Abstract
A glowing new era in cancer surgery may be dawning. Using fluorescently labelled markers, surgical molecular navigation means that tumours and nerves can be displayed in real time intra-operatively in contrasting pseudocolours, which allows more complete tumour resection while preserving important structures. These advances can potentially cause a paradigm shift in cancer surgery, improving patient outcome and decreasing overall health-care costs.
The primary treatment modality for most solid tumours is surgery1. If all the cancer cells are removed by surgery, the patient is cured of that cancer. The presence or absence of remaining tumour cells in the area surrounding the resection, also known as the surgical margin, is usually considered one of the strongest predictors of tumour recurrence and survival. Positive margins, defined as tumour cells being present at the cut edge of the surgical specimen, are associated with increased local recurrence and indicate poor prognoses for patients with head and neck cancer2, breast cancer3,4, non-small-cell lung cancer5, colorectal cancer6 and urogenital tract cancer7,8. In most cases, the poorer outcome as a result of positive surgical margins is not mitigated by salvage surgery (that is, re-excision of the positive margin) or by adjuvant chemotherapy and/or radiotherapy2–8. The reason for this observation is probably multifactorial and is related in part to the difficulty in identifying the residual cancer during repeat surgery.
Besides management of the primary tumour, treatment of metastatic disease is integral to cancer therapy and prognosis. Evaluation of metastases often requires surgical removal of all anatomically susceptible lymph nodes for ex vivo pathological examination. In the selected clinical situations described below, the sentinel lymph node (SLN; which is the first draining lymph node for a given anatomical site) is removed and examined by the pathologist for evidence of cancer invasion. If there is no cancer invasion of the SLN, the patient is spared from having the remainder of the lymph nodes in that region removed. Compared with complete removal of all susceptible lymph nodes, such as during axillary lymph node dissection for breast cancer, SLN surgery is considerably shorter in duration and carries a lower risk of lymphoedema (prolonged swelling) and nerve injury (resulting in decreased movement or sensation)9 because the extent of surgical dissection is more limited. Although the use of SLN dissection has greatly reduced the number of patients undergoing unnecessary lymph node removal, it is widely used only for breast cancer9 and melanoma10. The use of SLN dissection has not been adopted as standard of care for surgical management of other cancers. There are various reasons for this, which include the more complex lymphatic anatomy of other anatomical sites such as the pelvis and retroperitoneum. Furthermore, even when SLN dissection can be used, current detection methods give no information regarding cancer invasion into the lymph node at the time of surgery. These detection methods include the use of fluorescent dyes as markers of lymphatic flow (such as indocyanine green (ICG))11,12 or for the detection of reticuloendothelial-cell-specific receptor binding (such as technetium-99m tilmanocept)13. Instead, the SLN has to be surgically removed and sent to the pathology laboratory for sectioning and histological evaluation. Either the surgical team waits for the verdict while the patient is under anaesthesia, or the patient has to be brought back for removal of additional lymph nodes if the SLN is positive for cancer.
Although cure is the primary goal of cancer surgery, preservation of important structures such as nerves, blood vessels, ureters and bile ducts is equally crucial in achieving optimal patient outcome. Although surgery has advanced substantially in recent years to decrease patient morbidity through innovations in endoscopic, laparoscopic and robotic technologies, it is still largely based on anatomy as seen by the operating surgeon with white-light reflectance. Visually, different tissues in the body appear as various shades of white to pink (bone, nerves, cartilage, fat, connective tissue and muscle) or red to deep-red (blood vessels and more easily visible organs such as the liver, kidney and spleen), which limits easy visual distinction of the boundaries of each tissue type. Furthermore, working through an interface with cameras and instruments inserted into body cavities through narrow conduits has also sacrificed tactile feedback of directly working with tissue and dissection along tissue planes. Thus a medical speciality that is traditionally dependent on touch becomes even more dependent on vision. Enhancing the visual differences between tissues by using fluorescent probes based on structure or disease could be equated to colour-coding the surgical field. This type of structure and disease mapping would remove potential ambiguity during surgical dissection — in which most tissues are varying shades of white, pink and red when viewed using white-light reflectance alone — thus leading to decreased morbidity and surgical duration. Furthermore, the use of fluorescence to highlight anatomy or disease states during surgery has the added advantage of allowing the operating surgeon to visualize the fluorescence signal even if there is overlying tissue; that is, detecting a small area of residual cancer that is covered by normal overlying tissue or identifying a nerve that is buried beneath the surface before the dissection gets close enough to injure it. There have been numerous studies documenting the steep learning curve that is required for physicians to translate surgical skills between open and minimally invasive surgery14–16. Any real-time improvement in the intra-operative differentiation between different tissue types — including the differentiation between tumour and adjacent normal tissue and between nerve and adjacent non-nerve structures — would represent a considerable advance. Surgery is unsurpassed at overcoming intratumour microheterogeneity, at bypassing molecular resistance mechanisms and at allowing clinical judgment to ignore off-target uptake. Advances in fluorescence imaging using targeted probes to enhance the visual capability of the operating surgeon beyond that of white-light reflectance provide a major opportunity to improve outcomes. Better surgery might reduce chronic dosing with chemotherapy agents (which are usually administered after surgery), thus minimizing long-term toxicity and costs to the overall health-care system.
Non-optical technologies
There are ingenious and valuable non-optical technologies for intra-operative guidance, such as anatomical tracing of proximal draining lymph nodes (SLNs) by injection of technetium-99m colloid, radio-frequency spectroscopy17–19, or electromyographic (EMG) stimulation of motor nerves. However, these techniques carry their own risks and only sample radio activity, di-electric properties or muscle response intermittently at one location, and thus lack the wide-field coverage, spatial resolution and speed of optical imaging. Intra-operative magnetic resonance imaging (MRI) or ultrasound provide moderate-resolution images, which are challenging to register in real time with the conventional view that guides surgeons while they dissect.
Optical technologies
Endogenous contrast
Optical imaging techniques can be divided between those working on unstained tissue (endo genous contrast) versus those requiring the administration of extrinsic contrast agents. Endogenous contrast mechanisms include alterations in autofluorescence, Raman scattering, infrared reflectivity and micro-anatomical cyto-architecture20,21. The biggest problems with endogenous contrast are that it is hard to find robust spectroscopic differences between tumour and normal tissue, and that high-resolution microscopy is limited to small sample regions and does not provide a comprehensive, real-time view of the surgical field. Also, it is difficult to relate endogenous contrast to our increasing knowledge about the specific molecules and mechanisms that critically distinguish malignant from normal tissue. Thus, the field of intrinsic imaging tends to attract optical engineers but repel biochemists.
