Abstract
Bacterial efflux pumps are active transport proteins responsible for resistance to selected biocides and antibiotics. It has been shown that production of efflux pumps is up-regulated in a number of highly pathogenic bacteria, including methicillin resistant Staphylococcus aureus. Thus, the identification of new bacterial efflux pump inhibitors is a topic of great interest. Existing assays to evaluate efflux pump inhibitory activity rely on fluorescence by an efflux pump substrate. When employing these assays to evaluate efflux pump inhibitory activity of plant extracts and some purified compounds, we observed severe optical interference that gave rise to false negative results. To circumvent this problem, a new mass spectrometry-based method was developed for the quantitative measurement of bacterial efflux pump inhibition. The assay was employed to evaluate efflux pump inhibitory activity of a crude extract of the botanical Hydrastis Canadensis, and to compare the efflux pump inhibitory activity of several pure flavonoids. The flavonoid quercetin, which appeared to be completely inactive with a fluorescence-based method, showed an IC50 value of 75 μg/mL with the new method. The other flavonoids evaluated (apigenin, kaempferol, rhamnetin, luteolin, myricetin), were also active, with IC50 values ranging from 19 μg/mL to 75 μg/mL. The assay described herein could be useful in future screening efforts to identify efflux pump inhibitors, particularly in situations where optical interference precludes the application of methods that rely on fluorescence.
Introduction
Bacterial efflux pumps are active transport proteins that function to extrude toxic compounds, including antimicrobial drugs, from the cell. These pumps serve to protect bacteria from damage by toxins, and can play a role in the development of resistance to antimicrobials [1–5]. For example, it has been shown that production of efflux pumps is up-regulated in drug resistant strains of many bacteria, including methicillin resistant Staphylococcus aureus [6–10]. Compounds that inhibit bacterial efflux pumps are of interest because of their potential to increase antimicrobial effectiveness [11]. Thus, our laboratory has been engaged in experiments to find new efflux pump inhibitors (EPIs) from natural product sources.
Current methods for evaluating efflux pump inhibitory activity rely on an efflux pump substrate that fluoresces only when it is located inside a cell (due to intercalation with DNA) [12]. The majority of existing protocols operate by pre-loading cells with the efflux pump substrate ethidium bromide, which gives them a high initial fluorescent intensity. The extent of efflux pump inhibition is then measured by comparing the rate of decrease in fluorescence intensity over time in the presence of varying amounts of the putative EPI [4,9,13–18]. Related experiments utilizing measurements based on the intracellular accumulation of fluorescent substrates have also been reported [9,19]. For accumulation experiments, fluorescence increases over time as the substrate diffuses into cells.
Ethidium bromide is attractive as an indicator of efflux pump inhibition because of extensive literature precedent and also because it has been established to be active via intracellular action, with literature precedent stretching back to the 1950s [12,20,21]. However, the existing methods for testing efflux pump inhibition with ethidium bromide gave false results in our study due to matrix quenching effects (the suppression of fluorescence by various components of the mixture) in crude extracts and even with some pure compounds. We endeavored to circumvent these quenching effects by developing a new mass spectrometry-based efflux pump inhibition assay. There is extensive literature support for the efflux pump inhibitory activity of flavonoids and related compounds [9–11,16,22–29]; thus, we sought to validate the new assay by comparing efflux pump inhibitory activity of a series of pure flavonoids. In addition, to test the validity of the new assay in a more crude sample matrix, we compared the efflux pump inhibitory activity of an extract from the botanical goldenseal (Hydrastis canadensis), which is known to contain EPIs [10,16], using both fluorescence and mass spectrometry-based approaches for data collection.
Materials and Methods
Preparation of plant material
The goldenseal leaf and petiole material used was cultivated in a woodland setting in Hendersonville, North Carolina, (N 35°24.2770', W 082°20.9930', 702.4 m elevation) and was made available by William Burch; this population has been utilized in previously published work [10,16] and is represented by a voucher (NCU583414) curated in the University of North Carolina at Chapel Hill herbarium. The goldenseal extract was prepared using previously described methods [10,30]. Dried plant material was macerated for at least 24 hr, and the methanol extract was subsequently separated from the plant material. This extract was dried in a rotary evaporator to reduce the volume of methanol, and partitioned against an equal volume of hexane. The resulting mixture was stirred for at least one hr and the layers were collected separately using a separatory funnel. The methanol partition was added to water and chloroform in a ratio of 1:5:4, stirred for at least 1 hr, then separated. The chloroform partition from this step was evaporated and used as the starting material for all experiments described herein, and will be referred to as “goldenseal extract” from this point forward.
