Abstract
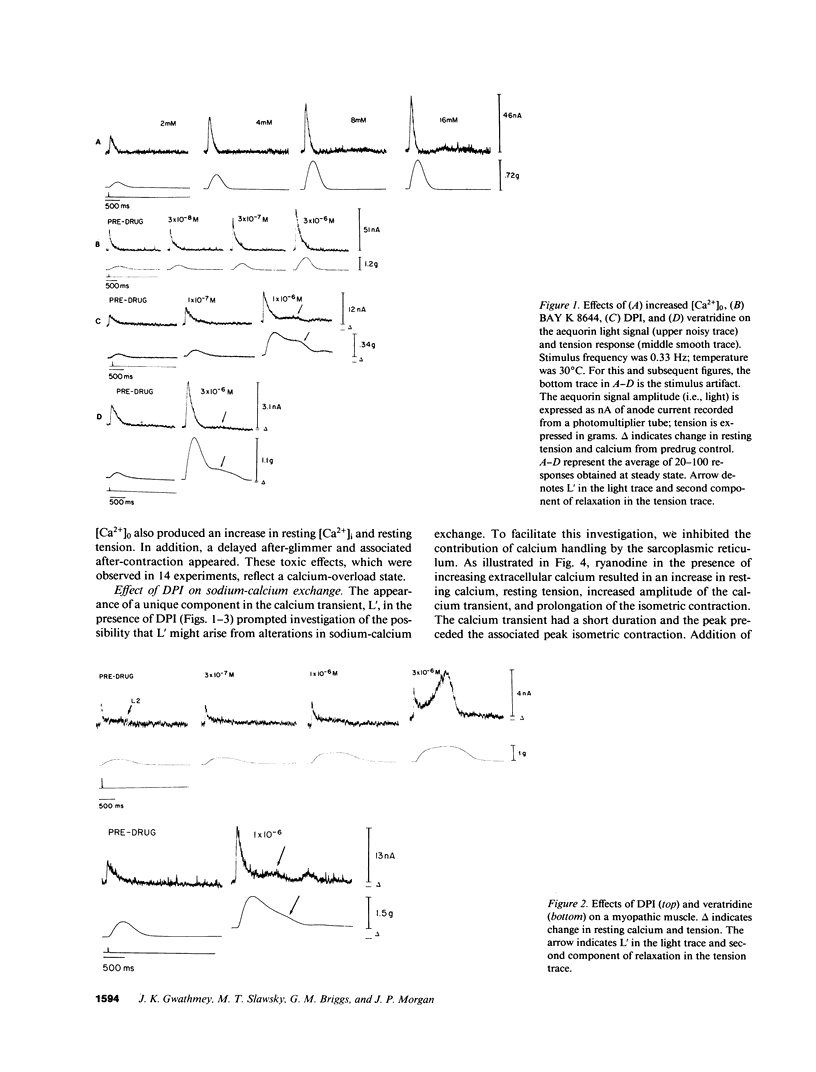
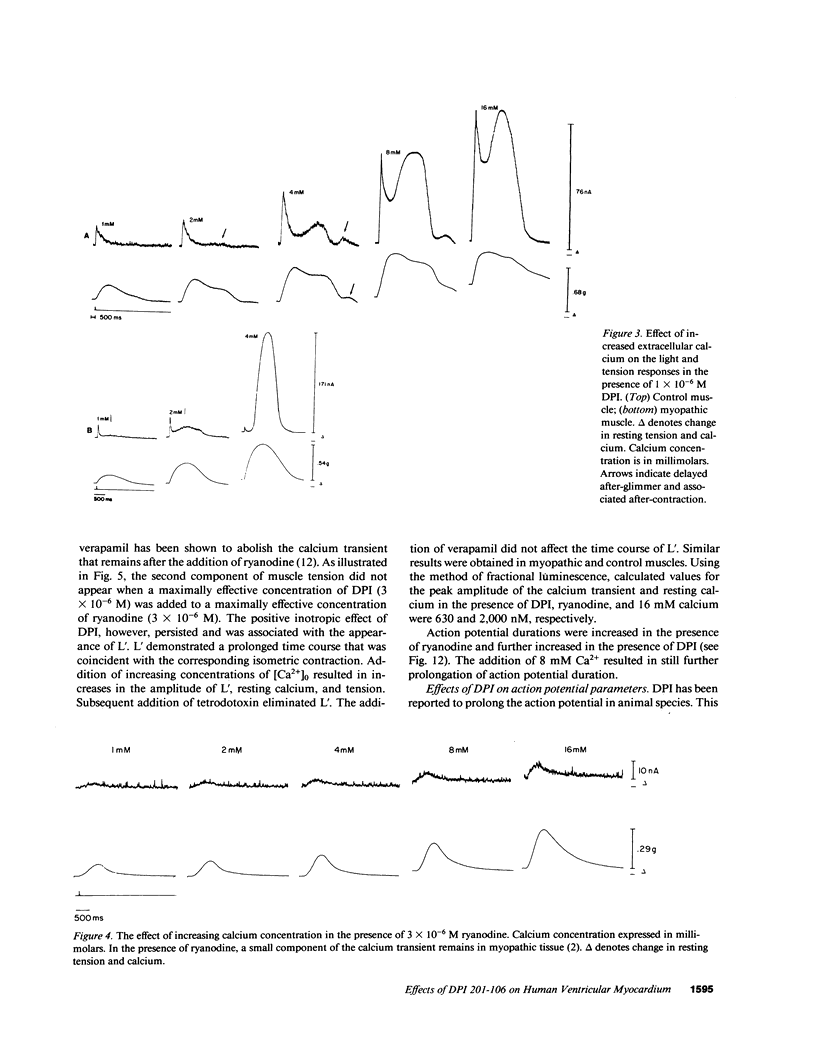
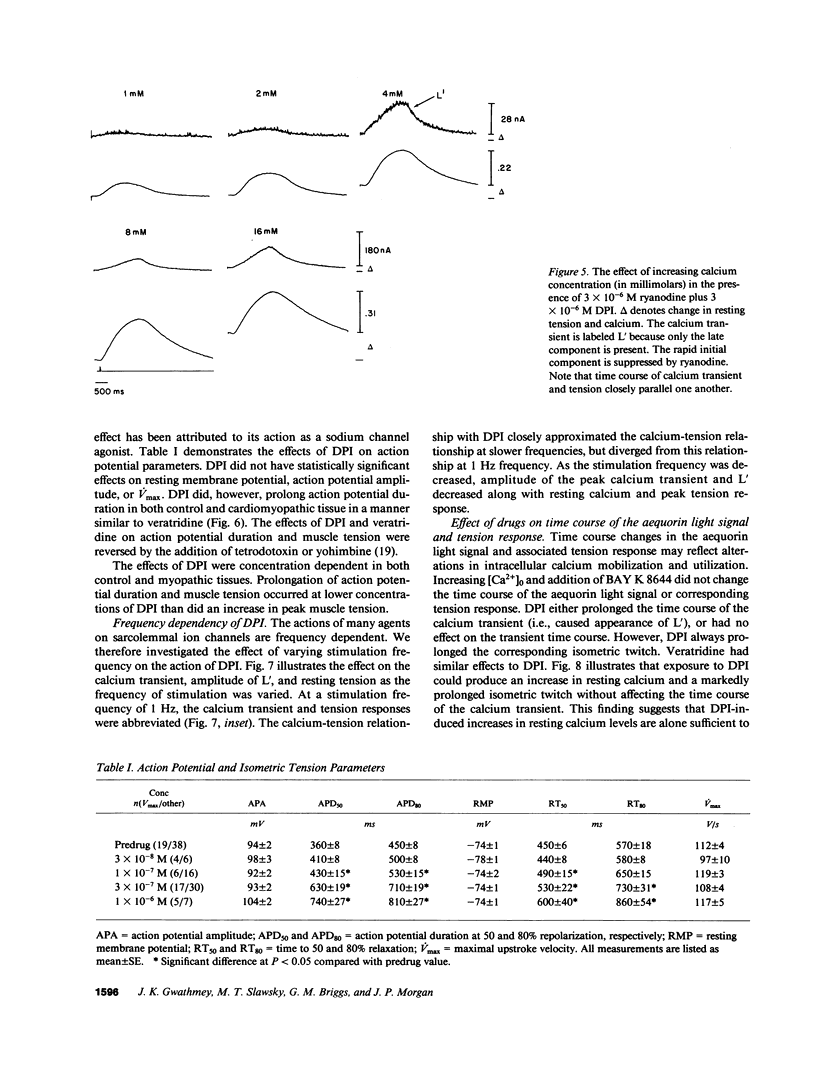
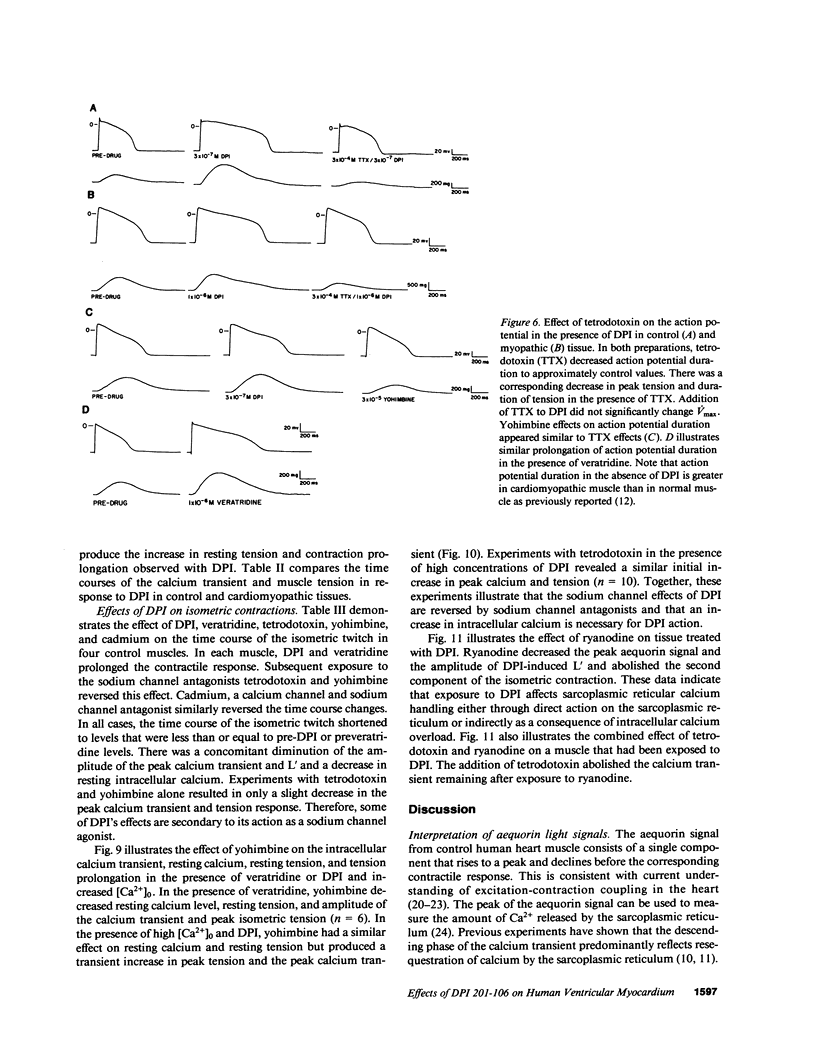
Experiments were performed to investigate the mechanism of action of DPI 201-106 on human heart muscle. In both control and myopathic muscles, DPI produced concentration-dependent increases in action potential duration, resting muscle tension, peak isometric tension, and duration of isometric tension. These changes were associated with increases in resting intracellular calcium and peak calcium transients as measured by aequorin. At higher concentrations of DPI, a second delayed Ca2+ transient (L') appeared. L' was inhibited by tetrodotoxin and ryanodine, suggesting that DPI acts at both the sarcolemma and the sarcoplasmic reticulum. DPI toxicity was manifested by after-glimmers and after-contractions reflecting a Ca2+-overload state: DPI effects were mimicked by veratridine, a Na+ channel agonist, and reversed by tetrodotoxin, yohimbine, and cadmium, Na+ channel antagonists. These results suggest that DPI acts primarily as a Na+ channel agonist. DPI may produce an increase in intracellular Ca2+ by increasing intracellular Na+ and altering Na+-Ca2+ exchange across the sarcolemma. DPI may also increase intracellular Ca2+ by directly altering sarcoplasmic reticulum Ca2+ handling.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984 Jul;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlock P., Katzung B. G. Effects of sodium substitutes on transient inward current and tension in guinea-pig and ferret papillary muscle. J Physiol. 1985 Mar;360:105–120. doi: 10.1113/jphysiol.1985.sp015606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Bayer R., Hennekes R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. I. Pattern of inotropic effects of the racemic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):49–68. doi: 10.1007/BF00499989. [DOI] [PubMed] [Google Scholar]
