Abstract
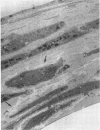
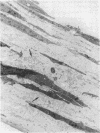
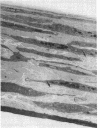
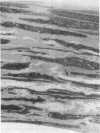
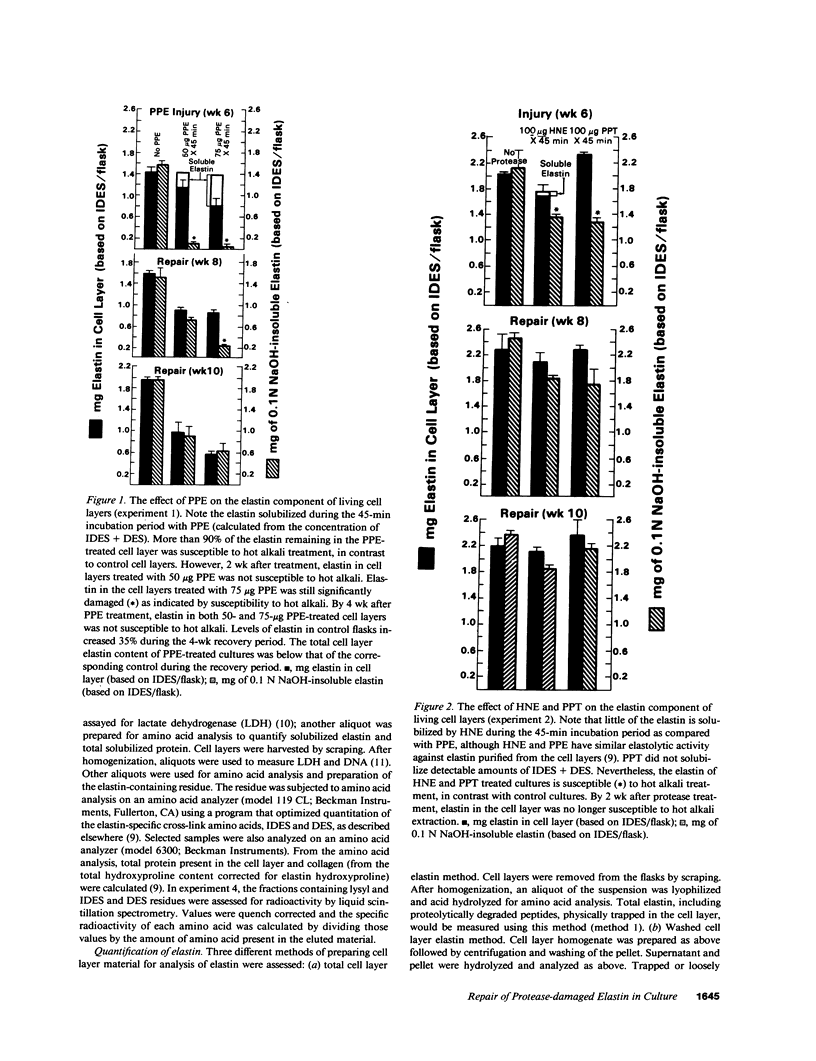
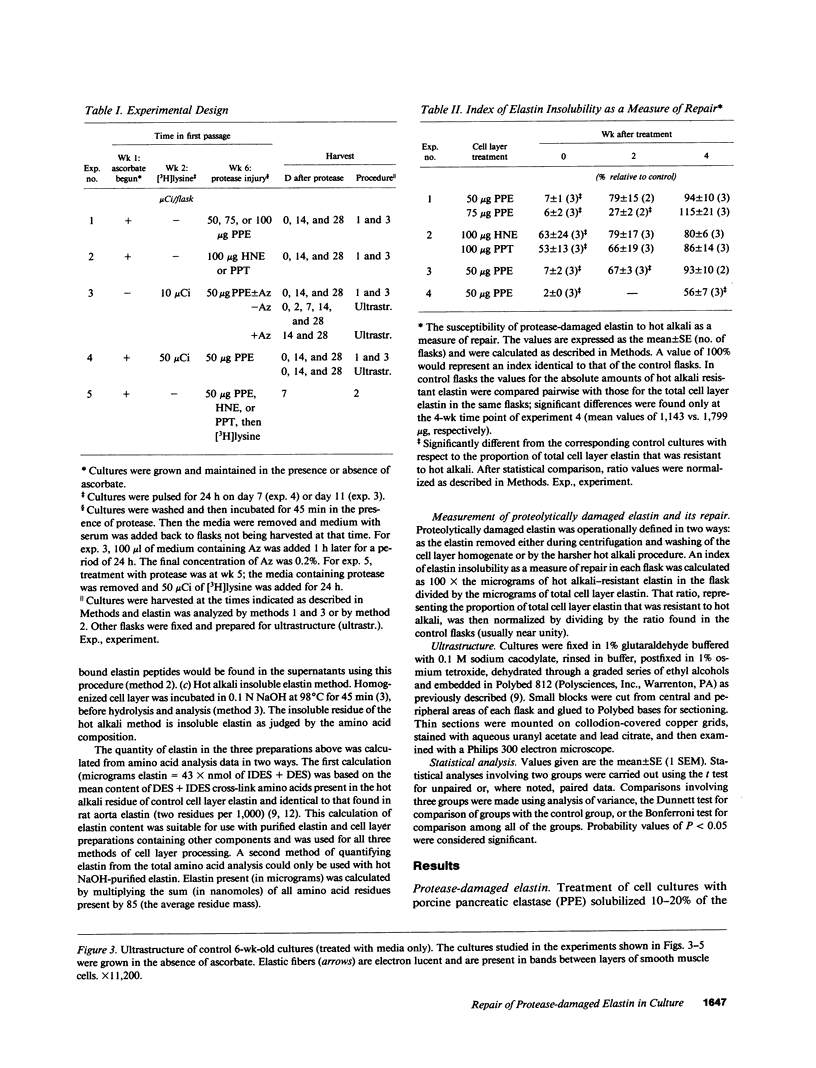
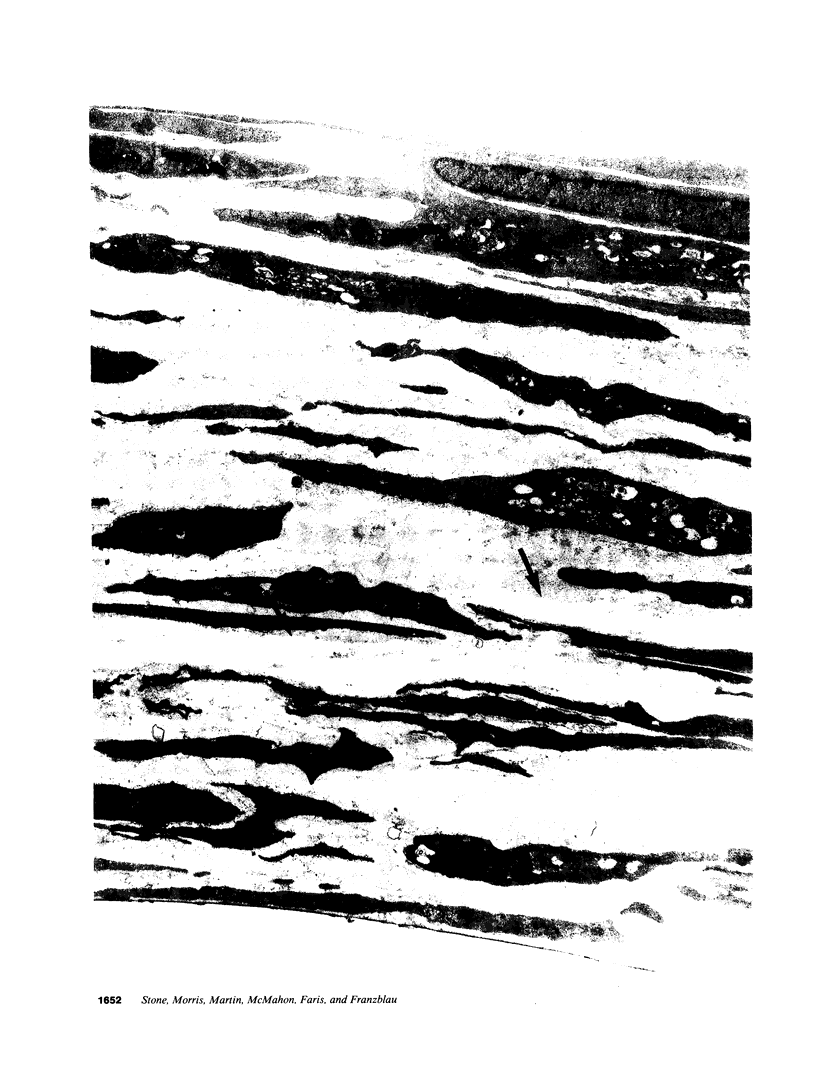
The objective of this study was to investigate the elastin repair process in the rat aortic smooth muscle cell culture after proteolytic injury. Although little studied in vivo, elastin repair is thought to occur through a sequential process involving enzymatic removal (debridement) of damaged fibers followed by synthesis of tropoelastin, its subsequent processing, and eventual incorporation into new insoluble elastin. A second repair mechanism of proteolytically damaged elastin in a culture system is reported here. Repair in this system relates directly to restoration of resistance to elastin solubilization by hot alkali. As expected, severe injuries were observed with porcine pancreatic elastase (PPE). Using PPE, only 6% of the elastin, relative to control, was resistant to hot alkali immediately after elastase treatment. 4 wk later, resistance to hot alkali had increased dramatically to a mean of 90%. Repair took longer after injury with 75 micrograms of PPE as compared with 50 micrograms of PPE. The limited elastic fiber proteolysis induced by either human neutrophil elastase or porcine trypsin was repaired in culture within 2 wk. Elastin that had been radiolabeled with [3H]lysine 4-5 wk before injury was converted from a hot NaOH-susceptible to a NaOH-resistant elastin fraction during recovery from PPE injury. At