Abstract
Objective
Define the neurocognitive features of primary central nervous system lymphoma (PCNSL) presenting with dementia, and compare with other causes of rapidly progressive dementia (RPD).
Background
PCNSL can present as an RPD. Differentiating PCNSL from other RPDs is critical because lymphomatous dementia may be reversible, and untreated PCNSL is fatal.
Methods
We performed a meta-analysis of case reports of dementia from PCNSL (between 1950 and 2013); 20 patients (14 with lymphomatosis cerebri) met our criteria. We compared these patients to a case series of patients with RPD from Creutzfeldt-Jakob disease and other non-PCNSL etiologies (Sala et al, 2012. Alzheimer Dis Assoc Disord. 26:267–271).
Results
Median age was 66 (range 41–81); 70% were men. Time from symptom onset to evaluation was < 6 months in 65%. No patients had seizures; 5% had headaches; 45% had non-aphasic speech difficulty. There was significantly more memory impairment in patients with PCNSL than other RPDs and significantly less myoclonus and parkinsonism. Behavioral changes and cerebellar signs were not significantly different. Significantly more patients with PCNSL than other RPDs had white matter changes; significantly fewer had atrophy. Elevated CSF protein and pleocytosis were more frequent in PCNSL; patients with other RPDs tended to have normal CSF ± 14-3-3 protein.
Conclusions
Unlike patients with RPD from other causes, those with PCNSL commonly present with impaired memory, apathy, and abnormal speech and gait, without headache, seizure, or myoclonus. White-matter changes and CSF abnormalities predominate. Improved clinical awareness of PCNSL can prompt earlier diagnosis and treatment.
Keywords: dementia, lymphoma, neurologic manifestations, neuropsychiatry
Infiltrating tumors are an under-recognized cause of rapidly progressive dementia (RPD), defined as a 1- to 2-year course from symptom onset to cognitive and functional debilitation (Geschwind, 2010). Many patients with RPD have a fast deterioration of cognition over weeks to months, with death within 2 years. Clinicians evaluating patients with RPD usually consider sporadic Creutzfeldt-Jakob disease (CJD), autoimmune encephalitides, paraneoplastic encephalitis, infectious conditions, and even neurodegenerative disorders before they consider neoplastic dementias.
An important potentially treatable neoplastic dementia is primary central nervous system lymphoma (PCNSL); however, there is little information to guide clinicians on the frequency and characteristics of PCNSL presenting as an RPD. Identifying this lymphomatous dementia as soon as possible and distinguishing it from other RPDs is critical because lymphomatous dementia is treatable but untreated PCNSL is rapidly fatal.
PCNSL is a type of non-Hodgkin lymphoma that affects only the central nervous system (CNS), without systemic involvement. PCNSL is uncommon, accounting for only 4% of newly diagnosed CNS tumors, with an incidence of 0.47 per 100,000 person-years (Villano et al, 2011). Brain magnetic resonance imaging (MRI) often shows a single contrast-enhancing lesion and/or restricted diffusion on diffusion weighted imaging (DWI), but the disease can instead be multifocal (Degnan and Levy, 2014b; Lai et al, 2002). The most common sites of involvement are the deep periventricular white matter and the corpus callosum, as well as the thalamus and basal ganglia (Eichler and Batchelor, 2006).
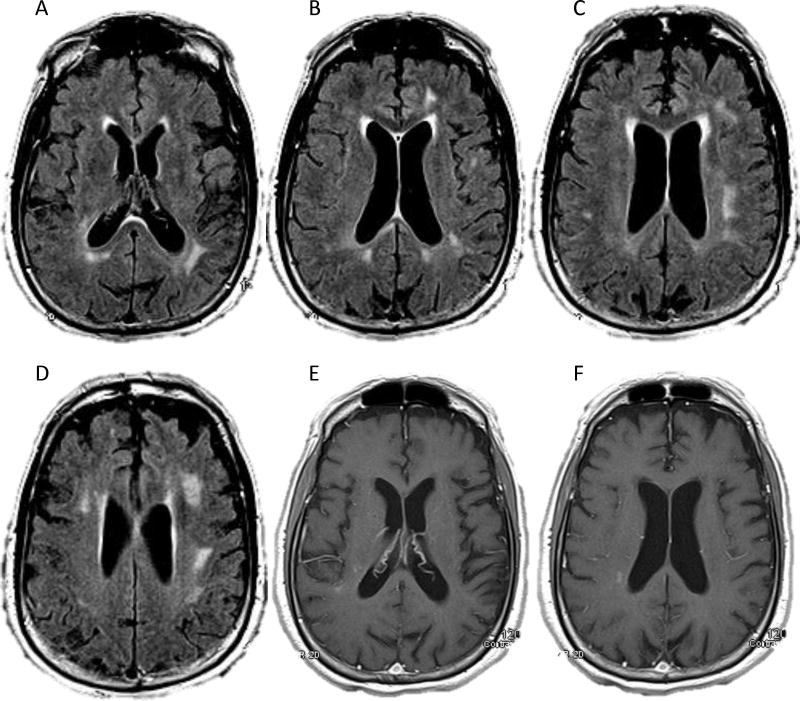
Homogeneous contrast enhancement, the classic appearance, can help distinguish PCNSL from other conditions, such as glioblastoma (heterogeneous enhancement) (Omuro and DeAngelis, 2013), progressive multifocal leukoencephalopathy (non-enhancing) (Shah et al, 2010), and Creutzfeldt-Jakob disease (CJD) (FLAIR and DWI hyperintensities in the cortical gyri as well as the caudate, putamen, or thalamus) (Degnan and Levy, 2014a; Geschwind et al, 2008). Uncommonly, PCNSL is widespread, with or without contrast enhancement, and may show only nonspecific T2 hyperintense lesions, known as lymphomatosis cerebri (LC) (Bakshi et al, 1999; Vital and Sibon, 2007; Weaver et al, 2007). In patients with LC, diagnosis can be especially challenging, as illustrated by Figure 1.
