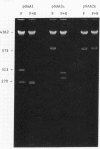
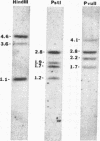
Abstract
Serum amyloid A protein (SAA) is a major acute-phase protein in humans and most other mammals. In addition, it is the serum precursor of the major protein constituent of reactive amyloid fibrils. Sequence analyses have identified a number of polymorphic forms of human SAA and amyloid A protein (AA), but the question of the number of genes encoding SAA in the human has not been addressed. In addition, there are insufficient data to predict whether one form of SAA predisposes to amyloid fibril formation. In the present study three separate SAA proteins have been isolated from the plasma of one individual and completely sequenced. While two of the SAA forms (SAA2 alpha and SAA2 beta) differ from each other only at position 71, they differ from the most abundant form (SAA1) at seven and eight other positions, respectively. Nucleotide sequencing of cDNAs from a liver library of this individual identified all three mRNs coding for these proteins and proved that: (a) the often-reported absence of arginine at the amino terminus of SAA proteins must result from proteolytic processing of the protein; (b) the polymorphism involving histidine and arginine at position 71 is present at the DNA level and therefore is not due to an event at the translational level; (c) there are at least two genes coding for human SAA. Comparison of these data to published sequences of SAA and AA proteins may help in identifying genetically determined forms of SAA which predispose to reactive amyloid fibril formation.
Full text
PDF

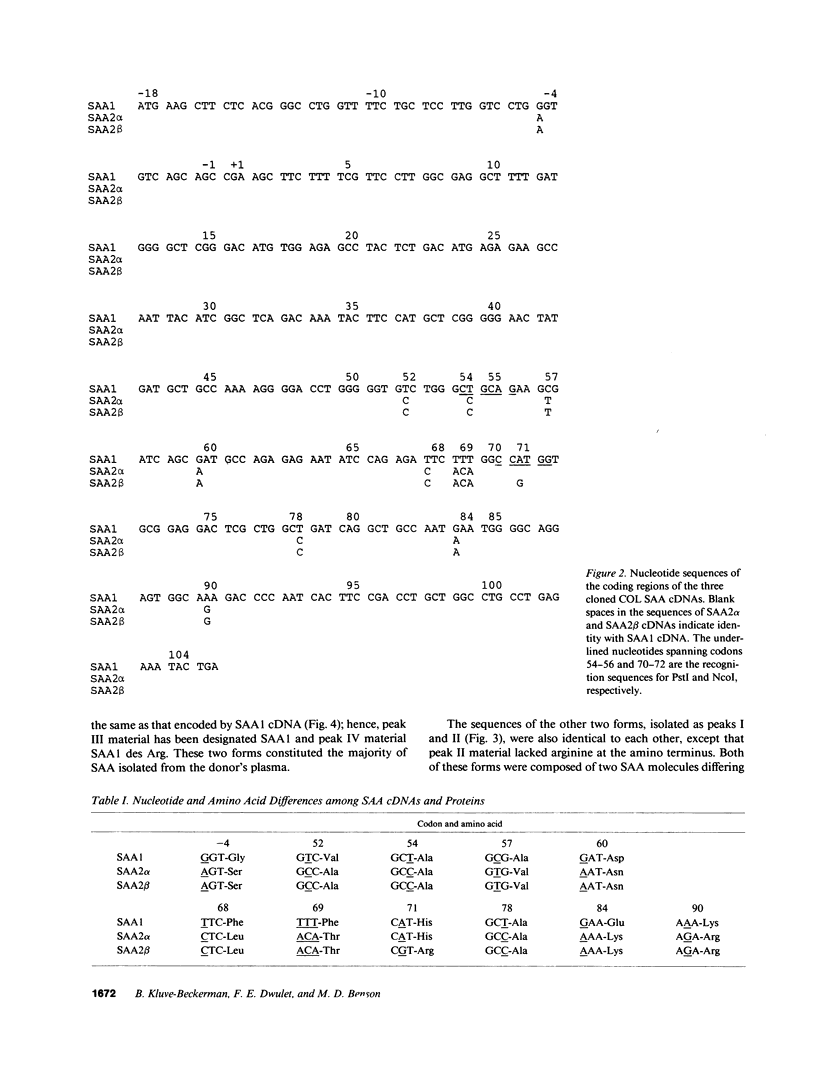
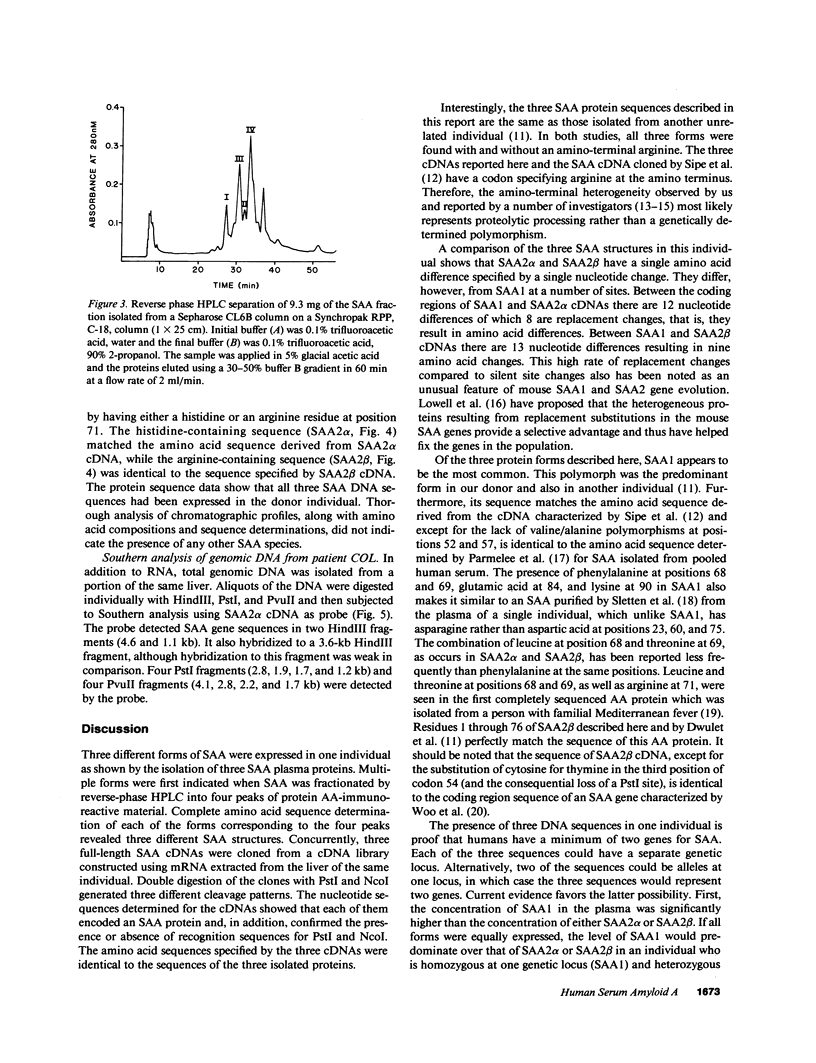


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of amyloid fibril protein AA in azocasein-induced amyloidosis of CBA/J mice. J Lab Clin Med. 1987 Sep;110(3):322–329. [PubMed] [Google Scholar]