the same time, the frayed elastic fibers that were seen with the electron microscope immediately after PPE treatment were replaced by continuous bands of elastin that resembled those in control cultures. Restoration of NaOH resistance did not require a net increase in total cell layer elastin, suggesting that relatively little new tropoelastin incorporation into the cell layer was required for this type of repair. These results suggested a salvage repair mechanism for proteolytically damaged elastin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris B., Toselli P., Kispert J., Wolfe B. L., Pratt C. A., Mogayzel P. J., Jr, Franzblau C. Elastase effect on the extracellular matrix of rat aortic smooth muscle cells in culture. Exp Mol Pathol. 1986 Oct;45(2):105–117. doi: 10.1016/0014-4800(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Fülöp T., Jr, Jacob M. P., Varga Z., Foris G., Leövey A., Robert L. Effect of elastin peptides on human monocytes: Ca2+ mobilization, stimulation of respiratory burst and enzyme secretion. Biochem Biophys Res Commun. 1986 Nov 26;141(1):92–98. doi: 10.1016/s0006-291x(86)80339-4. [DOI] [PubMed] [Google Scholar]
- Gosline J. M. The physical properties of elastic tissue. Int Rev Connect Tissue Res. 1976;7:211–249. doi: 10.1016/b978-0-12-363707-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Halme T., Jutila M., Vihersaari T., Oksman P., Light N. D., Penttinen R. The borohydride-reducible compounds of human aortic elastin. Demonstration of a new cyclic amino acid in alkali hydrolysate, and changes with age and in patients with annulo-aortic ectasia including one