96 well plate ethidium bromide accumulation assay
This assay is an adaptation of published ethidium bromide efflux-based assays [16–18] and previously published reports of measurements on intracellular accumulation of berberine and chloramphenicol [9,19]. All experiments presented here were performed in the 96 well plate format. Activity was tested using Staphylococcus aureus strain NCTC 8325–4 [31]. The final assay composition was 10% DMSO, 50% Muller-Hinton broth, 40% water (by volume), an estimated 1.6–1.8x108 CFU/mL S. aureus, 1.25 μg/mL ethidium bromide, and a range of analyte concentrations. Data collection, and hence bacterial exposure to these conditions, was limited to 30 min. The alkaloid piperine was used as a positive control, as it is well established in the literature to be an EPI [25,32,33]. Each analyte concentration was tested in triplicate, and the positive control (a piperine dilution series ranging from 4.7 μg/mL to 300 μg/mL prepared via 2-fold dilution) was included on each plate. Fluorescence was measured using a BioTek SynergyH1 microplate reader (BioTek, Winooski, VT) with an excitation wavelength of 520 nm and emission wavelength of 600 nm at 1 min intervals for a total of 30 min. All experiments were performed in triplicate and error bars reported as standard deviation.
Use of mass spectrometry to measure ethidium bromide accumulation
To enable mass spectrometric measurements of ethidium bromide accumulation, the experimental parameters were identical to those described in the previous section, except that the method of data collection was modified. The prepared samples were incubated at room temperature in a EMD Millipore MultiScreen fritted-bottom 96-well filter plate (pore size 0.22 μm, EMD Millipore, Darmstadt, Germany). At the conclusion of the 30 min incubation period, these were filtered simultaneously under vacuum into a receiving 96 well plate. All solutions were stored at 4°C prior to analysis.
Ethidium bromide in the bacterial supernatant was analyzed using high performance liquid chromatography (HPLC) electrospray ionization-mass spectrometry (ESI-MS). Liquid chromatography separations were achieved using a ThermoFinnigan Surveyer HPLC system (Thermo Finnigan, San Jose, CA). The autosampler was temperature controlled at 8°C, and the column (Agilent Prevail C18, 3 μm packing, 50 x 2.1 mm) was heated to 40°C. Sample injection volume was 5 μL and a flow rate of 0.2 mL/min was employed. Samples were eluted using binary gradients consisting of acetonitrile acidified with 1% acetic acid and deionized water (CH3CN:H2O) as follows:—0 min, 0:100; 1.5 min, 0:100; 2 min, 95:5; 10 min, 95:5; 10.5 min, 0:100; 18 min, 0:100. Mass spectrometry analyses were conducted with an LCQ DECA XP Plus ion trap mass spectrometer with electrospray ionization source (Thermo Fisher Scientific) using the following conditions: capillary temperature, 250°C; sheath gas flow, 10 (arbitrary units); no auxiliary gas; source voltage 4.5 kV; capillary voltage, 42 V; tube lens offset, -25 V. The instrument was operated in the positive ion mode with two scan events. The first was full scan, followed by the data-dependent CID fragmentation (50% collision energy) of m/z 314.20 (the [M]+ ion of ethidium). The selected ion chromatogram was plotted for the main product ion m/z 286, and its peak area was determined. All experiments were performed in triplicate and error bars set to standard deviation.
Mass spectrometry data were analyzed to determine an IC50 value for each test compound. The IC50 of piperine was defined as the midpoint between the peak area for vehicle control and that of the 300ppm piperine sample, similar to an approach employed previously [34]. Once determined for piperine, the same peak area was used as a set point for determining IC50 values of the test compounds on the same plate.
Bacterial growth inhibition
MICs were determined according to Clinical Laboratory Standards Institute guidelines [35]. Solutions were prepared in 96 well plates with a final well volume of 250 μL, 2% DMSO in Mueller-Hinton broth, and variable concentrations of test compound or extract ranging from 4.7 to 150 μg/mL, prepared in triplicate. Duplicate plates of each experiment were employed, one inoculated with a bacterial concentration of 5x105 CFU/mL, the other containing only analyte and vehicle. All plates were incubated for 18 hr at 37°C, after which turbidity at 600nm (OD600) was measured with a BioTek Synergy H1 microplate reader. To correct for background due to absorbance of the analyte compounds, the mean OD600 for each treatment without addition of bacteria was subtracted from the mean OD600 of treated wells. MIC was determined as the concentration where there was no statistically significant difference between the mean absorbance of the treated wells and that of the negative control (vehicle in broth).
