Abstract
Background
Pneumonia is a major cause of mortality among HIV-infected patients. Pneumonia severity scores are promising tools to assist clinicians in predicting patients’ 30-day mortality, but existing scores were developed in populations infected with neither HIV nor tuberculosis (TB) and include laboratory data that may not be available in resource-limited settings. The objective of this study was to develop a score to predict mortality in HIV-infected adults with pneumonia in TB-endemic, resource-limited settings.
Methods
We conducted a secondary analysis of data from a prospective study enrolling HIV-infected adults with cough ≥2 weeks and <6 months and clinically suspected pneumonia admitted to Mulago Hospital in Kampala, Uganda from September 2008 to March 2011. Patients provided two sputum specimens for mycobacteria, and those with Ziehl-Neelsen sputum smears that were negative for mycobacteria underwent bronchoscopy with inspection for Kaposi sarcoma and testing for mycobacteria and fungi, including Pneumocystis jirovecii. A multivariable best subsets regression model was developed, and one point was assigned to each variable in the model to develop a clinical predictor score for 30-day mortality.
Results
Overall, 835 patients were studied (mean age 34 years, 53.4% female, 30-day mortality 18.2%). A four-point clinical predictor score was identified and included heart rate >120 beats/minute, respiratory rate >30 breaths/minute, oxygen saturation <90%, and CD4 cell count <50 cells/mm3. Patients’ 30-day mortality, stratified by score, was: score 0 or 1, 12.6%, score 2 or 3, 23.4%, score 4, 53.9%. For each 1 point change in clinical predictor score, the odds of 30-day mortality increased by 65% (OR 1.65, 95% CI 1.39-1.96, p <0.001).
Conclusions
A simple, four-point scoring system can stratify patients by levels of risk for mortality. Rapid identification of higher risk patients combined with provision of timely and appropriate treatment may improve clinical outcomes. This predictor score should be validated in other resource-limited settings.
Introduction
Pulmonary complications are a major cause of morbidity and mortality among HIV-infected patients worldwide. In spite of the increasing availability of effective combination antiretroviral therapy (ART), pneumonia and tuberculosis (TB) remain major causes of mortality in HIV-infected patients in resource-limited settings.[1, 2] Several studies have shown that mortality is higher in HIV-infected patients with pneumonia than in HIV-uninfected patients.[3–7] Moreover, in high-income countries such as Denmark, in which ART is now widely available, the risk of first-time hospitalization for pneumonia has been shown to be six-fold higher in HIV-infected persons compared to the general population, and hospitalization for pneumonia is associated with an increased mortality for the following year.[8, 9] Given the high mortality of pneumonia in HIV-infected patients, the development of a clinical predictor score for mortality in HIV-infected persons hospitalized for pneumonia could be an important clinical tool.
Previous studies have developed pneumonia severity scores, such as the Pneumonia Severity Index (PSI) and the CURB-65, to predict mortality in patients with pneumonia in the general population.[10, 11] However, these studies were conducted in high-income countries in patients that were infected with neither HIV nor TB. Furthermore, these studies included laboratory data, such as arterial blood gas, that may not be available in resource-limited settings where the majority of HIV-infected persons reside. Several studies have investigated the use of pneumonia severity scores in HIV-infected patients in high-income countries. One study evaluated the American Thoracic Society criteria for pneumonia in HIV-infected patients in Spain, and studies from the United States and Canada have evaluated the use of the PSI in patients with HIV.[12–14] The PSI has also been studied in low-income countries, although patients with HIV and TB were excluded.[15] A prior study conducted in the United States developed a staging system to predict mortality in HIV-infected patients with pneumonia but excluded patients with a history of TB.[16] A severity score has been developed for use in HIV-infected children with respiratory infections in resource-limited settings.[17] However, little has been done to develop prognostic tools in HIV-infected adult patients hospitalized with pneumonia in resource-limited, TB-endemic settings, particularly in sub-Saharan Africa. Thus, the aim of this study was to develop a clinical score using data that are often available at the time of initial evaluation to predict 30-day mortality in HIV-infected adults hospitalized with pneumonia in a TB-endemic and resource-limited setting.
Methods
Study Population
We conducted a secondary analysis of a prospective cohort study of opportunistic pneumonias enrolling patients who were admitted to Mulago Hospital, the national referral hospital located in Kampala, Uganda, from September 2008 to March 2011. The study’s primary focus was on HIV-associated TB and Pneumocystis pneumonia (PCP), and so we enrolled consecutive adults at least 18 years of age with suspected HIV, cough for at least 2 weeks but fewer than 6 months, and clinically suspected pneumonia. All subjects provided written informed consent. Patients with a reduced level of consciousness were not enrolled, as these patients could not be consented for the study. Patients who were already receiving treatment for TB or who tested negative for HIV infection were excluded from this analysis.