with Marfan syndrome. Biochem J. 1985 Nov 15;232(1):169–175. doi: 10.1042/bj2320169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M. P., Fülöp T., Jr, Foris G., Robert L. Effect of elastin peptides on ion fluxes in mononuclear cells, fibroblasts, and smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Feb;84(4):995–999. doi: 10.1073/pnas.84.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan H. M., Sullivan K. A. Lysyl oxidase: preparation and role in elastin biosynthesis. Methods Enzymol. 1982;82(Pt A):637–650. doi: 10.1016/0076-6879(82)82092-2. [DOI] [PubMed] [Google Scholar]
- Kuhn C., Yu S. Y., Chraplyvy M., Linder H. E., Senior R. M. The induction of emphysema with elastase. II. Changes in connective tissue. Lab Invest. 1976 Apr;34(4):372–380. [PubMed] [Google Scholar]
- LANSING A. I., ROSENTHAL T. B., ALEX M., DEMPSEY E. W. The structure and chemical characterization of elastic fibers as revealed by elastase and by electron microscopy. Anat Rec. 1952 Dec;114(4):555–575. doi: 10.1002/ar.1091140404. [DOI] [PubMed] [Google Scholar]
- Lefevre M., Rucker R. B. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 1980 Jul 15;630(4):519–529. doi: 10.1016/0304-4165(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Lefevre M., Rucker R. B. Modification of arterial elastin in vivo. Effects of age and diet on changes in the N-terminal amino acid content of aorta elastin. Biochim Biophys Acta. 1983 Mar 30;743(3):338–342. doi: 10.1016/0167-4838(83)90391-6. [DOI] [PubMed] [Google Scholar]