Results and Discussion
Assay development and comparison of efflux pump inhibition assay methods
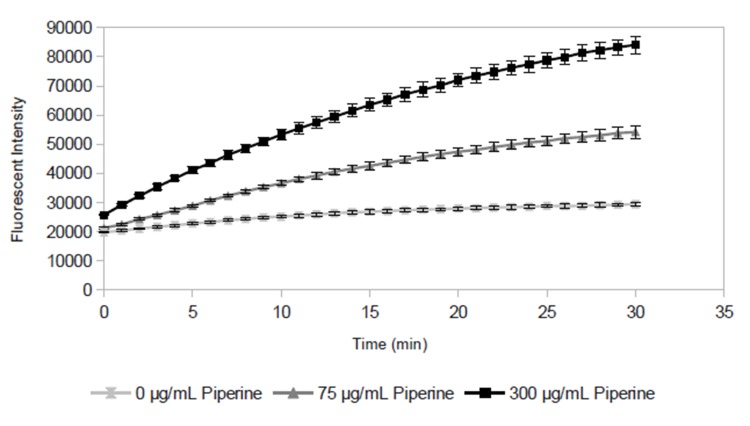
The first goal of our experiments was to determine the applicability of a fluorescence-based accumulation assay to measure the efflux pump inhibitory activity of various flavonoids. Towards this goal, we first validated the assay using a known efflux pump substrate (ethidium bromide) and a known EPI (piperine). As expected, when S. aureus is exposed to ethidium bromide, fluorescence increases over time (Fig 1). This increase is due to intracellular accumulation of ethidium, which fluoresces at 600 nm when it is intercalated with DNA [16]. Ethidium bromide is a substrate of NorA, a major chromosomally-encoded Staphylococcus aureus efflux pump [3,5]. Thus, the intracellular accumulation of ethidium bromide by S. aureus is counteracted by the action of NorA (and other efflux pumps). As evidence of this, the addition of piperine, a known NorA inhibitor, caused a more pronounced increase in fluorescence over time than was observed for the cells in the absence of the inhibitor (Fig 1).
Fig 1. Change in absolute fluorescent intensity over time for Staphylococcus aureus exposed to ethidium bromide in the efflux pump inhibitor piperine.
Fluorescence increases over time due to intracellular accumulation of ethidium bromide. The increase is more pronounced in the presence of piperine, which enhances intracellular accumulation of ethidium bromide by blocking efflux. Data points represent the mean of 3 samples, error bars represent standard deviation.
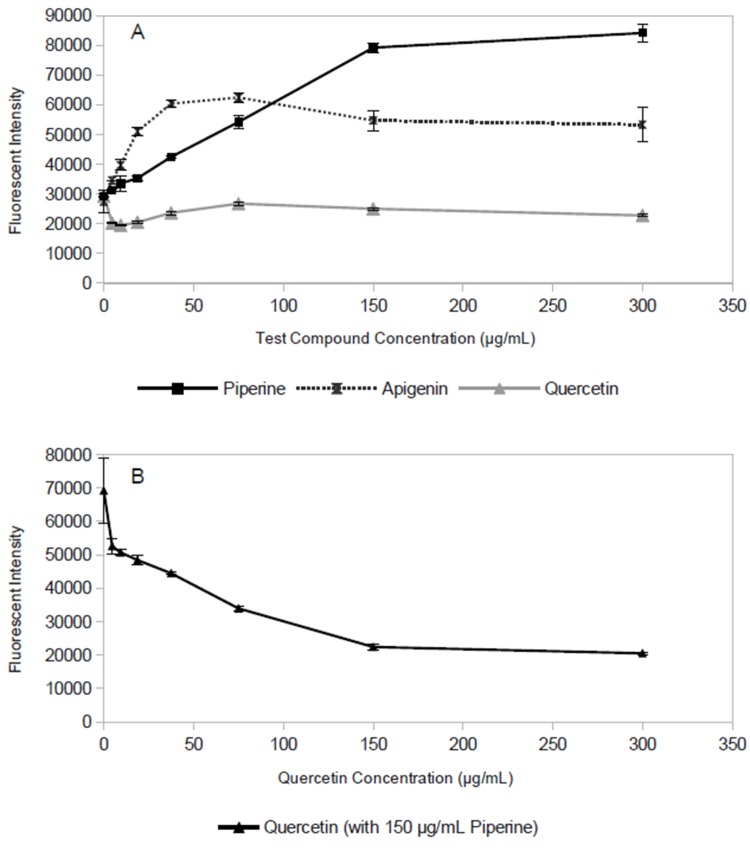
Plotting the time-dependent accumulation assay data (Fig 1) at only a single time point (30 min) allows them to be represented as a dose-response curve (Fig 2A), with mean fluorescent intensity at one concentration on the y-axis, and test compound concentration on the x-axis. These curves demonstrate that ethidium bromide efflux is inhibited in the presence of piperine, and that this inhibition is dose-dependent (Fig 2A).