Extrinsic contrast agents
Extrinsic contrast agents inevitably incur costs for the development and testing of efficacy versus potential side effects, and require regulatory approval, so they must greatly increase image quality and diagnostic accuracy to be justified. Detection of absorbance requires high dye concentrations that are attainable only using anatomical stains such as isosulphan blue or ink, so imaging of molecular targets generally relies on fluorescence, which can be detected at much lower probe concentrations and can report molecular events by a wider variety of spectroscopic mechanisms. Fluorescence instrumentation is fairly inexpensive, simple and adaptable to endoscopy and robotic surgery. Fields of view from single cells up to the entire surgical bed can be imaged in real time and integrated (that is, co-registered in a video screen) with the conventional view seen by white-light illumination. The limited depth penetration of fluorescence imaging — which prevents whole-body scanning, particularly in larger animals such as humans — is less of a drawback during surgery, in which overlying tissues are progressively reflected or resected. Currently the only fluorescent probes that are used to assist intra-operative dissection are the untargeted dyes ICG11,12 and fluorescein sodium22,23 (TABLE 1). Fluorescein sodium is approved for diagnostic angiography or angioscopy of the retina and iris vasculature. ICG is approved for determining cardiac output, hepatic function and liver blood flow and for ophthalmic angiography. Both of these entities are sometimes used ‘off-label’ to visualize structures that are filled with fluids such as lymph, cerebrospinal fluid or urine. The nuclear stain acriflavine observed at high magnification can histologically differentiate between normal and oral squamous cell carcinoma in ex vivo specimens24. Likewise, gliomas in animal models and freshly resected human glioma specimens can be distinguished from normal tissue25. However, acriflavine (the predominant component of which is proflavine) is a DNA-intercalator and mutagen and has not been approved by the US Food and Drug Administration (FDA) for administration to patients, so its application may be limited to freshly resected tissue26.
Table 1.
Extrinsic fluorescent contrast agents
| Name | Emission wavelength | FDA approved? | EMA approved? | Whole body? | Details |
|---|---|---|---|---|---|
| Non-targeted dyes | |||||
| ICG | 820 nm | Yes | Yes | No | FDA and EMA approved for injection, indicated for determining cardiac output, hepatic function and liver blood flow and for ophthalmic angiography. Off-label or research use to monitor fluid (for example, blood, lymph, cerebrospinal fluid or urine)-filled structures or as a vascular, renal or excretory pathway contrast agent11,12 |
| Fluorescein sodium | 520 nm | Yes | Yes | No | FDA and EMA approved for injection, indicated for diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature. Off-label or research use to monitor fluid (for example, blood, lymph, cerebrospinal fluid or urine)-filled structures or as a vascular, renal or excretory pathway contrast agent23,24 |
| Methylene blue | 680 nm | Yes | No | No | FDA approved for injection, indicated for drug-induced methaemoglobinaemia. Research use as an intraluminal gastrointestinal tract contrast agent74 |
| Acriflavine | 510 nm | No | No | No | Not FDA or EMA approved. It is a DNA intercalator and nuclear stain21,25,26 |
| Amino acids and peptides | |||||
| 5-ALA and its esters | 635 nm | Yes | Yes | No | FDA approved as a topical solution. Aminolevulinic acid hydrochloride as a topical solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is indicated for the treatment of minimally to moderately thick actinic keratoses (grade 1 or 2) of the face or scalp. EMA approved 5-ALA as an orally administered drug. 5-ALA is metabolized by certain tumours including glioblastoma into protoporphyrin IX, which is fluorescent48,49 |
| 5-ALA | Yes | Yes | No | Mechanism of accumulation by tumours is unclear. Heterogeneous accumulation in tumours | |
| cRGD | Varies | No | No | Maybe | Binds to integrins present on certain tumour cells64–66 |
| Folate | Varies | No | No | Maybe | FDA approved in combination with ethinyl oestradiol and drospirenone in an oral formulation for contraception. Also available as a dietary supplement. Binds to FRα, which is overexpressed by certain cancer cells47 |
| Chlorotoxin | Varies | No | No | Maybe | Unclear mechanism of targeting, which may involve MMP2 (REFS 53,54,62,63) |
| Cyclic RPMC | Varies | No | No | Maybe | RPMC-F peptide may bind to an integrin, specifically α5β161 |
| NP41 | Varies | No | No | Maybe | Binds to the extracellular matrix of the epineurium, perineurium and endoneurium of nerves57,67 |
| BMB | 540 nm (excitation at 360 nm) | No | No | No | Binds to myelin, crosses the BBB, cannot detect non-myelinated nerves56 |
| GE3082 | 600 nm (excitation at 360 nm) | No | No | No | Binds to myelin, crosses the BBB, cannot detect non-myelinated nerves68 |
| Synthetic macromolecules | |||||
| Technetium- 99m tilmanocept | Varies | Yes | Pending | No | Technetium-99m tilmanocept injection is a radioactive diagnostic agent indicated for lymphatic mapping with a hand-held γ-ray counter to assist in the localization of lymph nodes that drain a primary tumour site in patients with breast cancer or melanoma. Binds to mannose receptors of lymphatic-resident reticuloendothelial cells13 |
| ACPP | Varies | No | No | Yes | Uses protease activity present in the microenvironment of tumours and atherosclerotic plaques to accumulate fluorescence. May be combined with chemotherapeutic or photodynamic agents34,36,37,39,40,55 |
| ProSense MMPSense |
700 nm 780 nm |
No | No | No | Uses protease activity present in the microenvironment of tumours and atherosclerotic plaques to dequench fluorescence. No mechanism for preferential intracellular accumulation60 |
| GB119 | Cy5 (680 nm) | No | No | No | Uses cathepsin protease activity present in the tumour microenvironment to dequench fluorescence. No mechanism for intracellular accumulation33,59 |
| BMV083 | Cy5 (680 nm) | No | No | No | Non-peptidic scaffold-based molecule that uses cathepsin-S protease activity in the tumour microenvironment to dequench fluorescence. No mechanism for intracellular accumulation34 |
| γGlu-HMRG | 515–550 nm | No | No | No | Topically active fluorogenic substrate for GGT46,73 |
| Antibodies | |||||
| Anti-CA19-9 | Varies | No | No | Maybe | Binds to CA19-9, which is overexpressed in certain cancers43 |
| Anti-CEA | Varies | No | No | Maybe | Binds to CEA, which is overexpressed in certain cancers42 |
| Anti-EPCAM | Varies | No | No | Maybe | Binds to EPCAM, which is overexpressed in certain cancers44 |
| Anti-PSMA | Varies | No | No | Maybe | Binds to PSMA, which is overexpressed in certain cancers41 |
| Anti-EGFR | Varies | No | No | Maybe | Binds to EGFR, which is overexpressed in certain cancers45. Specific formulations are FDA and EMA approved; others are used either in clinical trials or for research only. Cetuximab is an EGFR antagonist indicated for the treatment of cancer of the head and neck and of colorectal cancer |
5-ALA, 5-aminolevulenic acid; ABP, activity-based probes; ACPP, activatable cell-penetrating peptide; BBB, blood–brain barrier; BMB, 4,4′-[(2-methoxy-1,4-phenylene)di-(1E)-2,1-ethenediyl]bis-benzenamine; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; cRGD, cyclic Arg–Gly–Asp peptide; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; EPCAM, epithelial cell adhesion molecule; FDA, US Food and Drug Administration; FRα, folate receptor-α; GE3082, 4-[(1E)-2-[4-[(1E)-2-[4-aminophenyl]ethenyl]-3-methoxyphenyl]ethenyl]-benzonitrile; γGlu-HMRG, γ-glutamyl hydroxymethyl rhodamine green; GGT, γ-glutamyltranspeptidase; ICG, indocyanine green; MMP2, matrix metalloproteinase 2; NIH, US National Institutes of Health; NP41, nerve peptide 41; PSMA, prostate-specific membrane antigen; RPMC-F, fluorescently labelled peptide containing the sequence Arg–Pro–Met–Cys.