Data Collection
Enrolled participants underwent a standardized evaluation of their respiratory symptoms. Demographic data were collected using a standardized patient questionnaire. Clinical data gathered included symptoms and signs of pneumonia and clinical characteristics available for most patients at the time of presentation. Patients without a known, confirmed HIV diagnosis were tested for HIV infection. HIV-infected patients had a chest radiograph and CD4 cell count measurement performed. Patients provided two sputum specimens for smear examination with Ziehl-Neelsen staining for acid-fast bacilli (AFB), per World Health Organization (WHO) guidelines recommending the collection of two, rather than, three sputa.[18] For additional diagnostic yield, patients with sputum smears that were negative for mycobacteria were referred for bronchoscopy with bronchoalveolar lavage (BAL). Mulago Hospital has personnel and equipment to perform bronchoscopy and this test is available to clinicians. Bronchoscopic inspection for Kaposi sarcoma (KS) was performed and BAL fluid was tested for mycobacteria, Pneumocystis jirovecii (modified Giemsa stain), and other fungi (potassium hydroxide smear, India ink stain, and culture on Sabouraud agar).
Diagnosis of TB was based on positive sputum or BAL culture on Lowenstein-Jensen media, which was performed throughout the study, or detection by mycobacterial growth indicator tube (MGIT), which was performed on specimens starting in May 2009, or Gene Xpert (Cepheid, Sunnyvale, CA), which was performed starting in August 2009. Diagnosis of fungal pneumonia was based on a positive BAL fungal culture. Diagnosis of PCP was based on microscopic visualization of the characteristic Pneumocystis cysts and trophic forms on Diff-Quik-stained BAL specimens. Diagnosis of pulmonary KS was based on visualization of the characteristic KS lesions during bronchoscopic inspection of the tracheobronchial tree. Cases were reviewed by study physicians at two months and after microbiologic results were known and final diagnoses were assigned according to standardized criteria. In addition to the above diagnostic classification, patients who improved after taking TB medications, in whom no alternate diagnosis was found, and whose AFB cultures were negative were presumed to have had culture-negative TB. Patients who had pulmonary infiltrates on chest radiography, improved after taking antibiotics and no other antimicrobials, and in whom no alternate diagnosis was found were presumed to have had bacterial pneumonia. Patients were evaluated either in-person or by telephone at two months to determine their vital status. Patients who were lost to follow-up are listed as having an unknown final diagnosis.
Statistical Analysis and Derivation of the Clinical Predictor Score
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA). Vital sign cutoffs entered into the model were selected based on clinically meaningful values. Univariate associations between demographic and clinical variables and 30-day mortality were tested using the χ2 test; risk ratios (RR) and 95% confidence intervals (CI) were calculated using the Mantel-Haenszel method. Statistically or clinically significant variables were retained in a stepwise sequence of models using a logistic procedure. Akaike information criterion (AIC) and Schwarz information criterion (SC) scores were used to determine the optimal number of variables to include in the most predictive model. A best subsets logistic regression was then performed to identify the model with the optimal number of variables based on AIC and SC scores that had the highest χ2 value.[19] We used the bootstrap method to simulate 200 populations with the number of subjects in our cohort. One point was assigned to each variable in the final model to develop a clinical predictor score for 30-day mortality. The score’s ability to predict mortality in this cohort was assessed using logistic regression with score as a continuous factor (values ranging from 0 to 4) and then as a categorical factor (values grouped into 3 categories: 0–1, 2–3, and 4) for simplification of risk strata into lower, intermediate, and higher risk. The calibration of the predictor score was evaluated by the Hosmer-Lemeshow goodness of fit test and discrimination was evaluated by determining the proportion of patients in each score category among those who died and among those who survived.[20]
Ethics Approval
The Institutional Review Board at Mulago Hospital, the Makerere University School of Medicine Research Ethics Committee, the Uganda National Council for Science and Technology, and the Committee on Human Research at the University of California, San Francisco approved the study protocol.