FIGURE 1.
Our evaluation for primary central nervous system lymphoma in a 74-year-old right-handed man with 6 months of progressive personality change, confusion, and impaired gait. On a magnetic resonance imaging scan of his brain, axial FLAIR (Panels A–D) and T1 post-contrast (Panels E–F) sequences showed subcortical and deep periventricular white matter hyperintensities and subtle right periatrial enhancement. Cerebrospinal fluid analysis showed lymphocytic pleocytosis (7 white blood cells/cu mm, 93% lymphocytes), elevated protein (119 mg/dL), normal glucose (52 mg/dL), and negative cytology. Empiric steroids did not improve the patient’s symptoms; however, cerebrospinal fluid analysis while the patient was taking steroids showed resolution of the pleocytosis, persistently elevated protein (96 mg/dL), normal glucose, negative cytology, negative flow cytometry and gene rearrangement from limited specimens, and elevated beta-2 microglobulin (4.6 mg/L). We concluded that the patient had clinically probable lymphomatosis cerebri.
A finding of neoplastic lymphocytes in cerebrospinal fluid (CSF), by either cytomorphology or immunophenotyping, is sufficient for a diagnosis of PCNSL with meningeal dissemination (Kiewe et al, 2010); however, because these CSF studies are not very sensitive, multiple samples may be needed to confirm the diagnosis. If the CSF is diagnostic, then a brain biopsy can be avoided. In diagnostically challenging cases, patients may be treated empirically with steroids for presumed demyelinating or autoimmune conditions, but the steroids’ lympholytic effects will further delay definitive diagnosis by CSF or biopsy.
Neuropsychiatric symptoms account for about 40% of the presenting symptoms in patients with PCNSL (Bataille et al, 2000), but the distinguishing neurocognitive features have not been further characterized, particularly in comparison to other RPDs. Physicians may not suspect PCNSL, particularly if the neuroimaging is nonspecific and does not show a typical contrast-enhancing lesion.
We analyzed case reports of patients with PCNSL presenting with dementia so that we could define their cognitive impairment and neurologic features. Then we compared these characteristics to those of CJD and other causes of RPD.
METHODS
Figure 2 shows how we selected the case reports for this retrospective analysis. We began with a PubMed search of “dementia and brain tumor,” “cancer mimicking dementia,” “cancer presenting as dementia,” and “dementia [MeSH] and brain neoplasm [MeSH]” for all articles up through our search date in September 2013. From 3238 results, with the earliest paper published in 1950, we winnowed down to 16 articles that met our inclusion criteria of English-language case reports describing dementia as the presenting syndrome in adults with PCNSL. These 16 articles, published between 1990 and 2013, contained 20 original case reports of patients with pathologically confirmed PCNSL: Bakshi et al, 1999; Carlson, 1996; Kanai et al, 2008; Kim et al, 2006; Leschziner et al, 2011; Lewerenz et al, 2007; Matosevic et al, 2010; Nakasu et al, 1990; Pandit et al, 2010; Rollins et al, 2005; Sugie et al, 2009; Sugino et al, 2013; Vital and Sibon, 2007; Weaver et al, 2007; Widdess-Walsh et al, 2005; and Zarabi et al, 1992.
FIGURE 2.
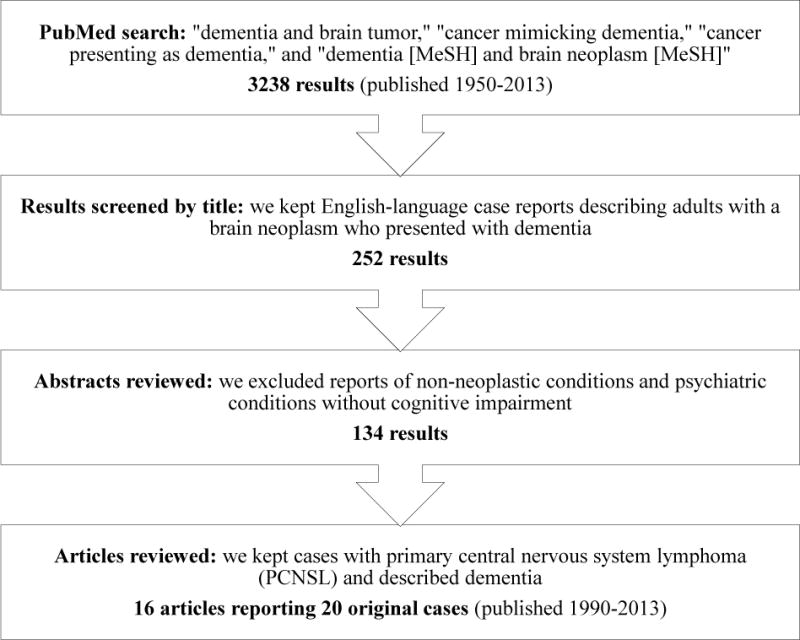
Inclusion and exclusion criteria for articles chosen for this review. We searched PubMed in September 2013.