This Opinion article focuses on surgery guided by extrinsic fluorescent probes that are targeted to molecular features that distinguish different tissues, for example, diseased versus healthy. In our opinion, such molecules offer the greatest rewards for future development. We will use the term ‘live molecular navigation’ (LMN) to indicate the in vivo imaging of targeted probes (as opposed to ex vivo imaging of resected specimens) in the context of fluorescence-guided surgery (FGS). FGS requires the use of a fluorescent molecule for imaging or visualization, and LMN requires molecules that not only specifically localize to target tissues but also that can do so while the tissue is attached and perfused in the living patient, rather than following tissue excision. Because this field is progressing rapidly, we summarize current probes in TABLE 1 and instrumentation in TABLE 2, leaving most of our discussion to more general criteria that we believe will guide future developments.
Table 2.
Instrumentation for fluorescence-guided surgery
| Category | Name | Fluorescence capability | FDA approved? | EMA approved? |
|---|---|---|---|---|
| Open-field surgery | SurgOptix T3-platform | 520 nm | Clinical trial | Clinical trial |
| FLARE | 820 nm | Clinical trial | Clinical trial | |
| Multispectral fluorescence camera system47 | 520 nm | Clinical trial | Clinical trial | |
| ArteMIS | 400–1000 nm | Clinical trial | Yes | |
| Fluoptics | 520 nm; 670 nm | No | ? | |
| SPY imaging system22 | 820 nm | Yes | Yes | |
| Hamamatsu PDE | 820 nm | Yes | ? | |
| Microscopic | Zeiss Pentero 900 | 560 nm; 635 nm; 820 nm | Yes | Yes |
| Leica OH5 | 635 nm; 820 nm | Yes | Yes | |
| Robotic | da Vinci | 820 nm | Yes | Yes |
| Endoscopic | ArteMIS | 400–1000 nm | ? | ? |
| PINPOINT® endoscopic fluorescence imaging system | 820 nm | Yes | Yes | |
| Olympus | Autofluorescence | Yes | Yes | |
| Velscope | Autofluorescence | Yes | Yes |
EMA, European Medicines Agency; FDA, US Food and Drug Administration; FLARE, Fluorescence-Assistance Resection and Exploration; PDE, Photodynamic Eye.
When is molecular guidance needed?
Which types of surgery?
Although surgery is the first-line treatment for the majority of solid tumours, not every tumour resection requires precise margin identification to achieve cure. For example, clear surgical margins are relatively easy to achieve for tumours that are well delineated (for example, early-stage skin cancers), and are not crucial for tumours that have good adjuvant therapy (for example, thyroid tumours that are treatable using 131I). By contrast, surgery to excise tumours that are difficult to differentiate from adjacent normal tissue (such as for breast cancer) and/or are next to crucial structures (such as for brain tumours) will benefit greatly from probes that augment their visual differences. Thus, the incidence of positive margins and isolatable metastases, such as in nearby lymph nodes, and their impact on overall prognosis are two crucial factors that need to be considered before the use of LMN. FGS with LMN will be most helpful to surgeries that currently have moderate cure rates that are dependent on clinical luck and surgical skill. Operations that are invariably successful or futile are less likely to benefit. Examples of surgeries that have high local recurrence (that is, recurrence at the site of the initial surgery) or positive margin rates are resections of malignant gliomas (80%)27, radical prostatectomy for prostate cancer (10–20%)28–30 and conservation surgery for breast cancer (20–40%)31,32. In these situations, a fluorescently labelled, molecularly targeted probe would provide real-time, intra-operative distinction of the molecular edge between cancer and adjacent normal tissue, potentially decreasing the incidence of leaving residual cancer cells behind (that is, positive margins) (FIG. 1). Fluorescently labelled protease-sensitive probes have improved tumour detection in animal models33–35. In particular, activatable cell-penetrating peptides that are responsive to tumour-associated matrix metalloproteinases (MMPs) have been shown to decrease the incidence of positive margins and increase tumour-free survival36–40 when used to guide the resection of breast cancers and melanomas in mice. Several fluorophore-conjugated antibodies have improved tumour detection in animal models41–44 and in freshly resected human specimens45. Ovarian cancers overexpressing γ-glutamyltranspeptidase (GGT) have been detected in mouse models by a topically applied, fluorogenic substrate for this enzyme46. In human patients, fluorescein-conjugated folate lights up ovarian cancers that overexpress folate receptor-α (FRα)47 (FIG. 1). So far, the only example for which clinical FGS improved tumour-free survival, albeit modest and short-term, was after administration of 5-aminolevulinic acid (5-ALA) to elevate fluorescent protoporphyrin IX in glioblastoma (an aggressive form of brain cancer)48,49. We speculate that FGS in those early studies may have been limited by the variability of protoporphyrin IX upregulation between tumours, the reliance on the surgeon’s own colour vision to distinguish short-wavelength reflectance and scattering from long-wavelength fluorescence, and the aggressiveness of the glioblastoma that enables recurrence from very small invasive tendrils.
Figure 1. Live molecular detection of surgical margins.
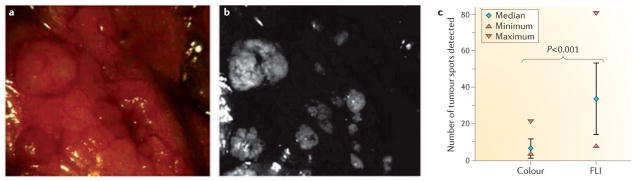
a,b | Colour image captured using white light reflectance (a) with the corresponding tumour-specific fluorescence image (b) of a representative area in the abdominal cavity. c | Quantitation of the number of tumour spots using both methods. Scoring was based on three different colour images (median 7 tumour spots, range 4–22) and their corresponding fluorescence images (FLI) (median 34 tumour spots, range 8–81); P < 0.001 by five independent surgeons47. Figure is reproduced, with permission, from REF. 47 © (2011) Macmillan Publishers Ltd. All rights reserved.