Results
Patient Characteristics
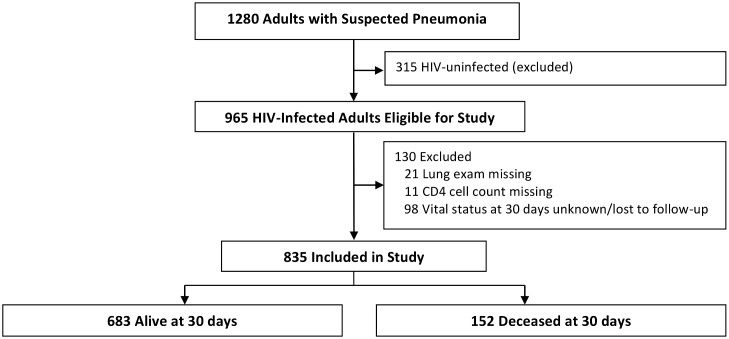
Of 1280 patients enrolled in the study, 965 were infected with HIV and therefore eligible for this study (Fig 1). We excluded HIV-infected patients who had a missing lung exam (21 patients) or a missing CD4 cell count (11 patients). We included HIV-infected patients who died within 30 days but excluded those who had missing data on vital status at 30 days or were lost to follow-up (98 patients). Baseline characteristics (e.g., age, proportion on ART at enrollment, proportion on PCP prophylaxis at enrollment) were not significantly different between the included and excluded patients with the exception of baseline CD4 cell count (lower in enrolled subjects) and sex (more men in enrolled subjects).
Fig 1. Screening and follow-up of study participants.
The included study population of 835 HIV-infected patients was predominantly female (53.4%) and below the age of 45 years (87.5%) (Table 1). HIV was newly diagnosed in 256 patients (30.7%). The majority of patients reported fever, chills, or night sweats (88.1%), subjective weight loss (95.5%), sputum production (91.7%), dyspnea (52.2%), and chest pain (64.6%). Only 13.7% of patients had been on ART for at least 30 days prior to enrollment and 44.2% had been taking PCP prophylaxis for at least 30 days prior to enrollment. Overall, 39% of patients had a temperature below 35.5°C or above 38°C, 27.8% had a heart rate above 120 beats/minute, 45.3% had a respiratory rate above 30 breaths/minute, and 16.4% had an oxygen saturation below 90% on room air at the time of study enrollment. Most patients (62.4%) had an abnormal lung examination. Nearly half (49.6%) of patients had a CD4 cell count below 50 cells/mm3 at enrollment. Over half (51.3%) of patients were diagnosed with pulmonary TB, whereas 16 (1.9%) had pulmonary Kaposi sarcoma, 11 (1.3%) had PCP, and 80 (9.6%) patients were presumed to have had bacterial pneumonia.
Table 1. Baseline characteristics and final diagnoses of 835 subjects.
| Characteristic | n (%) |
|---|---|
| Female | 446 (53.4) |
| Male | 389 (46.6) |
| Age | |
| < 25 years | 111 (13.3) |
| 25–34 years | 369 (44.2) |
| 35–44 years | 251 (30.1) |
| ≥ 45 years | 104 (12.5) |
| New HIV diagnosis | 256 (30.7) |
| Fever, chills, or night sweats | 736 (88.1) |
| Weight loss (subjective) | 797 (95.5) |
| Cough* | 835 (100.0) |
| Sputum production | 766 (91.7) |
| Dyspnea | 436 (52.2) |
| Wheezing | 201 (24.1) |
| Chest pain | 539 (64.6) |
| Smoked > 99 cigarettes in lifetime | 191 (22.9) |
| Ever consumed alcohol | 544 (65.2) |
| ART ≥ 30 days prior to enrollment | 114 (13.7) |
| PCP prophylaxis ≥ 30 days prior to enrollment | 369 (44.2) |
| Temperature <35.5°C or >38°C | 326 (39.0) |
| Heart rate >120 beats/minute | 232 (27.8) |
| Respiratory rate >30 breaths/minute | 378 (45.3) |
| Oxygen Saturation <90% | 137 (16.4) |
| Abnormal lung exam** | 521 (62.4) |
| CD4 cell count <50 cells/mm3 | 414 (49.6) |
| Diagnosis *** | |
| Pulmonary tuberculosis | 429 (51.3) |
| Pneumocystis pneumonia | 11 (1.3) |
| Pulmonary Kaposi’s sarcoma | 16 (1.9) |
| Presumed bacterial pneumonia | 80 (9.6) |
| Other | 27 (3.2) |
| Unknown | 277 (33.2) |
* Cough was a criterion for study inclusion.
** Rhonchi, crepitations, or bronchial breath sounds.
*** Four subjects had more than one diagnosis: two had pulmonary tuberculosis (TB) & Pneumocystis pneumonia, one had pulmonary TB & pulmonary Kaposi’s sarcoma (KS), one had pulmonary KS & Pneumocystis pneumonia, and one had pulmonary KS and presumed bacterial pneumonia.
ART, antiretroviral therapy; PCP, Pneumocystis pneumonia.