As detailed in Tables 1 and 3, we reviewed the 20 cases for:
Time from initial symptoms to the diagnostic evaluation
Neurologic symptoms: headache, seizure, and incontinence
Cognitive impairment: disorientation, altered level of arousal, fluctuating cognition, relative sparing of memory, problems with attention or concentration, aphasia, and apraxia (excluding constructional apraxia)
Neuropsychiatric symptoms: disengagement or apathy, other personality change, agitation, hallucinations, and other psychiatric symptoms
Neurologic signs: non-aphasic speech disorder (dysarthria, slowed speech), extrapyramidal signs (bradykinesia, masked facies), strength abnormality, tone abnormality, tremor, limb ataxia, myoclonus, gait disorder (ataxia, shuffling, imbalance, unsteadiness), eye movement abnormality, frontal release signs (snout, suck, grasp, palmomental, glabellar), and cerebellar signs
Imaging abnormalities
Electroencephalogram (EEG) abnormalities
CSF abnormalities
Treatment
Cognitive improvement as a result of treatment
Whether or not the diagnosis was established before death
TABLE 1.
Neurocognitive Presentation in 20 Patients with Dementia Caused by Primary Central Nervous System Lymphoma (PCNSL)
| n (%) | Neurocognitive Feature |
|---|---|
| Time from first symptoms to evaluation | |
| 3 (15) | ≤ 1 month |
| 5 (25) | > 1 month to < 3 months |
| 5 (25) | 3 months to < 6 months |
| 6 (30) | 6 months to ≤ 12 months |
| 1 (5) | > 12 months |
| Neurologic symptoms | |
| 1 (5) | Headache |
| 0 | Seizure |
| 5 (25) | Incontinence (any) |
| 3 (15) | Bladder |
| 0 | Bowel |
| 1 (5) | Both |
| 1 (5) | Unspecified |
| Cognitive symptoms and signs | |
| 11 of 16* (69) | Disorientation |
| 8 (40) | Altered level of arousal |
| 4 (20) | Fluctuating cognition |
| 0 of 16* | Relative sparing of memory |
| 6 (30) | Attention or concentration problem |
| 5 (25) | Aphasia (any) |
| 1 (5) | Dysnomia |
| 1 (5) | Expressive aphasia |
| 0 | Receptive aphasia |
| 0 | Mixed aphasia |
| 3 (15) | Unspecified |
| 1 (5) | Apraxia (excluding constructional) |
| Neuropsychiatric symptoms | |
| 12 (60) | Behavioral change (any) |
| 8 (40) | Disengagement or apathy |
| 5 (25) | Personality change other than apathy: irritability (2 patients), angry outbursts (1), unspecified abnormal behavior (1), unspecified (2) |
| 1 (5) | Agitation |
| 3 (15) | Hallucinations: visual (2 patients), unspecified (1) |
| 4 (20) | Psychiatric symptoms other than hallucinations: depression (2 patients), delusions (1), paranoia with a fear of being burglarized (1) |
| Neurologic signs | |
| 9 (45) | Non-aphasic speech disorder: dysarthria (7 patients), slowed or hesitant speech (1), unspecified (1) |
| 4 (20) | Extrapyramidal signs |
| 6 (30) | Strength abnormality: hemiparesis (3 patients), diffuse weakness (2), monoparesis (1) |
| 3 (15) | Tone abnormality: hypertonia (2 patients), hypotonia (1) |
| 0 | Tremor |
| 4 (20) | Limb ataxia |
| 1 (5) | Myoclonus |
| 13 (65) | Gait disorder: ataxia (4 patients), shuffling (1), ataxia and shuffling (1), imbalance (1), inability to stand (2), unspecified (4) |
| 3 (15) | Eye movement abnormality: unilateral gaze-evoked nystagmus (1 patient), bilateral cranial nerve VI palsies (1), abnormal smooth pursuit and limited upgaze (1) |
| 5 (25) | Frontal release signs: snout (1 patient); grasp (2); grasp, snout, and palmomental (1); snout, palmomental, and glabellar (1) |
| 7 (35) | Cerebellar signs: limb ataxia (1 patient), ataxic gait (3), limb ataxia and ataxic gait (3) |
Not reported in 4 patients. Percentages reflect ratio of known cases.
TABLE 3.