Which patients are candidates?
The next consideration in FGS with LMN is heterogeneity between patients. For example, it has been shown that 72% of primary and 81% of recurrent epithelial ovarian carcinomas express FRα50. 86% of grade III and 63% of grade IV astrocytomas showed immunocytochemical staining for GGT51. 57% of grade III or grade IV but only 6% of grade II gliomas were positive for protoporphyrin IX production after dosing with the metabolic precursor 5-ALA52. This heterogeneity may also contribute to the relatively modest improvement in tumour-free survival that was seen when 5-ALA was used to guide glioblastoma resection48,49. A personalized assay that verifies the presence or activity of the molecular target in any given patient being considered for FGS with LMN will be valuable or even essential for confidence in intra-operative fluorescence guidance.
Examples of such assays to document biochemical activity before surgery include non-invasive scanning using a closely related molecular imaging agent, or an ex vivo assay on patient-derived tissues. Examples of molecularly related non-invasive imaging include positron-emission tomography (PET) of radiolabelled antibodies or MRI of chlorotoxin53,54 or activatable cell-penetrating peptides55. Such whole-body scanning would aid early detection when tumours are still operable, assist with pre-operative staging and therapy planning, and facilitate postoperative monitoring and adjuvant treatment. Pre-operative staging would exclude patients whose tumours do not accumulate the targeted agent or have metastasized beyond eligibility for resection. To maximize comparability between PET, MRI or intra-operative imaging, and to minimize the number of injections, the molecular probe should ideally carry labels for both the whole-body modality and fluorescence.
Preservation of normal tissues
Surgical outcome depends both on removing the cancer and limiting patient morbidity and iatrogenic injury. Nerves are particularly important because they can be very difficult to see using white-light imaging. Damage to motor nerves inevitably leads to weakness or paralysis, whereas damage to sensory nerves leads to loss of sensation. Regeneration of nerves is never fast and often fails altogether. The earliest approach to nerve-highlighting agents was based on small, hydrophobic molecules that bind to myelin (which forms a sheath around many axons)56. These dyes cross the blood–brain barrier and can label both central and peripheral myelinated nerves, but are likely to miss clinically important unmyelinated nerves, such as the autonomic nerves that are responsible for preserving urinary continence and erectile function after radical prostatectomy. A more recent approach has been to use phage display to find peptides with affinity for peripheral nerves (FIG. 2), then label those peptides with fluorescent dyes57. The resulting probes allow the targeting peptide and fluorophore to be optimized separately. They highlight both myelinated and non-myelinated peripheral nerves including those to be spared during prostatectomy, but they do not stain tracts within the central nervous system. These probes have only been tested in animals and have not yet been used in humans.
Figure 2. Live molecular detection of nerves.
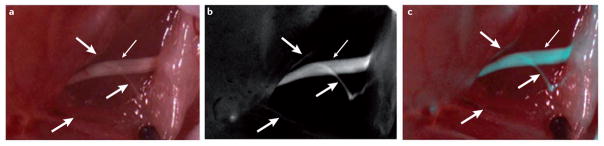
a | A white-light reflectance image showing a sciatic nerve branching within muscle planes. The main trunk of the nerve (thin white arrow) is clearly visible but the smaller branches (thick white arrows) are much more difficult to discriminate from surrounding tissue. b | A fluorescence image following systemic injection of a nerve-highlighting agent showing clear visualization of both the main nerve trunk and small branches57. c | An overlay of both fluorescence (pseudocoloured in blue) and white-light reflectance images.
Lymphatics, ureters, bile ducts and blood vessels conduct fluids and are therefore visualizable using untargeted tracers such as ICG. The main challenge with lymphatics is to distinguish nodes containing metastatic cells from healthy nodes, because unnecessary resection of healthy nodes carries considerable risk of subsequent lymphoedema. However, we believe that it will be more valuable to distinguish metastases in lymph nodes using a cancer-targeting probe than to highlight lymphatic anatomy without the ability to distinguish healthy from invaded nodes.
Desirable characteristics of FGS probes
Dosing
Because of the logistics that are associated with surgery scheduling, fluorescence imaging agents for a given surgical procedure should ideally be administered topically during the surgery itself, or intravenously 0.5–2 hours before the start of the operation. Contrast should ideally last the duration of the surgery or at least during the period of tumour resection and margin evaluation (typically 1–4 hours); if contrast is lost, re-administration of the agent may be necessary. There are several topical agents that can be used to highlight tumours in animal models33,41,46 and one that can be used in patients58. Such local administration minimizes the overall dose and the risk of systemic side effects, but requires their strong, direct binding to the target or rapid enzymatic cleavage for their activation. Also, probe penetration into the targeted tissue is likely to be limited to a fraction of a millimetre, owing to poor diffusion, which would sacrifice the discrimination of deeper structures or would require frequent re-application. Finally, topical application to the cut surface of the tumour would not identify skip lesions that are separated from the main tumour mass by normal tissue. Systemic administration allows more time for contrast to develop in the target tissues and for nonspecific fluorescence to wash out. There are several systemically administered agents available that can highlight tumour margins in animal models35–39,55,59–61. Systemic administration the day before surgery might be acceptable, especially as this would allow time for PET or MRI imaging of multimodal probes (probes that carry both a PET or MRI contrast agent in addition to a fluorescent marker so that they can be imaged using both the whole-body imaging modalities and during surgery). Ideally, surgical planning with a targeted probe using a pre-operative whole-body scan (PET or MRI) would be carried out in conjunction with intra-operative fluorescence guidance for tumour resection.
Targeting strategies
LMN requires molecules that specifically localize to target tissues. There is a hierarchy of specificity in the current strategies that are used to visualize cancer lesions. Tumour-specific molecules in or on cancer cells are usually the most specific (that is, those detected by antibodies; TABLE 1). However, they have the drawback of usually being applicable only to a particular type of cancer. For example, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) may be good markers for pancreatic or hepatobiliary cancers but cannot be generalized to other cancers such as breast cancer or melanoma. Membrane-associated or excreted enzymes such as MMPs are second best in terms of specificity but may have the advantage of being generalizable to all cancers. Cancer-activated metabolism pathways including glycolysis (imaged using fluoro-deoxyglucose (FDG) or 2-deoxyglucose (2-DG) probes) and porphyrin metabolism (imaged using 5-ALA) are the third most specific type. Physiological parameters including blood flow (imaged using fluorescein sodium) and vascular permeability (imaged using ICG) are the least specific. For tumour margins, current targeting strategies (summarized in TABLE 1) have focused on cell-surface markers such as chlorotoxin62,63, integrin αvβ364–66, tumour-associated antigens (including prostate-specific membrane antigen (PSMA)41, CEA and CA19-9 (REFS 42,43)), FRα47 or tumour-associated enzymes33–36.