Fig 2. Change in fluorescence due to ethidium accumulation by S. aureus in the presence of putative inhibitors.
(A) Dose-response curves for the flavonoids apigenin and quercetin. (B) Fluorescence observed for 150 μg/mL piperine in the presence of increasing concentrations of quercetin. Decrease in fluorescence with increasing quercetin concentration in (B) can be attributed to fluorescence quenching by the flavonoid. Data points represent the mean of triplicate measurements (biological replicates), error bars represent standard deviation.
After the positive control was validated with the above methodology, the same approach was used to measure efflux inhibitory activity of several purified flavonoids. Flavonoids were chosen as test compounds because they are ubiquitous in plants, and by extension in plants that are used for food and for medicine [36,37]; and because numerous reports indicate that many flavonoids possess EPI activity [9–11,16,22–29]. Consistent with this precedent, the flavonoid apigenin displayed clear evidence of efflux pump inhibition (Fig 2A). However, the flavonoid quercetin appeared to be inactive. The unexpected negative result for quercetin led to the suspicion that optical matrix interference (quenching of ethidium bromide fluorescence) could interfere with accurate determinations of EPI activity of some compounds. To evaluate this possibility, a series of samples was prepared that included constant concentrations (150 μg/mL) of piperine, along with varying concentrations of the suspected quenching agent quercetin (Fig 2B). It is clear that as the concentration of quercetin increases in these solutions, the overall fluorescence of the mixture decreases, presumably due to quenching. Consistent with this observation, there are many reports of quenching in fluorescence-based assays, especially when plant extracts are involved [38–42].
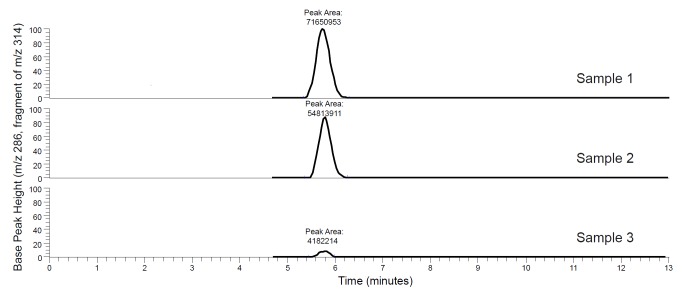
To circumvent the problem with optical matrix interference, a method was developed using liquid chromatography—mass spectrometry (LC-MS) to measure ethidium bromide concentrations in the spent broth filtrate. The concentration-response relationship for the data generated by the LC-MS method was expected to be the inverse of that generated by the measurement of fluorescence—as the concentration of an inhibitor increases, it traps the ethidium inside the bacterial cells, and the concentration of ethidium in the spent broth filtrate (as measured by LC-MS) should decrease. The known EPI piperine was again used for assay validation and it was observed that, as expected, the concentration of ethidium in the filtrate decreased with increasing concentration of piperine (Fig 3A). These results were plotted in a dose-response curve, with peak area displayed on the y-axis and concentration on the x-axis (Fig 3B).
Fig 3. Representative selected chromatograms of filtered, spent broth showing a peak for the ethidium ion (MS-MS transition of m/z 314 to 286).
Sample 1 is the negative control (S. aureus cultured for 30 min in Mueller Hinton broth with 1.25 μg/mL ethidium bromide and 10% DMSO), samples 2 and 3 were cultured under the same conditions as sample 1 with the addition of 75 μg/mL and 300 μg/mL piperine, respectively. All three peaks are normalized to a signal intensity of 3.45 x 106. As piperine is added, efflux pumps are blocked, trapping the ethidium inside the cells and decreasing the quantity of ethidium (as indicated by the area of the ethidium peak) in the spent broth.
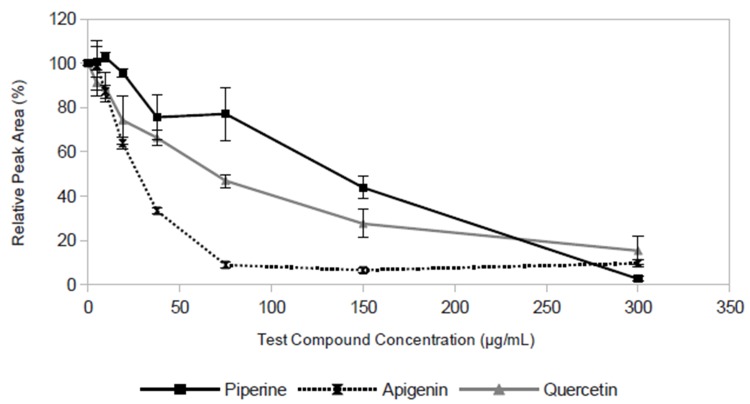
With the LC-MS assay, it is possible to quantify the extracellular levels of ethidium without relying on fluorescence. Thus, if quercetin is in fact an active EPI and the negative results observed in the fluorescence based assay (Fig 2) are due to quenching, quercetin should have demonstrable activity in the new LC-MS based assay. Consistent with this expectation, the LC-MS based method showed quercetin to be active as an EPI (Fig 3B). Once the confounding effect of quenching is removed, it is apparent that quercetin is similar in its EPI activity to piperine, and apigenin is the most active EPI of the three. Furthermore, the LC-MS method showed a more typical dose-response relationship for apigenin (Fig 3B) compared to the fluorescence-based assay (Fig 2A), suggesting that apigenin quenches ethidium fluorescence at concentrations ≥ 38 μg/mL.