Ancillary Test Results: Patients with Primary Central Nervous System Lymphoma (PCNSL) Presenting as Dementia versus Patients with Other Rapidly Progressive Dementias (RPD), Both Creutzfeldt-Jakob Disease (CJD) and Non-CJD RPD
| PCNSL Current Meta-Analysis 1990–2013 (n = 20) | RPD Cases from Sala et al (2012) 1994–2009 (n = 46) |
P PCNSL vs RPD |
RPD Sala’s Subgroup with Probable or Definite CJD (n = 15) |
P PCNSL vs CJD |
RPD Sala’s Subgroup with Non-CJD Etiologies (n = 31) |
P PCNSL vs Non-CJD |
|
|---|---|---|---|---|---|---|---|
| Imaging* | |||||||
| Normal | 0 of 20 (0%) | 5 of 46 (11%) | 0.31 | 2 of 15 (13%) | 0.18 | 3 of 31 (10%) | 0.27 |
| Atrophy | 1 of 20 (5%) | 15 of 46 (33%) | 0.03 | 2 of 15 (13%) | 0.56 | 13 of 31 (42%) | < 0.01 |
| White matter abnormalities | 16 of 20 (80%) | 13 of 46 (28%) | < 0.01 | 0 of 15 (0%) | < 0.01 | 13 of 31 (42%) | 0.01 |
| Deep gray matter abnormalities | 5 of 20 (25%) | 5 of 46 (11%) | 0.16 | 5 of 15 (33%) | 0.71 | 0 of 31 (0%) | 0.01 |
| Cortical abnormalities | 3 of 20 (15%) | 6 of 46 (13%) | 1.00 | 4 of 15 (27%) | 0.43 | 2 of 31 (6%) | 0.37 |
| Restricted diffusion on diffusion weighted imaging (DWI) sequences† | 0 of 1 | 8 of 23 (35%) | 1.00 | 8 of 9 (89%) | 0.20 | 0 of 14 (0%) | 1.00 |
| Contrast enhancement† | 9 of 18 (50%) | 1 Number of studies performed with contrast: not reported |
Not applicable | 0 Number of studies performed with contrast: not reported |
Not applicable | 1 Number of studies performed with contrast: not reported |
Not applicable |
| Lacunar infarcts | 0 of 20 (0%) | 7 of 46 (15%) | 0.09 | 1 of 15 (7%) | 0.43 | 6 of 31 (19%) | 0.07 |
| Hydrocephalus | 1 of 20 (5%) | 3 of 36 (6%) | 1.00 | 0 of 15 (0%) | 1.00 | 3 of 31 (10%) | 1.00 |
| Electroencephalogram† | |||||||
| Normal | 0 of 4 (0%) | 12 of 38 (32%) | 0.31 | 2 of 14 (14%) | 1.00 | 10 of 24 (42%) | 0.27 |
| Diffuse slowing | 4 of 4 (100%) | 14 of 38 (37%) | 0.03 | 2 of 14 (14%) | < 0.01 | 12 of 24 (50%) | 0.11 |
| Periodic sharp complexes | 0 of 4 (0%) | 10 of 38 (26%) | 0.56 | 10 of 14 (71%) | 0.02 | 0 of 24 (0%) | 1.00 |
| Cerebrospinal fluid† | |||||||
| Normal | 1 of 17 (6%) | 18 of 46 (39%) | 0.01 | 1 of 15 (7%) | 1.00 | 17 of 31 (55%) | < 0.01 |
| Normal except positive for 14-3-3 protein | 1 of 3 (33%) | 13 of 46 (28%) | 1.00 | 10 of 15 (67%) | 0.53 | 3 of 31 (10%) | 0.32 |
| Abnormal and positive for 14-3-3 protein | 2 of 3 (67%) | 4 of 46 (9%) | 0.04 | 3 of 15 (20%) | 0.17 | 1 of 31 (3%) | 0.02 |
| Abnormal but negative for 14-3-3 protein | Unknown | 11 of 46 (24%) | Not applicable | 1 of 15 (7%) | Not applicable | 10 of 31 (32%) | Not applicable |
| Pleocytosis | 6 of 17 (35%) | 2 of 46 (4%) | < 0.01 | 1 of 15 (7%) | 0.09 | 1 of 31 (3%) | 0.01 |
| Elevated protein | 11 of 17 (65%) | 15 of 46 (33%) | 0.04 | 4 of 15 (27%) | 0.04 | 11 of 31 (35%) | 0.07 |
Data are shown as number (%). Significant P values (P < 0.05) are shown in bold type.
Magnetic resonance imaging was performed in 18 of 20 (90%) patients with PCNSL and 40 of 46 (87%) patients with RPD. Computed tomography was performed in the other patients.
Study was not performed in all patients.
For us to count relevant symptoms and signs in the study, patients had to have them between the onset of their illness and the time of their diagnostic evaluation and/or start of treatment. With the exceptions of orientation and memory, if the case report did not explicitly note a neurologic symptom or sign, we presumed that the patient did not have it. For example, if the report did not specify a patient’s having aphasia or a tremor, we presumed that the patient had not had either sign. If the report did not comment on a patient’s orientation and memory, we rated them as unknown and excluded them from that portion of the analysis. We assumed that authors reported their patients’ cases accurately.
For further clinical characterization, we used Fisher’s exact 2-sided test to compare the characteristics of our cases to those of a published series of 49 cases of RPD selected from a CSF 14-3-3 protein registry at a tertiary care hospital in Barcelona, Spain (Sala et al, 2012). Sala and colleagues defined RPD as a dementia that appeared < 1 year after the first cognitive symptom. We chose their study for comparison because it included prevalence of neurocognitive symptoms as well as listings of the individual patients’ age, sex, neurocognitive features, imaging findings, and CSF and EEG abnormalities.
We set statistical significance at P < 0.05.
RESULTS
We analyzed the 20 patients with PCNSL presenting with dementia who met our inclusion criteria. The patients’ median age at time of diagnostic evaluation was 66 years (range 41–81); 70% of the patients were men (Table 2).
TABLE 2.