In the context of cancer surgery, nerves are the only normal structures that have been studied for targeted probe development56,57,67,68. Other anatomical structures that are relevant during cancer surgery — such as ureters, bile and pancreatic ducts and blood vessels — may be visualized with non-targeted fluorescent dyes alone. Targeted probes exist for other disease states such as atherosclerosis, infection and inflammation but are outside the scope of this article.
Wavelengths and dyes
Fluorescent imaging for applications in vitro or in transparent biological milieu have traditionally relied on dyes that absorb at wavelengths ranging from ultraviolet to green because their emission at blue to orange wavelengths is easily visible to the naked human eye. Also, many such dyes are relatively small, rugged, easily synthesized molecules that are hallowed by long tradition (for example, fluorescein and rhodamines date back to the nineteenth century). However, in intact mammals, wavelengths below 600 nm (orange–red) encounter high autofluorescence owing to many endo genous fluorophores, strong scattering (which is proportional to the inverse fourth-power of the wavelength) and strong absorbance below 600 nm of haemoproteins such as haemoglobin, myoglobin and cytochromes. Therefore, longer excitation and emission wavelengths give less autofluorescence, deeper tissue penetration and easier separation from standard white-light reflectance, but require solid-state detectors (for example, cameras) rather than the naked eye. Some laboratories focus on the longest possible wavelengths, but our experience is that the corresponding fluorophores are dim, chemically fragile and more prone to nonspecific hydrophobic stickiness. Therefore, our laboratory has often used the cyanine dye Cy5 as a compromise fluorophore of greater brightness, stability and hydrophilicity, yet the far-red wavelengths (650 nm excitation, 670–680 nm emission) are almost as good at penetrating tissue as true near-infrared wavelengths.
Fluorescent proteins and quantum dots have attracted much interest as alternatives to organic synthetic fluorophores. However, the chief advantage of fluorescent proteins, their genetic encodability, is mainly useful in animal models in which the tumour can be genetically prelabelled. In clinical tumours, fluorescent-protein expression would be dependent on gene therapy. Even if the many technical obstacles to efficient, tumour-selective gene delivery were eventually overcome, a cytotoxic gene would be a more directly therapeutic payload than a fluorescent protein to guide surgery. The possible exception would be if the genetic payload could be expressed in only a fraction of tumour cells, which would be insufficient for cell-autonomous ablation but enough to visualize for resection.
Regarding quantum dots, their chief advantage is photostability, but our experience is that macroscopic surgical imaging with modern, highly sensitive solid-state cameras is easily feasible with excitation intensities that cause negligible-to-minor photobleaching of standard dyes. Owing to their inherent insolubility, quantum dots would have to overcome major concerns about poor diffusibility through solid tissue, nonspecific binding, long-term excretion and toxicity.
Specificity
Regardless of fluorophore wavelength, a major consideration in the use of a fluorescently labelled probe is the correlation between image intensity and target specificity. Thus, before clinical use, correlation of fluorescence intensity measurements for a given probe (using pre-delineated probe administration and imaging parameters) with target tissue specificity will need to be defined. Ratiometric imaging represents an advantage over single-wavelength intensity measurements because it allows cancer-specific discrimination relatively independently of dose, pharmacokinetics, optical variables and thresholding. The advantages of ratiometric versus single-intensity measurements are well known69 in fluorescence microscopy and flow cytometry but have hitherto been neglected in intra-operative molecular imaging. Quantitative discrimination between target tissues and background based on the ratio (of two fluorophores rather than one fluorophore) of intensities at different wavelengths, times or locations control for variability in factors such as the amount of probe administered, patient-specific pharmacokinetics and imaging parameters, and may provide more reproducible quantitative discrimination of target tissues.
Instrumentation
Our group and others have recently reviewed technical considerations in instrumentation for FGS70,71, hence this article will restrict the discussion to practical applications. A new medical device is ‘cleared’ if it is predicated on a similar, already approved device. This new similar device goes through a 510(k) procedure with the FDA to show similarities with a previously approved device. A device that is not able to show similarity with a previously approved device is required to go through premarket approval (PMA), which is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of class III medical devices. The rapidly evolving field of probe development for FGS will probably require both new devices to be ‘cleared’ through 510(k) and ‘approved’ through PMA action.
Ideally, new instruments will accommodate multiple wavelengths ranging from visible to infrared, given the wide variety of molecularly targeted probes being developed, the desirability of counterstaining healthy tissue with a contrasting colour and the possibility of ratiometric discrimination. Few investigators would buy an expensive fluorescence microscope that is permanently locked to a single pair of excitation and emission wavelengths, especially when additional wavelength pairs only require replacing a filter cube, yet surgical microscopes are too often inflexible with respect to wavelengths. Open-field surgery depends on the direct visualization of patient tissues, whereas in minimally invasive surgeries (that is microscopic, endoscopic, laparoscopic and robotic surgery), patient tissues are visualized through an interface (viewed through the ocular eyepiece or using cameras and digital displays). For the minimally invasive surgical procedures, the added hardware that is necessary for fluorescence imaging can be fitted into existing instrumentation (TABLE 2). The fluorescence signal display can dovetail nicely with the digital display of the white-light reflectance with minimal disruption to existing clinical instrumentation (FIG. 2). Display of different surgical field views (white light alone, fluorescence alone or combined) should be controllable by the operating surgeon in real time with flexibility in the placement of the video screen to accommodate both the surgeon and to support operating room staff. Each view (white light or fluorescence) should have independent control of intensity gain to compensate for variation in factors such as lighting, magnification, probe uptake and pigmentation.
What are the end points?
Cancer detection
The current gold standard for tumour margin evaluation is pathological examination of the surgical specimen. In order for fluorescence-guided detection of cancer to become the new ‘standard of care’, clinical trials will be necessary to define the steps that operating surgeons, pathologists and oncologists need to incorporate into their current practices. Molecular detection of cancer at the surgical borders to decrease the incidence of positive margins may be one clinical end point, although this will need to be weighed against excessive resection of normal tissue with nonspecific fluorescence uptake. Another clinical end point is the molecular detection of cancer in metastatic lymph nodes to dictate the level of lymph node dissection necessary, for example deciding whether to carry out an axillary lymph node dissection for breast cancer if the SLN is positive or whether to extend a neck dissection for a head and neck cancer if the lymph nodes close to the primary tumour are positive. Ultimately, increased tumour-free survival is the goal of all oncology procedures. However, given the cost and time associated with survival studies, it is likely that the aforementioned preliminary parameters will be clinically useful to define probe efficacy.
