The influence of ocular surface diseases in the management of glaucoma. J Fr Ophtalmol. 2011;34(4):230–237. doi: 10.1016/j.jfo.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 23.Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2013;23(1):47–54. doi: 10.5301/ejo.5000181. [DOI] [PubMed] [Google Scholar]
- 24.Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147(4):725–735.e1. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Van Went C, Alalwani H, Brasnu E, et al. Evaluation de la sensibilite corneenne chez les patients traites medicalement pour un glaucome ou une hypertonie oculaire [Corneal sensitivity in patients treated medically for glaucoma or ocular hypertension] J Fr Ophtalmol. 2011;34(10):684–690. doi: 10.1016/j.jfo.2011.07.011. French. [DOI] [PubMed] [Google Scholar]
- 26.Bron A, Chiambaretta F, Pouliquen P, Rigal D, Rouland JF. Interet de la substitution d’un traitement journalier de 2 instillations de timolol par 1 instillation quotidienne de betabloquant non conserve chez. [Efficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertension] J Fr Ophtalmol. 2003;26(7):668–674. French. [PubMed] [Google Scholar]
- 27.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 28.Beden C, Helleboid L, Marmouz F, Liard F. Etude comparative de la survenue d’effets indesirables suite a l’administration de collyres anti-allergiques sans ou avec conservateur. [A comparative study of the ocular tolerance after administration of anti-allergic eye drops with or without a preservative] Therapie. 2004;59(2):259–264. doi: 10.2515/therapie:2004050. French. [DOI] [PubMed] [Google Scholar]
- 29.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 30.European Glaucoma Society . Terminology and Guidelines for Glaucoma. 4th ed. Savona, Italy: Editrice Dogma Srl; 2014. [Google Scholar]
- 31.National Institute for Health and Care Excellence . CG85 Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. London, UK: National Collaborating Centre for Acute Care; 2009. [PubMed] [Google Scholar]
- 32.American Optometric Association Original Consensus Panel . Care of the Patient with Open Angle Glaucoma. St Louis, MI, USA: American Optometric Association; 2011. [Google Scholar]
- 33.European Medicines Agency . EMEA Public Statement on Antimicrobial Preservatives in Ophthalmic Preparations for Human Use (EMEA/622721/2009) London, UK: EMEA; 2009. [Google Scholar]