Neurocognitive Features: Patients with Primary Central Nervous System Lymphoma (PCNSL) Presenting as Dementia versus Patients with Other Rapidly Progressive Dementias (RPD)
| PCNSL Current Meta-Analysis 1990–2013 (n = 20) |
RPD Cases from Sala et al (2012) 1994–2009 (n = 49) |
P PCNSL vs RPD |
|
|---|---|---|---|
| Mean age (years) | 65.75 | 72.46 (n = 46*) | 0.02 |
| Men:women (%) | 14:6 (70:30) | 20:26 (43.5:56.5) (n = 46*) | 0.06 |
| Orientation (% impaired) | 69 (n = 16†) | 88 | 0.12 |
| Memory (% impaired) | 100 (n = 16†) | 69 | 0.01 |
| Behavioral changes (% affected) | 60 | 75 | 0.25 |
| Parkinsonism (% affected) | 20 | 65 | < 0.01 |
| Cerebellar signs (% affected) | 35 | 31 | 0.78 |
| Myoclonus (% affected) | 5 | 65 | < 0.01 |
Significant P values (P < 0.05) are shown in bold type.
Excludes 3 patients with unknown etiology for RPD.
Not reported in 4 of 20 PCNSL cases. Percentages reflect ratio of known cases.
Of the 20 patients, 14 were diagnosed with LC. Seventeen of the 20 patients had diffuse large B cell lymphomas, of which 11 were LC and 1 primary leptomeningeal. The remaining 3 of the 20 patients all had LC: 1 with small B cell lymphoma, 1 with T/natural killer cell lymphoma, and 1 with anaplastic large cell lymphoma.
Neurocognitive Features
Table 1 lists the 20 patients’ neurocognitive features.
Time course
Time from initial symptoms to the evaluation that led to the diagnosis (or attempted diagnosis in those patients whose true diagnosis remained uncertain before death) was < 6 months in 65% of the patients, and within 1 month for 15%.
Neurologic symptoms
Headache was uncommon, reported in only 1 (5%) patient. No patients had seizures.
Cognitive impairment
Of the 16 patients for whom orientation was reported, 11 (69%) were disoriented. The majority of patients were alert; 12 of the 20 (60%) had a normal level of arousal. Memory was impaired in all 16 patients in whom it was reported. Apraxia was reported in only 1 (5%) patient.
Neuropsychiatric symptoms
Apathy was reported in 8 (40%) of the 20 patients. Table 1 itemizes the types of personality changes and other psychiatric symptoms reported.
Neurologic signs
Non-aphasic speech disorders, most often dysarthria and slowed speech, were found in 9 (45%) of the 20 patients. Extrapyramidal signs and parkinsonism were reported in 4 (20%), and 6 (30%) had impaired strength. No one was reported to have tremor. Limb ataxia was noted in 4 (20%), and myoclonus in only 1 (5%). The majority of patients (65%) had abnormal gait.
Imaging Abnormalities
All 20 patients underwent brain imaging: MRI in 18 and computed tomography in 2. Our summary is shown in Table 3 under PCNSL Current Meta-Analysis 1990–2013. None of the scans were normal. Atrophy was reported in 1 patient (5%). White matter abnormalities were reported in 16 patients (80%), including all 14 with LC. Stroke was not reported at all.
DWI sequences were specifically mentioned for only 1 of the 18 MRIs performed, and did not show restricted diffusion. Contrast was administered to 18 of the 20 patients, including 13 of the 14 patients with LC. Contrast enhancement was seen in 9 of those 18 patients (50%), including 4 of the 13 patients with LC (31%).
EEG Abnormalities
EEG was performed in 4 of the 20 patients, and showed generalized diffuse slowing in all 4.
CSF Abnormalities
Lumbar puncture was performed in 17 of the 20 patients. Only 1 of the 17 lumbar punctures was normal. Elevated protein, the most common abnormality, was found in 11 of the 17 patients, followed by pleocytosis in 6.
CSF cytology was negative in 11 of the 17 patients. It showed numerous lymphoid cells without atypia in 3 patients, and atypical cells in 2. One patient had oligoclonal bands, and 3 had 14-3-3 protein.
Treatment and Cognitive Response
Fourteen of the 20 patients were treated for their lymphoma. The effect of treatment on cognitive function was reported for 12 of the 14 patients. Six of the 12 (50%) showed improvement. Of those 6 patients, 3 had been treated with corticosteroids alone, 1 with corticosteroids and radiation therapy, 1 with corticosteroids and azathioprine chemotherapy, and 1 with corticosteroids and chemotherapy in the form of cytosine arabinoside, leucovorin, and intravenous and intrathecal methotrexate.
Of the 6 patients whose cognitive function did not improve with treatment, 2 had been treated with corticosteroids alone, 1 with corticosteroids and radiation therapy, 1 with radiation therapy alone, 1 with high-dose methotrexate chemotherapy alone, and 1 with corticosteroids, cyclophosphamide chemotherapy, and radiation therapy.
Diagnostic Certainty Before Death
Of the 20 patients, 6 (30%) died before a diagnosis could be established; 4 of these 6 patients had LC. The premortem diagnoses considered in the patients with LC were CJD in 1, vasculitis in 1, and either leukoencephalopathy of unknown etiology or CNS sarcoid in 1; no diagnosis was reported for the other patient with LC. For the 2 undiagnosed patients who did not have LC, Alzheimer disease was considered in 1 and nothing was reported for the other.
PCNSL versus Other RPDs
We compared our 20 patients’ neurocognitive features and ancillary test results with those of the case series by Sala et al (2012). We obtained the Sala study data from the Supplemental Table in their article: Supplemental Digital Content 1, http://links.lww.com/WAD/A20.