Normal structure preservation
Although everybody in the field can agree that cancer lesions and cells by themselves — including minimal residual disease at the margins and lymph node metastasis — are the most important objects to ‘identify’ during surgical or endoscopic procedures; normal, crucial structures should be next on the list of important objects to identify (FIG. 2). Under the tight regulatory process for diagnostic agents, it will be challenging to use more than one imaging agent for a given surgical procedure until clinical efficacy for a combination of agents can be shown. For example, the dissection of a tumour with known nerve involvement may have better margins and postoperative neural function when both the nerves and the tumour are labelled instead of either one alone. For probes that target normal structures (such as nerves, ureters or lymphatics) to facilitate surgical preservation, clinical efficacy will probably need to be defined as a combination of decreased complication, decreased surgical time and increased surgeon confidence.
Regulatory concerns
The need for co-approval from regulatory bodies
A fluorescent probe has to be evaluated along with a proposed imaging instrument during a clinical trial. This process is easiest if the instrument already has regulatory approval (through PMA action) or can win acceptance as equivalent to a previously approved instrument (cleared through 510(k) action). Currently, such devices mainly work at wavelengths that are suitable for fluorescein and ICG, owing to the long history of these agents. Other wavelengths and more sophisticated forms of image processing and display would greatly expand surgical utility but are likely to require closer regulatory scrutiny. In addition, molecularly targeted probes typically have longer development times than the instruments that are used to visualize them in vivo, hence the developing probes will define the instruments in the foreseeable future, rather than the reverse. Eventually, standardization of imaging parameters — such as light-source intensity (brightness per given area), wavelength and efficacy of excitation and emission filters, imaging time and display processing — will be highly desirable to allow the use of different instruments with a given probe and of multiple probes with a single instrument. Such interchangeability exists in other clinical imaging modalities, so FGS should eventually be no different.
Costs
There is a substantial start-up cost to the surgical institution to consider before the implementation of FGS owing to the need for equipment purchase, personnel training and probe dose determination. Injectable probes for surgical navigation have to undergo the same regulatory scrutiny as any other drug; however, the financial reward may be far less than that for a drug that is useful for treating multiple types of cancer or other diseases72. A coordinated analysis of the risk and potential benefit to the patient (such as decreased unnecessary morbidity of inadvertent injury to important structures, and decreased rate of positive margins requiring adjuvant therapy or salvage surgery) and to the health-care system as a whole will be required for these novel fluorescent probes to become widely available. However, if the rate of complete resection of cancer is increased by FGS it may avoid the need for expensive chemotherapy or radiotherapy treatments.
Conclusions
Surgery has a vital role in the treatment of cancer. Insufficient visual differences between tumour and normal tissue when using white-light reflectance alone contribute to potentially preventable cancer persistence or recurrence, or unacceptable morbidity when too much tissue is resected. We are entering an exciting frontier in the field of cancer surgery. The increasing availability of novel, fluorescently labelled probes to identify crucial landmarks including tumour margins, lymph nodes and vital structures such as nerves, lymphatics and ureters, along with new instrumentation has expanded the surgeons’ visual capability beyond that of white-light reflectance. Currently, only two non-targeted dyes (ICG and fluorescein sodium) are widely adopted during FGS. Although the widespread off-label use of other drugs in FGS described in the literature does not make for an approved indication, the FDA or European Medicines Agency (EMA) have approved some of these drugs for other uses. Although 5-ALA is available for use through oral administration in Europe, it is only used topically in the context of photodynamic therapy or within clinical trials in the United States. Furthermore, we believe that there is a difference between topically and systemically applied drugs for FGS because topical compounds can only highlight the structures on the surface to which they are applied58,73. By contrast, systemically injectable drugs can highlight structures below the surface, thus directing the operating surgeon to structures that they have yet to uncover (that is, buried nerves or tumour foci). We believe that this topographically unbiased ability to highlight structures of interest is superior for surgical applications.
Although much more work is necessary to translate the exploding number of targeted probes that are currently in the research pipelines, FGS with LMN promises to be a major leap forwards in the field of surgery to improve patient clinical outcomes and to reduce overall health-care costs. Although the imaging agents themselves help patients only indirectly by helping surgeons to achieve better surgical outcomes through improving surgical margins or preserving nerves, a good outcome is in fact the goal of the surgery. Given that imaging agents should have higher safety profiles than therapeutic agents, the benefit of using multi-imaging agents must therefore outweigh the combined risks of the individual agents. We believe that the use of multi-imaging agents should be at the discretion of the operating surgeon and their patients following detailed discussion with full disclosure of the risks (TABLE 3). Finally, imaging agents to assist surgery will probably be dosed only once or twice at the time of surgery. This is unlike therapeutic drugs, which undergo similar regulatory hurdles but are typically dosed repeatedly over extended periods of time. This difference in single versus repeated use translates into financial considerations that, along with a likely higher safety profile requirement for an imaging agent, represent another challenge towards their development.
Table 3.
Considerations of surgery with live molecular navigation
| Parameter | Considerations |
|---|---|
| Surgery selection |
|
| Patient heterogeneity | Personalized screening |
| Pharmacokinetics |
|
| Imaging parameters |
|
| Defining end points |
|
Along with innovation in marker development, there is a parallel path of innovation in the field of instrumentation to merge the traditional surgical field with fluorescence. Although these advances in molecular navigation have the potential to shift the surgical paradigm, there are remaining challenges of defining clinical end points and identifying which patients and surgeries would most benefit from these molecular navigation techniques.
Acknowledgments
The authors would like to thank S. Howell and L. Simon for helpful discussions on the regulatory aspects discussed in this manuscript, and M. Whitney for helpful scientific discussions.