To further evaluate the applicability of the LC-MS based efflux pump inhibition assay, we measured IC50 values (Table 1) for a series of six structurally diverse flavonoids and two known EPIs, the aforementioned compound piperine, and carbonyl cyanide m-chloro-phenylhydrazone (CCCP) [9,10,14,16,19]. All six flavonoids were active (sample data shown in Fig 4), with IC50 values ranging from 19 μg/mL (kaempferol and rhamnetin) to 75 μg/mL (quercetin, luteolin, and myricetin.) (Table 1). Piperine demonstrated an IC50 value in this assay between 75 μg/mL and 150 μg/mL, and CCCP was the most active of all compounds tested (IC50 4.7 μg/mL, Table 1).
Table 1. Efflux pump inhibitory activity and antimicrobial activity of flavonoids.
| Flavonoid | IC50 for efflux inhibition a | MIC b |
|---|---|---|
| Apigenin | 38 μg/mL (140 μM) | >150 μg/mL (>560 μM) |
| Kaempferol | 19 μg/mL (66.0 μM) | >150 μg/mL (>520 μM) |
| Rhamnetin | 19 μg/mL (60.0 μM) | >150 μg/mL (>470 μM) |
| Quercetin | 75 μg/mL (250 μM) | >150 μg/mL (>500 μM) |
| Luteolin | 75 μg/mL (260 μM) | 75 μg/mL (260 μM) |
| Myricetin | 75 μg/mL (240 μM) | 150 μg/mL (470 μM) |
| CCCP c | 4.7 μg/mL (23 μM) | 0.29 μg/mL (1.4 μM) |
a: Efflux pump inhibition was measured via LC-MS analysis of ethidium in spent, filtered culture supernatant after a 30 min incubation in triplicate wells.
b: Growth inhibition was measured by optical density at 600nm (in triplicate) after an 18 hr incubation.
c: CCCP is an abbreviation for the compound carbonyl cyanide m-chloro-phenylhydrazone
Fig 4. Efflux pump inhibitory activity of apigenin, piperine, and quercetin as indicated by LC-MS measurement of residual ethidium bromide in spent broth after a 30 min incubation.
Relative peak area (expressed as a percentage) for ethidium is plotted as a function of concentration of the putative inhibitor. Data points represent the mean of 3 measurements (biological replicates), with error bars representing standard deviation.
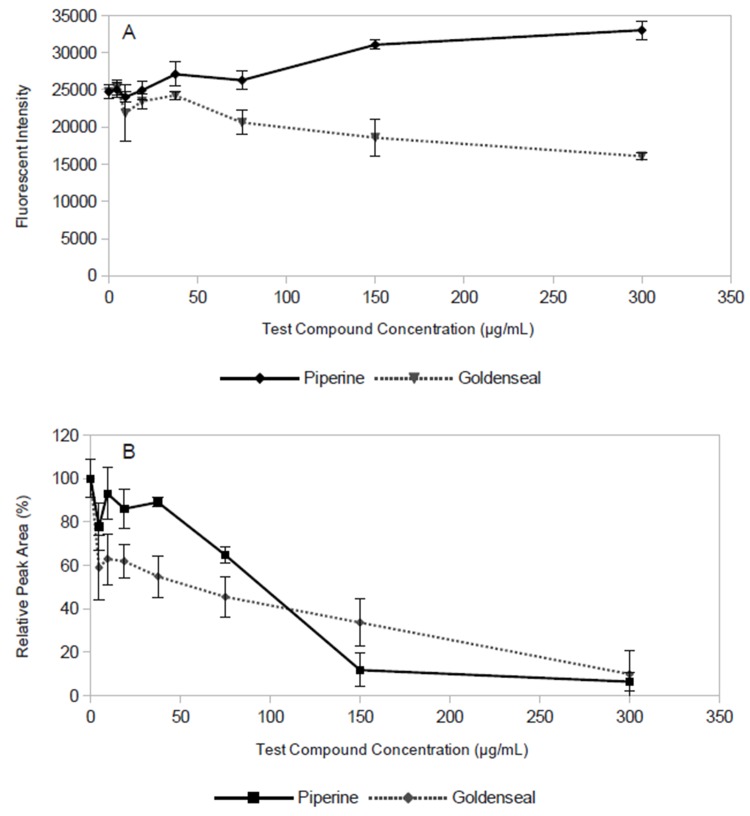
Finally, it was of interest to evaluate whether the mass spectrometry-based efflux assay would be applicable to samples with more complex matrices. Toward this goal, a botanical extract prepared from goldenseal (Hydrastis canadensis) was evaluated for EPI activity using both the fluorescence-based and mass spectrometry-based ethidium bromide accumulation assays. When assayed by the fluorescence-based method, the extract appeared inactive (no apparent IC50, Fig 5A), contradicting literature that indicates H. canadensis extracts contain EPIs [10,16]. However, when the activity of the extract was evaluated with the mass spectrometry-based assay, an IC50 value of 75 μg/mL was observed (Fig 5B). These results demonstrate that quenching can hamper the measurement of efflux pump inhibition for complex extracts using fluorescence, and that the interference can be overcome using the method developed herein. This finding is particularly important given that it may be useful in drug discovery efforts to screen complex extracts for the presence of EPIs.
Fig 5. Efflux pump inhibition in S. aureus by a goldenseal (Hydrastis canadensis) extract.
(A) Data collected using the fluorescence-based ethidium accumulation assay for a range of H. canadensis extract concentrations. (B) Data collected using the mass spectrometry-based ethidium accumulation assay. Incubation time was 30 min for both A and B, data represents mean of 3 samples, error bars represent standard deviation.