Mortality
At 30 days, 152 (18.2%) patients had died (Table 2), the majority (57%) of whom died within the first 10 days of their admission. Significantly more patients died who presented with dyspnea (21.1% vs. 15.0%, p = 0.02), heart rate above 120 beats/minute (23.7% vs. 16.1%, p = 0.01), respiratory rate above 30 breaths/minute (23.3% vs. 14.0%, p = 0.0005), and oxygen saturation below 90% (31.4% vs. 15.6%, p <0.0001). Among patients with a CD4 cell count below 50 cells/mm3, 22.7% died compared to 13.8% of patients with a CD4 cell count of 50 cells/mm3 or above (p = 0.0008). More patients with a temperature below 35.5°C or above 38°C also died as compared to normothermic patients, although this difference was not statistically significant (20.9% vs. 16.5%, p = 0.11). There was a trend toward lower mortality among patients on ART for at least 30 days prior to enrollment and patients taking PCP prophylaxis for at least 30 days prior to enrollment but neither association was statistically significant.
Table 2. Univariate analysis of the association between selected clinical characteristics and 30-day mortality.
| Clinical Characteristic | Died | |||
|---|---|---|---|---|
| n (%) | RR | 95% CI | p-value | |
| All subjects | 152 (18.2) | |||
| Female | 77 (17.3) | 0.89 | 0.67–1.19 | 0.45 |
| Mean age (SE) | 33.7 ±8.5 | 0.90 | 0.74–1.09 | 0.28 |
| New diagnosis of HIV | 43 (16.8) | 0.89 | 0.65–1.23 | 0.48 |
| Fevers, chills, night sweats | 130 (17.7) | 0.79 | 0.53–1.19 | 0.27 |
| Weight loss (subjective) | 148 (18.6) | 1.76 | 0.69–4.51 | 0.21 |
| Sputum production | 140 (18.2) | 1.05 | 0.62–1.80 | 0.86 |
| Dyspnea | 92 (21.1) | 1.40 | 1.04–1.89 | 0.02 |
| Wheezing | 37 (18.4) | 1.01 | 0.73–1.42 | 0.93 |
| Chest pain | 96 (17.8) | 0.94 | 0.70–1.27 | 0.69 |
| Smoked >99 cigarettes (lifetime) | 37 (19.4) | 1.08 | 0.78–1.51 | 0.63 |
| Ever consumed alcohol | 107 (19.7) | 1.27 | 0.93–1.75 | 0.13 |
| ART ≥ 30 days prior to enrollment | 18 (15.8) | 0.85 | 0.54–1.33 | 0.47 |
| PCP prophylaxis ≥ 30 days prior to enrollment | 65 (17.6) | 0.94 | 0.71–1.26 | 0.70 |
| Temperature <35.5°C or >38°C | 68 (20.9) | 1.26 | 0.95–1.69 | 0.11 |
| Heart rate >120 beats/minute | 55 (23.7) | 1.47 | 1.10–1.98 | 0.01 |
| Respiratory rate >30 breaths/minute | 88 (23.3) | 1.66 | 1.24–2.23 | 0.0005 |
| Oxygen saturation <90% | 43 (31.4) | 2.01 | 1.49–2.72 | <0.0001 |
| Abnormal lung exam | 101 (19.4) | 1.19 | 0.88–1.62 | 0.25 |
| CD4 cell count <50 cells/mm3 | 94 (22.7) | 1.65 | 1.22–2.22 | 0.0008 |
RR, risk ratio; CI, confidence interval; SE, standard error; PCP, Pneumocystis pneumonia.