- Bers D. M. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–H381. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- Bers D. M. Mechanisms contributing to the cardiac inotropic effect of Na pump inhibition and reduction of extracellular Na. J Gen Physiol. 1987 Oct;90(4):479–504. doi: 10.1085/jgp.90.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Briggs G. M., Meier C. F., Jr Effect of yohimbine on action potentials recorded from isolated canine ventricular myocytes. Eur J Pharmacol. 1986 Aug 7;127(1-2):125–128. doi: 10.1016/0014-2999(86)90213-x. [DOI] [PubMed] [Google Scholar]
- Brill D. M., Wasserstrom J. A. Intracellular sodium and the positive inotropic effect of veratridine and cardiac glycoside in sheep Purkinje fibers. Circ Res. 1986 Jan;58(1):109–119. doi: 10.1161/01.res.58.1.109. [DOI] [PubMed] [Google Scholar]
- Brown H. F., Noble D., Noble S. J., Taupignon A. I. Relationship between the transient inward current and slow inward currents in the sino-atrial node of the rabbit. J Physiol. 1986 Jan;370:299–315. doi: 10.1113/jphysiol.1986.sp015936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggisch D., Isenberg G., Ravens U., Scholtysik G. The role of sodium channels in the effects of the cardiotonic compound DPI 201-106 on contractility and membrane potentials in isolated mammalian heart preparations. Eur J Pharmacol. 1985 Dec 3;118(3):303–311. doi: 10.1016/0014-2999(85)90141-4. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Na-Ca exchange: stoichiometry and electrogenicity. Am J Physiol. 1985 Mar;248(3 Pt 1):C189–C202. doi: 10.1152/ajpcell.1985.248.3.C189. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. D., Copelas L., Gwathmey J. K., Phillips P., Warren S. E., Schoen F. J., Grossman W., Morgan J. P. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987 Feb;75(2):331–339. doi: 10.1161/01.cir.75.2.331. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Vassort G. The electrogenic Na-Ca exchange and the cardiac electrical activity. I--Simulation on Purkinje fibre action potential. J Physiol (Paris) 1981 Sep;77(6-7):705–709. [PubMed] [Google Scholar]
- Fozzard H. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1977;39:201–220. doi: 10.1146/annurev.ph.39.030177.001221. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey J. K., Copelas L., MacKinnon R., Schoen F. J., Feldman M. D., Grossman W., Morgan J. P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Holck M., Osterrieder W. Interaction of the cardiotonic agent DPI 201-106 with cardiac Ca2+ channels. J Cardiovasc Pharmacol. 1988 Apr;11(4):478–482. doi: 10.1097/00005344-198804000-00015. [DOI] [PubMed] [Google Scholar]
- Honerjäger P. Cardioactive substances that prolong the open state of sodium channels. Rev Physiol Biochem Pharmacol. 1982;92:1–74. doi: 10.1007/BFb0030502. [DOI] [PubMed] [Google Scholar]
- Honerjäger P., Reiter M. The relation between the effects of veratridine on action potential and contraction in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):1–28. doi: 10.1007/BF00498026. [DOI] [PubMed] [Google Scholar]