Potential interference by growth effects
Many botanical compounds possess antimicrobial activity. Thus, it was important to evaluate whether growth effects might confound the measurements of efflux pump inhibition. Towards this goal, the flavonoids, controls, and the goldenseal extract were screened for inhibition of bacterial growth across a range of concentrations, from 4.7 μg/mL to 150 μg/mL. Only three samples, the flavonoids luteolin and myricetin, and the known EPI CCCP, demonstrated measureable MICs under these conditions (Table 1). To determine whether growth inhibition by these compounds was likely to confound data interpretation, the two inhibitory flavonoids (luteolin and myricetin) were incubated with test strains under the experimental conditions at twice their IC50 values (Table 1). S. aureus cells were tested for loss of viability in the presence of these flavonoids (as well as in the presence of the flavonoid apigenin which does not inhibit bacterial growth) by replicating the experimental conditions of bacterial, ethidium bromide, broth and DMSO content, and plating aliquots of the resulting culture onto supplemented Mueller-Hinton agar at 0, 15 and 30 min time points. No loss of viability was observed after a 30 min exposure to the experimental conditions, as determined by colony count enumeration (S1 Fig). Additionally, to further evaluate the potential for simple toxicity to confound evaluation of data in this assay, the commercial antibiotics gentamicin and nafcillin were subjected to the mass spectrometry-based efflux pump inhibition assays, and no IC50 was observed to the maximum concentration tested of 100 μg/mL (S2 Fig). The highest tested concentration for the antibiotics was well above their reported MICs against Staphylococcus aureus (~0.5 μg/mL [43,44]). Collectively, these results suggest that growth inhibition does not confound the measurements of efflux pump inhibitory activity reported in Table 1.
Conclusions
In light of the health risks posed by the increased occurrence of antibiotic resistant bacterial strains, the need for reliable methods for their study is of high importance. The mass spectrometry-based method to quantitatively investigate efflux pump inhibition is just such a tool, and as such is expected to be of high value to the scientific community. Our study shows that misleading results (false negative in the case of assays that rely on ethidium bromide accumulation) can be obtained when screening crude extracts and even pure compounds with fluorescence-based efflux pump inhibition assays. The new method presented here circumvents these problems. Additionally, the ubiquity of activity in the flavonoids tested in the validation process of this assay reinforces the importance of this class of natural products in the reversal of efflux-pump mediated drug resistance.
Supporting Information
Conditions are as follows: 10% DMSO, 50% Muller-Hinton broth, 40% water (by volume), with 1.25 μg/mL ethidium bromide, for a maximum of 30 min. Test compounds include two flavonoids that inhibit the growth of this strain (luteolin and myricetin), and one that does not (apigenin) (Table 1). (EPS)
(TIF)
Also shown is a control dose-response curve performed on the positive control piperine.