Sala et al had reported 49 patients with RPD, none caused by PCNSL. Because 3 of Sala’s patients had RPD of unknown etiology, we excluded them from our analyses of demographics, imaging, EEG, and CSF. In the remaining 46 patients, the RPD in 15 had been caused by probable or definite CJD and in 31 by non-CJD etiologies. The non-CJD conditions were neurodegenerative disorders in 18 patients, vascular dementia in 4, toxic-metabolic conditions in 4, a paraneoplastic syndrome in 1, chronic meningoencephalitis in 1, autoimmune Hashimoto encephalopathy in 1, non-convulsive status epilepticus in 1, and chronic hydrocephalus in 1. Tables 2 and 3 compare our 20 patients with PCNSL to Sala’s 46 patients with RPD.
For Sala’s 46 patients with a known cause of RPD (Table 2), the median age of onset was 75.5 years (range 46–85), but younger in the patients with CJD (67 years, range 51–85) than those without CJD (76 years, range 46–84). Men accounted for 43.5% of patients with “all-cause” RPD, 33% of those with CJD, and 48% of those with non-CJD.
We included all 49 of Sala’s patients in the comparison of neurocognitive features because the authors did not specify these features for each patient in the Supplemental Table, but summarized them for the entire series. We found that 88% of the patients with RPD were disoriented, as opposed to 69% of our patients with PCNSL (P = 0.12). For impaired memory, we found a significant difference: 69% of the patients with RPD versus 100% of the patients with PCNSL (P = 0.01). Similar numbers of patients with RPD and PCNSL had behavioral changes (75% versus 60%, respectively) and cerebellar signs (31% versus 35%), but significantly more patients with RPD had parkinsonism (65% versus 20%) and myoclonus (65% versus 5%).
For ancillary tests (Table 3), we analyzed Sala’s 46 patients with RPD of known etiology. All 46 had undergone MRI (87%) or computed tomography (13%). The studies were normal in only 5 (11%), a nonsignificant difference from the 0 normal studies in PCNSL. One third of the 46 patients with RPD had atrophy, including 13 of 31 (42%) of those with non-CJD RPD; both numbers significantly exceeded those in PCNSL (P = 0.03 and P < 0.01, respectively). White matter abnormalities were seen in 13 of the 46 (28%) patients with RPD, all of them in the non-CJD group (13 of 31, 42%); this percentage was still significantly lower than the 80% in PCNSL (P = 0.01).
DWI sequences were performed in 23 of Sala’s 46 patients and were positive in 8 (35%), all in the CJD group. Lacunar infarcts were found in 15% of the 46 patients, mainly in the non-CJD group (19%); no lacunar infarcts were found in PCNSL, but this difference was not statistically significant. Contrast enhancement was reported in 1 patient with non-CJD RPD; the other 45 RPD reports did not specify whether the patients had been given contrast.
On EEG, periodic sharp complexes were found only in the patients with CJD (71%); none were found in 4 tested patients with PCNSL (P = 0.02).
On CSF evaluation, 39% of the total 46 patients with RPD had a normal CSF evaluation, which included a negative test for 14-3-3 protein; all but 1 of these patients (55%) were in the non-CJD group. CSF was normal in significantly more patients in the non-CJD group than in PCNSL (P < 0.01). In contrast, of patients whose only CSF abnormality was 14-3-3 protein, 67% had CJD versus 10% of the non-CJD group. Pleocytosis was reported in only 4% of the patients with all-cause RPD, as against 35% of the patients with PCNSL (P < 0.01). Likewise, elevated CSF protein was found in only 33% of patients with all-cause RPD, as opposed to 65% of patients with PCNSL (P = 0.04).
DISCUSSION
Patients with cognitive complaints should be thoroughly evaluated for reversible dementias. Neoplastic dementia presenting as an RPD can be treatable if the clinician considers and diagnoses it early. Neoplastic dementia can usually be identified on imaging if there is an obvious contrast-enhancing tumor; however, a lymphomatous cause of dementia, especially LC, can be difficult to differentiate from non-neoplastic etiologies. Being able to distinguish lymphomatous dementia from other causes of RPD through a signature pattern of neurocognitive impairment could help clinicians consider PCNSL earlier and allow proper workup with ancillary testing, just as the signature patterns of neurocognitive impairment in the neurodegenerative disorders allow for differentiation and diagnosis.
Paterson et al (2012) found the most common cause of RPD to be prion disease, accounting for 62% of referrals to their dementia center at the University of California San Francisco. Sala et al (2012) found the most common cause of RPD at their hospital in Barcelona, Spain, to be neurodegenerative disease (36.8%), followed by prion disease (30.6%).
In our meta-analysis of case reports, we focused exclusively on patients with dementia (as defined by each of the 16 sets of authors) caused by PCNSL. The time from symptom onset to dementia in all 20 of our patients was within 1 to 2 years. Thus, by Geschwind’s (2010) definition these patients all had RPD, with a course of < 6 months in most and ≤ 1 year in all but 1.
We compared demographics between our 20 patients and the 46 patients reported by Sala et al (2012). Age at onset in the PCNSL group was younger than in both the all-cause RPD group and its subgroup with non-CJD, but was similar to the subgroup with CJD. As for the sex distribution, we found more men than women affected with PCNSL, in agreement with prior reports (Villano et al, 2011). Men and women were equally affected in all-cause RPD as well as both the CJD and non-CJD subgroups.
Compared to the 46 total patients with RPD, our patients with PCNSL were significantly more likely to have memory impairment, less likely to have myoclonus or parkinsonism, and about as likely to have cerebellar signs and behavioral changes.
Although none of our patients with PCNSL had normal imaging studies, the difference between them and the 46 patients with RPD who had normal studies was not statistically significant. White matter abnormalities were significantly more common in PCNSL than all-cause RPD, and were not seen in any of the patients with CJD.