- January C. T., Riddle J. M., Salata J. J. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ Res. 1988 Mar;62(3):563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- King B. W., Bose D. Mechanism of biphasic contractions in strontium-treated ventricular muscle. Circ Res. 1983 Jan;52(1):65–75. doi: 10.1161/01.res.52.1.65. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Fröbe U., Herzig J. W. Modification of single cardiac Na+ channels by DPI 201-106. J Membr Biol. 1986;89(2):163–172. doi: 10.1007/BF01869712. [DOI] [PubMed] [Google Scholar]
- Malecot C. O., Bers D. M., Katzung B. G. Biphasic contractions induced by milrinone at low temperature in ferret ventricular muscle: role of the sarcoplasmic reticulum and transmembrane calcium influx. Circ Res. 1986 Aug;59(2):151–162. doi: 10.1161/01.res.59.2.151. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Ryanodine prolongs Ca-currents while suppressing contraction in rat ventricular muscle cells. Br J Pharmacol. 1984 Jan;81(1):13–15. doi: 10.1111/j.1476-5381.1984.tb10735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Blinks J. R. Intracellular Ca2+ transients in the cat papillary muscle. Can J Physiol Pharmacol. 1982 Apr;60(4):524–528. doi: 10.1139/y82-072. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Chesebro J. H., Pluth J. R., Puga F. J., Schaff H. V. Intracellular calcium transients in human working myocardium as detected with aequorin. J Am Coll Cardiol. 1984 Feb;3(2 Pt 1):410–418. doi: 10.1016/s0735-1097(84)80028-5. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., DeFeo T. T., Morgan K. G. A chemical procedure for loading the calcium indicator acquorin into mammalian working myocardium. Pflugers Arch. 1984 Mar;400(3):338–340. doi: 10.1007/BF00581571. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Calcium and cardiovascular function. Intracellular calcium levels during contraction and relaxation of mammalian cardiac and vascular smooth muscle as detected with aequorin. Am J Med. 1984 Nov 5;77(5A):33–46. doi: 10.1016/s0002-9343(84)80006-6. [DOI] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Salzmann R., Scholtysik G., Clark B., Berthold R. Cardiovascular actions of DPI 201-106, a novel cardiotonic agent. J Cardiovasc Pharmacol. 1986 Sep-Oct;8(5):1035–1043. doi: 10.1097/00005344-198609000-00023. [DOI] [PubMed] [Google Scholar]
- Scholtysik G., Quast U., Schaad A. Evidence for different receptor sites for the novel cardiotonic S-DPI 201-106, ATX II and veratridine on the cardiac sodium channel. Eur J Pharmacol. 1986 Jun 5;125(1):111–118. doi: 10.1016/0014-2999(86)90089-0. [DOI] [PubMed] [Google Scholar]
- Scholtysik G., Salzmann R., Berthold R., Herzig J. W., Quast U., Markstein R. DPI 201-106, a novel cardioactive agent. Combination of cAMP-independent positive inotropic, negative chronotropic, action potential prolonging and coronary dilatory properties. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):316–325. doi: 10.1007/BF00501887. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Chung M., Cohen C. J. A dihydropyridine (Bay k 8644) that enhances calcium currents in guinea pig and calf myocardial cells. A new type of positive inotropic agent. Circ Res. 1985 Jan;56(1):87–96. doi: 10.1161/01.res.56.1.87. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Tajima T., Yuen J., Pappano A. J. Positive inotropic effects of acetylcholine and BAY K 8644 in embryonic chick ventricle. Am J Physiol. 1987 Apr;252(4 Pt 2):H807–H815. doi: 10.1152/ajpheart.1987.252.4.H807. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]