- ROBERT L., POULLAIN N. ETUDES SUR LA STRUCTURE DE L''ELASTINE ET LE MODE D'ACTION DE L''ELASTASE. I. NOUVELLE M'ETHODE DE PR'EPARATION DE D'ERIV'ES SOLUBLES DE L''ELASTINE. Bull Soc Chim Biol (Paris) 1963;45:1317–1326. [PubMed] [Google Scholar]
- Rasmussen B. L., Bruenger E., Sandberg L. B. A new method for purification of mature elastin. Anal Biochem. 1975 Mar;64(1):255–259. doi: 10.1016/0003-2697(75)90426-1. [DOI] [PubMed] [Google Scholar]
- Robert L. Turnover and elastolysis in elastic tissue: introduction. Adv Exp Med Biol. 1977;79:139–143. [PubMed] [Google Scholar]
- Rucker R. B., Dubick M. A. Elastin metabolism and chemistry: potential roles in lung development and structure. Environ Health Perspect. 1984 Apr;55:179–191. doi: 10.1289/ehp.8455179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P., Wrenn D. S., Prasad K. U., Urry D. W. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984 Sep;99(3):870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskel N. T., Sandburg L. B. A comparison of six methods of extracting elastin residue from hamster lungs. Exp Lung Res. 1983 Feb;4(2):109–119. doi: 10.3109/01902148309055008. [DOI] [PubMed] [Google Scholar]
- Starcher B. C., Galione M. J. Purification and comparison of elastins from different animal species. Anal Biochem. 1976 Aug;74(2):441–447. doi: 10.1016/0003-2697(76)90224-4. [DOI] [PubMed] [Google Scholar]
- Stone P. J., McMahon M. P., Morris S. M., Calore J. D., Franzblau C. Elastin in a neonatal rat smooth muscle cell culture has greatly decreased susceptibility to proteolysis by human neutrophil elastase. An in vitro model of elastolytic injury. In Vitro Cell Dev Biol. 1987 Oct;23(10):663–676. doi: 10.1007/BF02620979. [DOI] [PubMed] [Google Scholar]