Contrast enhancement was found in only half of the patients with PCNSL who had received contrast, and in fewer than one third of the patients with LC. Only 1 patient among the 46 with RPD was reported to have been given contrast; this patient, who had chronic meningoencephalitis, did show enhancement.
Although many patients with PCNSL show restricted diffusion on DWI, only 1 of our 18 patients who underwent MRI was reported to have had DWI sequences acquired. Therefore, we cannot draw any conclusions about the comparative frequency of DWI changes in PCNSL.
EEG in the PCNSL group showed only diffuse slowing. By contrast, EEG showed periodic sharp complexes in 71% of the patients with CJD.
The PCNSL group had significantly more CSF pleocytosis than the all-cause RPD group and its non-CJD subgroup, and significantly more elevated protein than the all-cause RPD group and its CJD subgroup. We cannot draw conclusions about relative frequencies of 14-3-3 protein because, although all 46 patients with RPD were tested for this protein, only 3 patients with PCNSL were known to have been tested (and all 3 had the protein).
Our study is limited by its retrospective nature, analyzing others’ case reports. We had to rely on the authors’ descriptions of their patients’ neurocognitive features, and in several instances we had to assume that a patient did not have a particular symptom or sign because the authors had not specified it. We could not report on executive function because few studies explicitly commented on it. Further, we could not confirm the accuracy of the imaging or CSF findings, relying solely on the authors’ descriptions.
There is an inherent bias toward reporting interesting or atypical cases. Most of our patients had LC, a rare variant of the already uncommon PCNSL. Relying on case reports alone may misrepresent the frequencies of typical deficits. Our findings should be confirmed either by a prospective study or by a retrospective analysis of a large number of patients with RPD, including a substantial number with lymphomatous dementia, presenting to a particular center.
Finally, because Sala et al (2012) selected their cases from a 14-3-3 protein registry, CJD may have been over-represented in their series. Other etiologies of RPD may have been under-represented; for example, only 1 case of autoimmune encephalitis was included. Thus, we may have compared PCNSL to an unrepresentative sample of other RPDs.
Despite these limitations, ours is the only systematic assessment of the neurocognitive deficits in dementia caused by PCNSL. We found that the diagnosis was missed in 30% of the 20 patients whom we reviewed. Considering that PCNSL is a treatable cancer and that 50% of our patients had at least partial reversal of their dementia when their cancer was treated, it is important to improve diagnostic speed and accuracy.
In conclusion, as summarized in Table 4, we found that patients with RPD caused by PCNSL commonly present alert but with disorientation, impaired memory, apathy, a non-aphasic speech disorder, and abnormal gait. Patients do not present with headache, apraxia, myoclonus, or seizure. Imaging shows abnormalities including white matter changes (which may appear nonspecific), but rarely atrophy. CSF analysis reveals pleocytosis and elevated protein. We suggest that clinicians seek this profile in patients with RPD. They may have an underlying lymphomatous dementia that, if diagnosed early, is potentially reversible.
TABLE 4.
Most Common Abnormalities in Patients with Rapidly Progressive Dementia (RPD) Caused by Primary Central Nervous System Lymphoma (PCNSL) versus Creutzfeldt-Jakob Disease (CJD)
| PCNSL | CJD | |
|---|---|---|
| People most often affected | Men in mid-60s | Men or women in mid-60s |
| Presenting features | Alertness, disorientation, and impaired memory, with a behavioral change (especially apathy), dysarthria, and a gait disorder | Disorientation with visual symptoms, behavioral changes, parkinsonism, myoclonus, and cerebellar signs |
| Magnetic resonance imaging | Classic findings: periventricular contrast-enhancing lesion(s); in some patients, restricted diffusion on diffusion weighted imaging White matter lesions in lymphomatosis cerebri Atrophy uncommon |
Restricted diffusion on diffusion weighted imaging and T2 hyperintensities in cortical ribbon and deep gray matter |
| Electroencephalogram | Diffuse slowing in some patients | Periodic epileptiform discharges of 1 Hz; focal slowing; no early changes in some patients |
| Cerebrospinal fluid | Pleocytosis (often lymphocytic); elevated protein; variable cytology: negative, atypical, positive, or showing numerous lymphoid cells | Normal except for 14-3-3 protein; elevated total tau in some patients; less commonly, pleocytosis or elevated protein |
Acknowledgments
Supported in part by NIA Grant 5R01AG034499 (M.F. Mendez).
Glossary
- CJD
Creutzfeldt-Jakob disease
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DWI
diffusion weighted imaging
- EEG
electroencephalogram
- LC
lymphomatosis cerebri
- MRI
magnetic resonance imaging
- PCNSL
primary central nervous system lymphoma
- RPD
rapidly progressive dementia
Footnotes
The authors declare no conflicts of interest.