(TIF)
Acknowledgments
We are grateful to Dr. Elizabeth Lacey and Dr. Nicholas H. Oberlies for insightful suggestions, and Carol Ann McCormick for help with preserving voucher specimens. This research was supported in part by Grant Number 1 R01 AT006860 from the National Center for Complementary and Alternative Medicine (NCCAM), a component of the National Institutes of Health (NIH).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Grant Number 1 R01 AT006860 from the National Center for Complementary and Alternative Medicine (NCCAM), a component of the National Institutes of Health (NIH).
References
- 1. Yin Y, He X, Szewczyk P, Nguyen T, Chang G (2006) Structure of the Multidrug Transporter EmrD from Escherichia coli. Science 312: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenwald NP, Gill MJ, Wise R (1998) Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 42: 2032–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bambeke FV, Balzi E, Tulkens PM (2000) Antibiotic Efflux Pumps. Biochem Pharmacol 60: 457–470. [DOI] [PubMed] [Google Scholar]
- 4. Tegos G, Stermitz FR, Lomovskaya O, Lewis K (2002) Multidrug Pump Inhibitors Uncover Remarkable Activity of Plant Antimicrobials. Antimicrob Agents Chemother 46: 3133–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saidijam M, Benedetti G, Ren Q, Xu Z, Hoyle CJ, Palmer SL, et al. (2006) Microbial Drug Efflux Proteins of the Major Facilitator Superfamily. Curr Drug Targets 7: 793–811. [DOI] [PubMed] [Google Scholar]
- 6. Walsh C (2000) Molecular mechanisms that confer antibacterial drug resistance. Nature 406: 775–781. [DOI] [PubMed] [Google Scholar]
- 7. Piddock LJV (2006) Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4: 629–636. [DOI] [PubMed] [Google Scholar]
- 8. Piddock LJV (2006) Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19: 382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K (2000) Synergy in a medicinal plant: Antimicrobial action of berberine potentiated 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci 97: 1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, et al. (2011) Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis). J Nat Prod 74: 1621–1629. 10.1021/np200336g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simões M, Bennett RN, Rosab EAS (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep 26: 746–757. 10.1039/b821648g [DOI] [PubMed] [Google Scholar]
- 12. Olmsted J, Kearns DR (1977) Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry (Mosc) 16: 3647–3654. [DOI] [PubMed] [Google Scholar]
- 13. Sabatini S, Kaatz GW, Rossolini GM, Brandini D, Fravolini A (2008) From Phenothiazine to 3-Phenyl-1,4-benzothiazine Derivatives as Inhibitors of the Staphylococcus aureus NorA Multidrug Efflux Pump. J Med Chem 51: 4321–4330. 10.1021/jm701623q [DOI] [PubMed] [Google Scholar]
- 14. Lechner D, Gibbons S, Bucar F (2008) Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J Antimicrob Chemother 62: 345–348. 10.1093/jac/dkn178 [DOI] [PubMed] [Google Scholar]
- 15. Pereda-Miranda R, Kaatz GW, Gibbons S (2006) Polyacylated oligosaccharides from medicinal mexican morning glory species as antibacterials and inhibitors of multidrug resistance in Staphylococcus aureus. J Nat Prod 69: 406–409. [DOI] [PubMed] [Google Scholar]
- 16. Ettefagh KA, Burns JT, Junio HA, Kaatz GW, Cech NB (2011) Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med 77: 835–840. 10.1055/s-0030-1250606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaatz GW, Seo SM, O’Brien L, Wahiduzzaman M, Foster TJ (2000) Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob Agents Chemother 44: 1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schindler BD, Seo SM, Jacinto PL, Kumaraswami M, Birukou I, Brennan RG, et al. (2013) Functional Consequences of Substitution Mutations in MepR, a Repressor of the Staphylococcus aureus mepA Multidrug Efflux Pump Gene. J Bacteriol 195: 3651–3662. 10.1128/JB.00565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahamoud A, Chevalier J, Davin-Regli A, Barbe J, Pagès JM (2006) Quinoline Derivatives as Promising Inhibitors of Antibiotic Efflux Pump in Multidrug Resistant Enterobacter Aerogenes Isolates. Curr Drug Targets 7: 843–847. [DOI] [PubMed] [Google Scholar]
- 20. Newton BA (1957) The mode of action of phenanthridines: the effect of ethidiurn bromide on cell division and nucleic acid synthesis. J Gen Microbiol 17: 718–730. [DOI] [PubMed] [Google Scholar]
- 21. Waring MJ (1965) Complex formation between ethidium bromide and nucleic acids. J Mol Biol 13: 269–282. [DOI] [PubMed] [Google Scholar]