Derivation of the Clinical Predictor Score
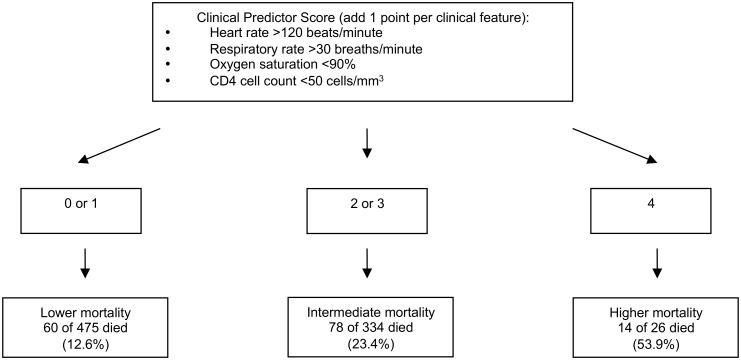
The primary outcome of this study was 30-day mortality. Variables included in the derivation of the clinical predictor score were: use of ART for at least 30 days prior to enrollment, use of PCP prophylaxis for at least 30 days prior to enrollment, temperature below 35.5°C or above 38°C, heart rate above 120 beats/minute, respiratory rate above 30 breaths/minute, oxygen saturation below 90%, and CD4 cell count below 50 cells/mm3. A separate model included the above variables and age and cigarette use, which had no effect on the variables included in the predictor score. Minimum AIC and SC scores indicated that a model with 3 to 5 variables would be optimal. Best subsets regression showed that the best model with 3 variables was: respiratory rate above 30 breaths/minute, oxygen saturation below 90%, and CD4 cell count below 50 cells/mm3 (χ2 = 35.1). The best model with 4 variables included these 3 variables with the addition of heart rate above 120 beats/minute and showed substantial improvement over the 3-variable model (χ2 = 38.1). The best models with 5 variables included all variables in the 4-variable model but no single fifth variable offered a substantial gain in predictive value over the 4-variable model (χ2 = 38.3 to 38.5 for all 5-variable models); thus, the 4-variable model was selected. The bootstrap method was then used to simulate 200 populations each comprised of 835 subjects. Based on the best subsets regression analysis, we identified a 4-variable clinical predictor score that included heart rate above 120 beats/minute, respiratory rate above 30 breaths/ minute, oxygen saturation below 90%, and CD4 cell count below 50 cells/mm3. One point was assigned for each clinical predictor present, with a range of scores from 0 to 4.
Association between Score and Mortality
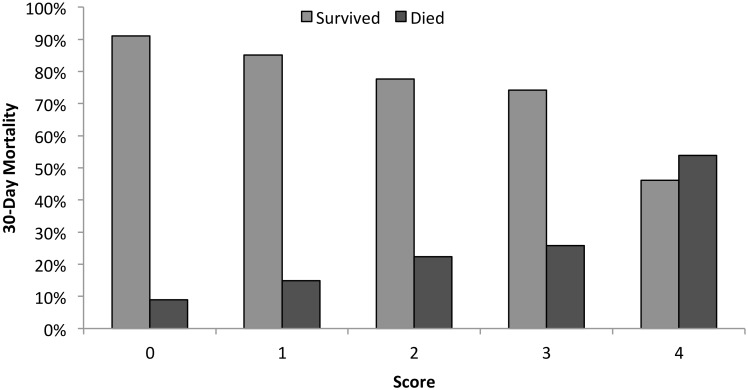
Patients with higher clinical predictor scores had higher mortality rates at 30 days (p <0.0001), (Fig 2). We stratified patients into groups of increasing risk for mortality based on score (Fig 3). Among patients with a clinical predictor score of 0 or 1, 12.6% had died at 30 days, as compared to 23.4% of patients with a score of 2 or 3, and 53.9% of patients with a score of 4 (p <0.001 for lower mortality vs. intermediate mortality vs. higher mortality groups). For each 1 point change in clinical predictor score, the odds of 30-day mortality increased by 65% (OR 1.65, 95% CI 1.39–1.96, p <0.001).
Fig 2. 30-day vital status for patients with each clinical predictor score.
p <0.0001 for the difference in mortality across clinical predictor score values.
Fig 3. Stratification of patients’ 30-day risk of mortality based on clinical predictor score.
The calibration of the predictor score was evaluated by the Hosmer-Lemeshow test, χ2 = 1.12 (p = 0.98), indicating that this model fit the data well. The discrimination of the predictor score was evaluated by determining the proportion of patients in each score category among those who died and among those who survived. Among patients who died, the percentage of patients with each score was as follows: 0 (10.5%), 1 (28.9%), 2 (35.5%), 3 (15.8%), 4 (9.2%). Among patients who survived, the percentage with each score was: 0 (23.9%), 1 (36.9%), 2 (27.4%), 3 (10.1%), 4 (1.8%).
Discussion
This study of HIV-infected patients admitted to an East African referral hospital with pneumonia and a high frequency of TB found that mortality was high overall and that a clinical predictor score using data that are often available at the time of initial evaluation can be used to stratify patients by levels of risk for mortality. We found an overall mortality rate of 18.2% at 30 days among patients in this study. This high mortality rate highlights the importance of rapidly identifying patients at an increased risk of death and targeting those individuals for timely interventions.