References
- Bakshi R, Mazziotta JC, Mischel PS, et al. Lymphomatosis cerebri presenting as a rapidly progressive dementia: clinical, neuroimaging and pathologic findings. Dement Geriatr Cogn Disord. 1999;10:152–157. doi: 10.1159/000017116. [DOI] [PubMed] [Google Scholar]
- Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- Carlson BA. Rapidly progressive dementia caused by nonenhancing primary lymphoma of the central nervous system. AJNR Am J Neuroradiol. 1996;17:1695–1697. [PMC free article] [PubMed] [Google Scholar]
- Degnan AJ, Levy LM. Neuroimaging of rapidly progressive dementias, part 1: neurodegenerative etiologies. AJNR Am J Neuroradiol. 2014a;35:419–423. doi: 10.3174/ajnr.A3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan AJ, Levy LM. Neuroimaging of rapidly progressive dementias, part 2: prion, inflammatory, neoplastic, and other etiologies. AJNR Am J Neuroradiol. 2014b;35:424–431. doi: 10.3174/ajnr.A3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler AF, Batchelor TT. Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus. 2006;21:E15. doi: 10.3171/foc.2006.21.5.16. [DOI] [PubMed] [Google Scholar]
- Geschwind MD. Rapidly progressive dementia: prion diseases and other rapid dementias. Continuum (Minneap Minn) 2010;16(2 Dementia):31–56. doi: 10.1212/01.CON.0000368211.79211.4c. [DOI] [PubMed] [Google Scholar]
- Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64:97–108. doi: 10.1002/ana.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Shibuya M, Hata T, et al. A case of ‘lymphomatosis cerebri’ diagnosed in an early phase and treated by whole brain radiation: case report and literature review. J Neurooncol. 2008;86:83–88. doi: 10.1007/s11060-007-9437-9. [DOI] [PubMed] [Google Scholar]
- Kiewe P, Fischer L, Martus P, et al. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010;12:409–417. doi: 10.1093/neuonc/nop053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kang JK, Lee SA. Hydrocephalus and hyponatremia as the presenting manifestations of primary CNS lymphoma. Eur Neurol. 2006;55:39–41. doi: 10.1159/000091425. [DOI] [PubMed] [Google Scholar]
- Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59:1557–1562. doi: 10.1212/01.wnl.0000034256.20173.ea. [DOI] [PubMed] [Google Scholar]
- Leschziner G, Rudge P, Lucas S, et al. Lymphomatosis cerebri presenting as a rapidly progressive dementia with a high methylmalonic acid. J Neurol. 2011;258:1489–1493. doi: 10.1007/s00415-011-5965-5. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Ding X, Matschke J, et al. Dementia and leukoencephalopathy due to lymphomatosis cerebri. J Neurol Neurosurg Psychiatry. 2007;78:777–778. doi: 10.1136/jnnp.2006.106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosevic B, Knoflach M, Furtner M, et al. Progressive leukoencephalopathy caused by primary CNS lymphoma. J Clin Pathol. 2010;63:359–361. doi: 10.1136/jcp.2009.072082. [DOI] [PubMed] [Google Scholar]
- Nakasu Y, Nakasu S, Isozumi T, et al. Periventricular spread of malignant lymphoma: report of two cases. Nihon Geka Hokan. 1990;59:323–329. [PubMed] [Google Scholar]
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Pandit L, Chickabasaviah Y, Raghothaman A, et al. Lymhomatosis cerebri–a rare cause of leukoencephalopathy. J Neurol Sci. 2010;293:122–124. doi: 10.1016/j.jns.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Paterson RW, Takada LT, Geschwind MD. Diagnosis and treatment of rapidly progressive dementias. Neurol Clin Pract. 2012;2:187–200. doi: 10.1212/CPJ.0b013e31826b2ae8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins KE, Kleinschmidt-DeMasters BK, Corboy JR, et al. Lymphomatosis cerebri as a cause of white matter dementia. Hum Pathol. 2005;36:282–290. doi: 10.1016/j.humpath.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Sala I, Marquie M, Sanchez-Saudinos MB, et al. Rapidly progressive dementia: experience in a tertiary care medical center. Alzheimer Dis Assoc Disord. 2012;26:267–271. doi: 10.1097/WAD.0b013e3182368ed4. [DOI] [PubMed] [Google Scholar]
- Shah R, Bag AK, Chapman PR, et al. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol. 2010;65:431–439. doi: 10.1016/j.crad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Sugie M, Ishihara K, Kato H, et al. Primary central nervous system lymphoma initially mimicking lymphomatosis cerebri: an autopsy case report. Neuropathology. 2009;29:704–707. doi: 10.1111/j.1440-1789.2009.01004.x. [DOI] [PubMed] [Google Scholar]
- Sugino T, Mikami T, Akiyama Y, et al. Primary central nervous system anaplastic large-cell lymphoma mimicking lymphomatosis cerebri. Brain Tumor Pathol. 2013;30:61–65. doi: 10.1007/s10014-012-0094-0. [DOI] [PubMed] [Google Scholar]
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary cns lymphoma. Br J Cancer. 2011;105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital A, Sibon I. A 64-year-old woman with progressive dementia and leukoencephalopathy. Brain Pathol. 2007;17:117–118. 121. doi: 10.1111/j.1750-3639.2007.00044_2.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Vinters HV, Koretz B, et al. Lymphomatosis cerebri presenting as rapidly progressive dementia. Neurologist. 2007;13:150–153. doi: 10.1097/01.nrl.0000254706.85609.95. [DOI] [PubMed] [Google Scholar]
- Widdess-Walsh P, Nair A, Staugaitis SM. October 2004: a 49-year-old man with progressive dementia. Brain Pathol. 2005;15:167–168. 173. doi: 10.1111/j.1750-3639.2005.tb00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarabi CM, Parker JC, Jr, Wasylenko M. Primary cerebral lymphoma manifested by dementia. South Med J. 1992;85:1249–1251. doi: 10.1097/00007611-199212000-00023. [DOI] [PubMed] [Google Scholar]