Glossary
- Approved
An approved drug is a drug that has been authorized for a therapeutic use by a ruling authority. In Europe, the European Medicines Agency (EMA) is the ruling authority. In the United States, the Food and Drug Administration (FDA), an agency within the Department of Health and Human Services, provides this approval. A list of FDA-approved drugs is updated regularly on the FDA Orange Book website (see Further information)
- Autofluorescence
Fluorescence that is inherent to different tissues (such as muscle, fat or liver) in the absence of any exogenously applied compounds
- Chlorotoxin
A peptide that is found in the venom of the scorpion Leiurus quinquestriatus that blocks small-conductance chloride channels. Chlorotoxin binds preferentially to gliomas and tumours of neuroectodermal origin
- Indication
A drug is approved by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for certain uses or indications following approved Phase I, II and III clinical trials. These indications are supplied with the package inserts for the drugs
- Infrared reflectivity
The fraction of incident infrared light that is reflected, which can vary between different tissues
- Intratumour microheterogeneity
The idea that within a given neoplasm, different tumour cells may display differences in genetic mutations and/or protein expression that may confer advantages and disadvantages for metastatic potential or response to chemotherapy or radiotherapy
- Lymphoedema
A condition of localized fluid retention and tissue swelling caused by a compromised lymphatic system. Lymphoedema is a common consequence of lymph node removal (for example, during neck dissection or axillary lymph node dissection) in the surgical treatment of cancer
- Medical device
An instrument that is used to diagnose or treat disease or other medical conditions and that does not achieve its purpose through chemical action within or on the body. In the United States, the Food and Drug Administration (FDA) is responsible for medical device evaluation, through premarket evaluation (510(k)) to show similarity with a previously approved device or through premarket approval (PMA) applications. PMA approval for a new device is based on a determination by the FDA that the PMA contains sufficient valid scientific evidence to ensure that the device is safe and effective for its intended use or uses
- Micro-anatomical cyto-architecture
The shapes and sizes of cells, nuclei, organelles and extracellular structures, which are used to distinguish tumour from normal tissue at microscopic resolution
- Nonspecific hydrophobic stickiness
Nonspecific binding to tissue of hydrophobic molecules such as most near-infrared dyes (which emit at wavelengths >700nm), thus typically resulting in increased background fluorescence
- Off-label
The clinical practice of prescribing pharmaceuticals for an unapproved indication or in an unapproved age group, at an unapproved dose or through an unapproved form of administration. After a drug has been approved for one purpose, licensed physicians are able to prescribe it for other purposes that in their professional judgment are both safe and effective, and are not limited to the official, approved indications
- Open-field surgery
A type of surgery in which the surgeon’s view of the patient’s tissue is through direct visualization through a large skin incision. This is in contrast to minimally invasive surgery (that is, laparoscopic surgery, endoscopic surgery or robotic surgery) in which the surgeon views the patient’s tissues through fibre-optic probes or cameras, which are inserted through natural orifices or small skin incisions and the images are displayed on a computer screen
- Quantum dots
Inorganic nanocrystals that typically have a diameter of 2–8 nm. Most are composed of an inner semiconductor core of CdSe, an outer shell of ZnS, and an organic coating to make the quantum dot biocompatible. Quantum dots can be engineered to emit fluorescent light in the ultraviolet to infrared spectrum by varying their size
- Raman scattering
A spectroscopic technique that differentiates different tissue types depending on the infrared spectra of different molecules (such as fat and collagen) within that tissue
- Ratiometric imaging
Measurement of the ratio of fluorescence intensities at two wavelengths. When the response of the fluorescent probe to the desired signal (for example, protease activity) is markedly different at two wavelengths, calculating the ratio of the fluorescence at those wavelengths isolates the desired signal from confounding factors such as the absolute concentration of the fluorescent probe, the thickness of tissue, the intensity of illumination and the sensitivity of detection, which all affect intensities at both wavelengths by equal proportions. Ratiometric activatable cell-penetrating peptides (RACPPs)40 use pairs of dyes such as Cy5 and Cy7 as donor and acceptor dyes for fluorescence resonance energy transfer, thus enabling emission ratiometric discrimination of protease activity
- Sentinel lymph node
(SLN). The first draining lymph node for a given anatomical site. For breast cancer, the SLNs are located in the axilla
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Quyen T. Nguyen, Email: q1nguyen@ucsd.edu, The Division of Otolaryngology — Head and Neck Surgery, University of California at San Diego, La Jolla, California 92093–0647, USA
Roger Y. Tsien, Email: rtsien@ucsd.edu, The Department of Pharmacology, Howard Hughes Medical Institute, University of California at San Diego, La Jolla, California 92093–0647, USA
References
- 1.National Cancer Institute. Previous version: SEER cancer statistics review, 1975–2005. 2008 [online], http://seer.cancer.gov/csr/1975_2005.
- 2.Haque R, Contreras R, McNicoll MP, Eckberg EC, Petitti DB. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. 2006;16:2. doi: 10.1186/1472-6815-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singletary S. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 4.Meric F, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003;97:926–933. doi: 10.1002/cncr.11222. [DOI] [PubMed] [Google Scholar]
- 5.Snijder R, de la Riviere A, Elbers H, van den Bosch J. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann Thorac Surg. 1998;65:212–216. doi: 10.1016/s0003-4975(97)01114-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 7.Dotan Z, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308–2312. doi: 10.1016/j.juro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- 9.Kumar A, Puri R, Gadgil PV, Jatoi I. Sentinel lymph node biopsy in primary breast cancer: window to management of the axilla. World J Surg. 2012;36:1453–1459. doi: 10.1007/s00268-012-1635-8. [DOI] [PubMed] [Google Scholar]
- 10.Wong SL, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–2918. doi: 10.1200/JCO.2011.40.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutteman M, et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127:163–170. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vorst JR, et al. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99m technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol. 2012;19:4104–4111. doi: 10.1245/s10434-012-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson DK, et al. A receptor-targeted fluorescent radiopharmaceutical for multireporter sentinel lymph node imaging. Radiology. 2012;265:186–193. doi: 10.1148/radiol.12120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph M, Phillips M, Farrell TM, Rupp CC. Can residents safely and efficiently be taught single incision laparoscopic cholecystectomy? J Surg Educ. 2012;69:468–472. doi: 10.1016/j.jsurg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kandil EH, Noureldine SI, Yao L, Slakey DP. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg. 2012;214:558–564. doi: 10.1016/j.jamcollsurg.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee JC, Jang HD, Shin BJ. Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: our experience in 86 consecutive cases. Spine. 2012;37:1548–1557. doi: 10.1097/BRS.0b013e318252d44b. [DOI] [PubMed] [Google Scholar]
- 17.Pappo I, et al. Diagnostic performance of a novel device for real-time margin assessment in lumpectomy specimens. J Surg Res. 2010;160:277–281. doi: 10.1016/j.jss.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Rivera RJ, Holmes DR, Tafra L. Analysis of the impact of intraoperative margin assessment with adjunctive use of MarginProbe versus standard of care on tissue volume removed. Int J Surg Oncol. 2012;2012:868623. doi: 10.1155/2012/868623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dotan ZA, et al. Detection of prostate cancer by radio-frequency near-field spectroscopy in radical prostatectomy ex vivo specimens. Prostate Cancer Prostatic Dis. 2012;16:73–78. doi: 10.1038/pcan.2012.34. [DOI] [PubMed] [Google Scholar]