In our study, we identified four clinical predictors (heart rate above 120 beats/minute, respiratory rate above 30 breaths/minute, oxygen saturation below 90%, and CD4 cell count below 50 cells/mm3) that were combined into a predictor score that stratifies patients by levels of risk for mortality. The clinical predictor score developed in our study can be implemented with limited laboratory data, in contrast to prior scoring systems, which often require laboratory parameters that may not be readily available in resource-limited settings. For example, CURB-65 requires the measurement of uremia, although a simplified version, CRB-65, does not require the use of this laboratory data.[11] The PSI incorporates 20 parameters, including arterial blood gas, and a score to predict mortality due to HIV-associated PCP makes use of the alveolar-arterial (A-a) oxygen gradient.[10, 21] Rather than requiring the use of blood gases, our clinical predictor score incorporates the measurement of oxygen saturation with a pulse oximeter. Other studies have shown that low oxygen saturation is a risk factor for mortality; for example, oxygen saturation below 90% was significantly associated with in-hospital mortality among HIV-infected patients with pneumonia in the United States.[16] Oxygen supplies are often inconsistently available in resource-limited settings. Given the strong association between hypoxemia and mortality in this study, patients may benefit from routine evaluation of oxygen saturation at the time of triage and from the provision of supplemental oxygen to those with a low saturation. Our study also highlights the need for expanded oxygen delivery capability in similar settings. Moreover, this relatively low cost investment could reap significant benefits with regard to patient outcomes.
A CD4 cell count below 50 cells/mm3 was significantly associated with increased 30-day mortality and is also a component of this clinical predictor score. Low CD4 cell count has been shown to be associated with higher rates of mortality in prior studies of HIV-infected patients with pneumonia, including a study of patients with bacterial pneumonia conducted in Spain.[22] Point of care CD4 testing enables rapid testing of patients upon presentation with results available within 20 minutes, has been shown to be feasible in pilot testing, and is increasingly becoming available in resource-limited settings.[23] Given the strong association between low CD4 cell count and risk of mortality in this cohort, increased availability of point of care CD4 testing could help to rapidly identify patients at increased risk of mortality.
In our study, although nearly 70% of patients had been diagnosed with HIV prior to enrollment and nearly half had a CD4 cell count below 50 cells/mm3, only 18.7% of patients who were aware of their HIV status were on ART for at least 30 days prior to entry. At the time that this study began enrollment, national antiretroviral treatment guidelines for Uganda recommended ART initiation for all HIV-infected patients with a CD4 cell count below 200 cells/mm3; patients with a CD4 cell count between 200 and 350 cells/mm3 who were co-infected with TB, pregnant, or had severe bacterial infection (e.g. requiring hospitalization); and in patients with WHO Stage IV disease irrespective of CD4 cell count.[24] Since the completion of this study, in 2013, the WHO recommended ART initiation in all individuals with a CD4 cell count less than 500 cells/mm3, and in December 2013, the Ugandan antiretroviral treatment guidelines raised the CD4 threshold to less than 500 cells/mm3, in line with WHO guidelines.[25] [26]
The significance of CD4 cell count as a predictor of 30-day mortality in our study raises the question of whether the prompt initiation of ART, and subsequent improvement in CD4 cell count, could improve outcomes in this population. A previous study showed that starting ART within 14 days of diagnosis of an acute opportunistic infection (OI) other than TB resulted in less AIDS progression and death versus starting ART after completion of treatment for an OI.[27] The majority of subjects were enrolled in the United States; 63% of patients had PCP and 12% had bacterial infections including pneumonia. The CAMELIA, SAPIT, and STRIDE studies showed that treatment of HIV and pulmonary TB improves survival and reduces HIV-related illnesses.[28–30] The STRIDE trial showed a significant reduction in AIDS-defining illness or death among patients with a CD4 cell count below 50 cells/mm3 in patients who started ART within two weeks of initiation of treatment for tuberculosis.[28] TB was the most common diagnosis in our cohort, which is consistent with prior studies showing that TB is a leading cause of death among HIV-infected patients in sub-Saharan Africa.[31, 32] Our data reflect the persistent need for timely diagnosis of HIV infection and earlier initiation of ART, which may also reduce rates of TB and other HIV-related complications in this patient population.
Strengths of this study include the prospective nature, the standardized diagnostic evaluation and 30-day outcome assessment, and the large cohort size, which improved the study’s power. To our knowledge, this is the only clinical predictor score for pneumonia that has been developed in adult patients with HIV from a resource-limited setting with a high prevalence of TB. The relatively young age of patients in this study is representative of the HIV-infected population in Uganda, as well as Uganda’s demographics as a whole.[33] A limitation of this study is that we only enrolled patients admitted to a single referral hospital. Although this is a single site study, the size of the study population (835) is comparable to the initial studies of well-validated pneumonia severity scores. The CURB-65 investigators studied 1068 patients at three sites.[11] The Pneumonia Severity Index was initially derived from a study of 347 patients at three Pittsburgh hospitals, and later went on to be validated and refined using hospital databases containing thousands of patient records. [10, 34–36] Our standardized clinical and diagnostic evaluation (e.g., bronchoscopy) and follow-up in a resource-limited setting are strengths of the study, and we hope that our results are validated in additional settings much like the initial Pneumonia Severity Index study was subsequently validated. Furthermore, as we are currently enrolling participants in another cohort study in Uganda of HIV-infected adults with lung disease, we will be able to test and validate this score on those participants. Whereas our clinical predictor score would be expected to perform well in individuals with a subacute presentation of cough, future studies should be conducted to validate our findings in HIV-infected patients with respiratory complaints of shorter duration (i.e. less than 2 weeks) than those in our study. Predictive models generally perform better in the specific population in which they were developed; thus, this clinical predictor score should also be validated in similar populations and in other settings. Another potential limitation of the study is that approximately 10% of patients did not have complete information on vital status at 30 days despite attempts by study staff to contact these individuals.