- 22. Gibbons S (2004) Anti-staphylococcal plant natural products. Nat Prod Rep 21: 263–277. [DOI] [PubMed] [Google Scholar]
- 23. Stermitz FR, Scriven LN, Tegos G, Lewis K (2002) Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med 68: 114–1141. [DOI] [PubMed] [Google Scholar]
- 24. Gibbons S (2005) Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem Rev 4: 63–78. [Google Scholar]
- 25. Stavri M, Piddock LJV, Gibbons S (2007) Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother 59: 1247–1260. [DOI] [PubMed] [Google Scholar]
- 26. Guz NR, Stermitz FR, Johnson JB, Beeson TD, Willen S, Hsiang JF, et al. (2001) Flavonolignan and Flavone Inhibitors of a Staphylococcus aureus Multidrug Resistance Pump: Structure-Activity Relationships. J Med Chem 44: 261–268. [DOI] [PubMed] [Google Scholar]
- 27. Stermitz FR, Beeson TD, Mueller PJ, Hsiang JF, Lewis K (2001) Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem Syst Ecol 29: 793–798. [DOI] [PubMed] [Google Scholar]
- 28. Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz L, Lewis K, et al. (2000) 5‘-Methoxyhydnocarpin-D and pheophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J Nat Prod 63: 1146–1149. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton-Miller JMT, Shah S (2000) Activity of the tea component epicatechin gallate and analogues against methicillin- resistant Staphylococcus aureus. J Antimicrob Chemother 46: 847–863. [DOI] [PubMed] [Google Scholar]
- 30. Gu JQ, Graf TN, Lee D, Chai HB, Mi Q, Kardono LBS, et al. (2004) Cytotoxic and Antimicrobial Constituents of the Bark of Diospyros maritima Collected in Two Geographic Locations in Indonesia. J Nat Prod 67: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 31. Novick R (1967) Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33: 155–166. [DOI] [PubMed] [Google Scholar]
- 32. Khan IA, Mirza ZM, Kumar A, Verma V, Qazi GN (2006) Piperine, a Phytochemical Potentiator of Ciprofloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 50: 810–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nargotra A, Sharma S, Koul JL, Sangwan PL, Khan IA, Kumar A, et al. (2009) Quantitative structure activity relationship (QSAR) of piperine analogsfor bacterial NorA efflux pump inhibitors. Eur J Med Chem 44: 4128–4135. 10.1016/j.ejmech.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 34. Turner RJ, Charlton SJ (2005) Assessing the Minimum Number of Data Points Required for Accurate IC50 Determination. ASSAY Drug Dev Technol 3: 525–531. [DOI] [PubMed] [Google Scholar]
- 35.Wikler MA, Cockerill FR, Craig WA, Dudley MN, Eliopoulos GM, Hecht DW, et al. (2006) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved standards—Seventh Edition M7-A7. Clin Labratory Stand Inst 26.
- 36. lwashina T (2000) The structure and distribution of the flavonoids in plants. J Plant Res 113: 287–299. [Google Scholar]
- 37. Hollman PCH, Katan MB (1999) Dietary flavonoids: Intake, health efects and bioavailability. Food Chem Toxicol 37: 937–942. [DOI] [PubMed] [Google Scholar]
- 38. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 426: 191–196. [DOI] [PubMed] [Google Scholar]
- 39. Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J, et al. (2002) Screening System for the Maillard Reaction Inhibitor from Natural Product Extracts. J Health Sci 48: 520–526. [Google Scholar]
- 40. Baer GR, Meyers SP, Molin WT, Schrader LE (1982) A Simple and Sensitive DNA Assay for Plant Extracts. Plant Physiol 70: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Remans T, Schenk PM, Manners JM, Grof CPL, Elliott AR (1999) A Protocol for the Fluorometric Quantification of mGFP5-ER and sGFP(S65T) in Transgenic Plants. Plant Mol Biol Report 17: 385–395. [Google Scholar]
- 42. Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276: 5960–5972. 10.1111/j.1742-4658.2009.07307.x [DOI] [PubMed] [Google Scholar]
- 43. Mulazimoglu L, Drenning SD, Muder RR (1996) Vancomycin-Gentamicin Synergism Revisited: Effect of Gentamicin Susceptibility of Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother 40: 1534–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LaPlante KL, Rybak MJ (2004) Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 58: 4665–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conditions are as follows: 10% DMSO, 50% Muller-Hinton broth, 40% water (by volume), with 1.25 μg/mL ethidium bromide, for a maximum of 30 min. Test compounds include two flavonoids that inhibit the growth of this strain (luteolin and myricetin), and one that does not (apigenin) (Table 1). (EPS)
(TIF)
Also shown is a control dose-response curve performed on the positive control piperine.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.