- 20.Pavlova I, et al. Multiphoton microscopy and microspectroscopy for diagnostics of inflammatory and neoplastic lung. J Biomed Opt. 2012;17:036014. doi: 10.1117/1.JBO.17.3.036014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce MC, et al. Accuracy of in vivo multimodal optical imaging for detection of oral neoplasia. Cancer Prev Res. 2012;5:801–809. doi: 10.1158/1940-6207.CAPR-11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurtner GC, et al. Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res. 2013;7:1. doi: 10.1186/1750-1164-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda K, et al. Intra-arterial injection fluorescein videoangiography in aneurysm surgery. Neurosurgery. 2012;72:141–150. doi: 10.1227/NEU.0b013e3182752f32. [DOI] [PubMed] [Google Scholar]
- 24.Shin D, Vigneswaran N, Gillenwater A, Richards-Kortum R. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Future Oncol. 2010;6:1143–1154. doi: 10.2217/fon.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foersch S, et al. Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. PLoS ONE. 2012;7:e41760. doi: 10.1371/journal.pone.0041760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vila PM, et al. Discrimination of benign and neoplastic mucosa with a High-Resolution Microendoscope (HRME) in head and neck cancer. Ann Surg Oncol. 2012;19:3534–3539. doi: 10.1245/s10434-012-2351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker FG, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709–720. doi: 10.1097/00006123-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Swindle P, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2008;179:S47–S51. doi: 10.1016/j.juro.2008.03.137. [DOI] [PubMed] [Google Scholar]
- 29.Warner JN, et al. Impact of margin status at 37 months after robot assisted radical prostatectomy. Can J Urol. 2011;18:6043–6049. [PubMed] [Google Scholar]
- 30.Chalfin HJ, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012;110:1684–1689. doi: 10.1111/j.1464-410X.2012.11371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pleijhuis RG, et al. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16:2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkins J, et al. Positive margin rates following breast-conserving surgery for stage I–III breast cancer: palpable versus nonpalpable tumors. J Surg Res. 2012;177:109–115. doi: 10.1016/j.jss.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cutter JL, et al. Topical application of activity-based probes for visualization of brain tumor tissue. PLoS ONE. 2012;7:e33060. doi: 10.1371/journal.pone.0033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdoes M, et al. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19:619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheth RA, et al. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol Oncol. 2009;112:616–622. doi: 10.1016/j.ygyno.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen QT, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang T, et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson ES, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol. 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilera TA, Olson ES, Timmers MM, Jiang T, Tsien RY. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr Biol. 2009;1:371–381. doi: 10.1039/b904878b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savariar EN, et al. Real-time in vivo molecular detection of primary tumors and metastases with ratiometric activatable cell-penetrating peptides. Cancer Res. 2013;73:855–864. doi: 10.1158/0008-5472.CAN-12-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima T, et al. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22:1700–1705. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran Cao HS, et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology. 2012;59:1994–1999. doi: 10.5754/hge11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElroy M, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall MA, et al. Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: quantitative small-animal PET/CT and NIRF. J Nucl Med. 2012;53:1427–1437. doi: 10.2967/jnumed.112.106302. [DOI] [PubMed] [Google Scholar]
- 45.Rosbach KJ, Williams MD, Gillenwater AM, Richards-Kortum RR. Optical molecular imaging of multiple biomarkers of epithelial neoplasia: epidermal growth factor receptor expression and metabolic activity in oral mucosa. Transl Oncol. 2012;5:160–171. doi: 10.1593/tlo.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urano Y, et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3:110ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nature Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 48.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 49.Stummer W, et al. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 50.Kalli KR, et al. Folate receptor α as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619–626. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schafer C, et al. γ-glutamyl transferase expression in higher-grade astrocytic glioma. Acta Oncol. 2001;40:529–535. doi: 10.1080/028418601750288271. [DOI] [PubMed] [Google Scholar]
- 52.Floeth FW, et al. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging. 2011;38:731–741. doi: 10.1007/s00259-010-1690-z. [DOI] [PubMed] [Google Scholar]
- 53.Sun C, et al. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomed. 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun C, et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson ES, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibbs-Strauss SL, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging. 2011;10:91–101. [PMC free article] [PubMed] [Google Scholar]
- 57.Whitney M, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nature Biotech. 2011;29:352–356. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturm MB, et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci Transl Med. 2013;5:184ra61. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nature Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 60.Figueiredo JL, Alencar H, Weissleder R, Mahmood U. Near infrared thoracoscopy of tumoral protease activity for improved detection of peripheral lung cancer. Int J Cancer. 2006;118:2672–2677. doi: 10.1002/ijc.21713. [DOI] [PubMed] [Google Scholar]
- 61.Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res. 2004;64:6247–6251. doi: 10.1158/0008-5472.CAN-04-0817. [DOI] [PubMed] [Google Scholar]
- 62.Veiseh M, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 63.Stroud MR, Hansen SJ, Olson JM. In vivo bio-imaging using chlorotoxin-based conjugates. Curr Pharm Des. 2011;17:4362–4371. doi: 10.2174/138161211798999375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye Y, et al. Near-infrared fluorescent divalent RGD ligand for integrin αvβ3-targeted optical imaging. Bioorg Med Chem Lett. 2012;22:5405–5409. doi: 10.1016/j.bmcl.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao J, et al. A novel clinically translatable fluorescent nanoparticle for targeted molecular imaging of tumors in living subjects. Nano Lett. 2012;12:281–286. doi: 10.1021/nl203526f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L, et al. Dual-functional, receptor-targeted fluorogenic probe for in vivo imaging of extracellular protease expressions. Bioconjugate Chem. 2011;22:1001–1005. doi: 10.1021/bc200005w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu AP, et al. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121:805–810. doi: 10.1002/lary.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotero VE, et al. Intraoperative fluorescence imaging of peripheral and central nerves through a myelin-selective contrast agent. Mol Imaging Biol. 2012;14:708–717. doi: 10.1007/s11307-012-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsien RY, Harootunian AT. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990;11:93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 70.Orosco RK, Tsien RY, Nguyen QT. Fluorescence imaging in surgery. IEEE Rev Biomed Eng. 2013;6:178–187. doi: 10.1109/RBME.2013.2240294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taruttis A, Ntziachristos V. Translational optical imaging. Am J Roentgenol. 2012;199:263–271. doi: 10.2214/AJR.11.8431. [DOI] [PubMed] [Google Scholar]
- 72.Nunn AD. The cost of developing imaging agents for routine clinical use. Invest Radiol. 2006;41:206–212. doi: 10.1097/01.rli.0000191370.52737.75. [DOI] [PubMed] [Google Scholar]
- 73.Mitsunaga M, et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut. 2013;62:1179–1186. doi: 10.1136/gutjnl-2011-301795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashitate Y, et al. Simultaneous assessment of luminal integrity and vascular perfusion of the gastrointestinal tract using dual-channel near-infrared fluorescence. Mol Imaging. 2012;11:301–308. [PMC free article] [PubMed] [Google Scholar]