Identifying patients at high risker for short-term mortality may allow for appropriate allocation of additional targeted interventions in resource-limited settings. These interventions may include the use of diagnostic testing (such as GeneXpert for rapid TB diagnosis) and therapeutics (including early goal directed therapy for sepsis, supplemental oxygen for hypoxemic patients, and ensuring early ART for all patients). Mortality was high overall in this cohort and further research is needed to optimize both diagnostic and therapeutic strategies in this population in order to improve outcomes and reduce mortality.
In conclusion, we found that a simple, four-point scoring system can stratify HIV-infected patients with pneumonia by increasing levels of risk for 30-day mortality. The rapid identification of patients at an increased risk for short-term mortality, combined with timely and appropriate diagnosis and treatment, may improve clinical outcomes. With further validation in other clinical settings, this clinical predictor score may help to improve the management of pneumonia in HIV-infected patients in resource-limited settings.
Acknowledgments
The authors would like to thank the study participants and the staff and administration of Mulago Hospital and the Infectious Disease Research Collaboration for supporting this study. The International HIV-associated Opportunistic Pneumonias (IHOP) Study team includes: Laurence Huang, William Worodria, Lucian Davis, Adithya Cattamanchi, Irene Ayakaka, Alfred Andama, Patrick Byanyima, Nelson Kalema, Winceslaus Katagira, Alfred Andama, Margaret Awor, Ingvar Sanyu, Sylvia Kaswabuli, and Catherine Nabakiibi.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by grants from the National Institutes of Health (K24 087713, L.H. and R01 090335, L.H.) and the Mount Zion Global Health Project Fund (C.A.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Curtis AJ, Marshall CS, Spelman T, Greig J, Elliot JH, Shanks L, et al. Incidence of WHO stage 3 and 4 conditions following initiation of anti-retroviral therapy in resource limited settings. PLoS One. 2012;7(12):e52019 10.1371/journal.pone.0052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall CS, Curtis AJ, Spelman T, O'Brien DP, Greig J, Shanks L, et al. Impact of HIV-associated conditions on mortality in people commencing anti-retroviral therapy in resource limited settings. PLoS One. 2013;8(7):e68445 10.1371/journal.pone.0068445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman C, Glatthaar M, Morar R, Mahomed AG, Kaka S, Cassel M, et al. Bacteremic pneumococcal pneumonia in HIV-seropositive and HIV-seronegative adults. Chest. 1999;116(1):107–14. [DOI] [PubMed] [Google Scholar]
- 4. Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–51. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DH, Carriere KC, Houston S, Jin Y, Predy G, Gill J, et al. Hospitalization for community-acquired pneumonia in Alberta patients with human immunodeficiency virus infections: a case control study. Can Respir J. 2003;10(5):265–70. [DOI] [PubMed] [Google Scholar]
- 6. Park DR, Sherbin VL, Goodman MS, Pacifico AD, Rubenfeld GD, Polissar NL, et al. The etiology of community-acquired pneumonia at an urban public hospital: influence of human immunodeficiency virus infection and initial severity of illness. J Infect Dis. 2001;184(3):268–77. [DOI] [PubMed] [Google Scholar]
- 7. Touchie C, Marrie TJ. Comparison of community-acquired pneumonia requiring admission to hospital in HIV-and non-HIV-infected patients. Can J Infect Dis. 1996;7(4):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sogaard OS, Lohse N, Gerstoft J, Kronborg G, Ostergaard L, Pedersen C, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995–2007: a Danish population-based, nationwide cohort study. Clin Infect Dis. 2008;47(10):1345–53. 10.1086/592692 [DOI] [PubMed] [Google Scholar]
- 9. Sogaard OS, Lohse N, Gerstoft J, Kronborg G, Ostergaard L, Pedersen C, et al. Mortality after hospitalization for pneumonia among individuals with HIV, 1995–2008: a Danish cohort study. PLoS One. 2009;4(9):e7022 10.1371/journal.pone.0007022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. [DOI] [PubMed] [Google Scholar]
- 11. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chew KW, Yen IH, Li JZ, Winston LG. Predictors of pneumonia severity in HIV-infected adults admitted to an Urban public hospital. AIDS Patient Care STDS. 2011;25(5):273–7. 10.1089/apc.2010.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cordero E, Pachon J, Rivero A, Giron JA, Gomez-Mateos J, Merino MD, et al. Community-acquired bacterial pneumonia in human immunodeficiency virus-infected patients: validation of severity criteria. The Grupo Andaluz para el Estudio de las Enfermedades Infecciosas. Am J Respir Crit Care Med. 2000;162(6):2063–8. [DOI] [PubMed] [Google Scholar]
- 14. Sanders KM, Marras TK, Chan CK. Pneumonia severity index in the immunocompromised. Can Respir J. 2006;13(2):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zuberi FF, Khan JA. Prospective comparison of prediction rules of mortality risk for CAP in a developing country. Int J Tuberc Lung Dis. 2008;12(4):447–52. [PubMed] [Google Scholar]
- 16. Arozullah AM, Parada J, Bennett CL, Deloria-Knoll M, Chmiel JS, Phan L, et al. A rapid staging system for predicting mortality from HIV-associated community-acquired pneumonia. Chest. 2003;123(4):1151–60. [DOI] [PubMed] [Google Scholar]
- 17. Reed C, Madhi SA, Klugman KP, Kuwanda L, Ortiz JR, Finelli L, et al. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. PLoS One. 2012;7(1):e27793 10.1371/journal.pone.0027793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Reduction of number of smears for the diagnosis of pulmonary TB. Geneva: World Health Organization, 2007. [Google Scholar]
- 19. Shtatland E, Cain E, Barton M. The perils of stepwise logistic regression and how to escape them using informational criteria and the output delivery system. SUGI. 2001;26. [Google Scholar]
- 20. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 2004. [Google Scholar]
- 21. Fei MW, Kim EJ, Sant CA, Jarlsberg LG, Davis JL, Swartzman A, et al. Predicting mortality from HIV-associated Pneumocystis pneumonia at illness presentation: an observational cohort study. Thorax. 2009;64(12):1070–6. 10.1136/thx.2009.117846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curran A, Falco V, Crespo M, Martinez X, Ribera E, Villar del Saz S, et al. Bacterial pneumonia in HIV-infected patients: use of the pneumonia severity index and impact of current management on incidence, aetiology and outcome. HIV Med. 2008;9(8):609–15. 10.1111/j.1468-1293.2008.00603.x [DOI] [PubMed] [Google Scholar]
- 23. Manabe YC, Wang Y, Elbireer A, Auerbach B, Castelnuovo B. Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PLoS One. 2012;7(4):e34319 10.1371/journal.pone.0034319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uganda Ministry of Health. National antiretroviral treatment and care guidelines for adults, adolescents, and children. Uganda: Ministry of Health, 2008. [Google Scholar]
- 25. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 26. Uganda Ministry of Health. Addendum to the national antiretroviral treatment guidelines. Uganda: Ministry of Health, 2013. [Google Scholar]
- 27. Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575 10.1371/journal.pone.0005575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. 10.1056/NEJMoa0905848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81. 10.1056/NEJMoa1013911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91. 10.1056/NEJMoa1013607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cox JA, Lukande RL, Nelson AM, Mayanja-Kizza H, Colebunders R, Van Marck E, et al. An autopsy study describing causes of death and comparing clinico-pathological findings among hospitalized patients in Kampala, Uganda. PLoS One. 2012;7(3):e33685 10.1371/journal.pone.0033685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C, Murdoch DM, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One. 2012;7(10):e47542 10.1371/journal.pone.0047542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. 2011 Uganda AIDS Indicator Survey: Key Findings. Calverton, Maryland, USA: Uganda Ministry of Health and ICF International, 2012. [Google Scholar]
- 34. Fine MJ, Hanusa BH, Lave JR, Singer DE, Stone RA, Weissfeld LA, et al. Comparison of a disease-specific and a generic severity of illness measure for patients with community-acquired pneumonia. J Gen Intern Med. 1995;10(7):359–68. [DOI] [PubMed] [Google Scholar]
- 35. Fine MJ, Orloff JJ, Arisumi D, Fang GD, Arena VC, Hanusa BH, et al. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med. 1990;88(5N):1N–8N. [PubMed] [Google Scholar]
- 36. Fine MJ, Singer DE, Hanusa BH, Lave JR, Kapoor WN. Validation of a pneumonia prognostic index using the MedisGroups Comparative Hospital Database. Am J Med. 1993;94(2):153–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.