- Dwulet F. E., Wallace D. K., Benson M. D. Amino acid structures of multiple forms of amyloid-related serum protein SAA from a single individual. Biochemistry. 1988 Mar 8;27(5):1677–1682. doi: 10.1021/bi00405a044. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Pras M., Levin M., Frangione B. The partial amino acid sequence of the major low molecular weight component of two human amyloid fibrils. FEBS Lett. 1972 Apr 15;22(1):121–123. doi: 10.1016/0014-5793(72)80235-7. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Long G. L., Benson M. D. DNA sequence evidence for polymorphic forms of human serum amyloid A (SAA). Biochem Genet. 1986 Dec;24(11-12):795–803. doi: 10.1007/BF00554519. [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Naylor S. L., Marshall A., Gardner J. C., Shows T. B., Benson M. D. Localization of human SAA gene(s) to chromosome 11 and detection of DNA polymorphisms. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1196–1204. doi: 10.1016/0006-291x(86)90352-9. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Madisen L., Hoar D. I., Holroyd C. D., Crisp M., Hodes M. E. DNA banking: the effects of storage of blood and isolated DNA on the integrity of DNA. Am J Med Genet. 1987 Jun;27(2):379–390. doi: 10.1002/ajmg.1320270216. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J. D., Colten H. R., Goldberger G., Edge M. D., Tack B. F., Cohen A. S., Whitehead A. S. Human serum amyloid A (SAA): biosynthesis and postsynthetic processing of preSAA and structural variants defined by complementary DNA. Biochemistry. 1985 Jun 4;24(12):2931–2936. doi: 10.1021/bi00333a018. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G., Natvig J. B. The complete amino acid sequence of an amyloid fibril protein AA1 of unusual size (64 residues). Biochem Biophys Res Commun. 1976 Mar 8;69(1):19–25. doi: 10.1016/s0006-291x(76)80266-5. [DOI] [PubMed] [Google Scholar]
- Sletten K., Marhaug G., Husby G. The covalent structure of amyloid-related serum protein SAA from two patients with inflammatory disease. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):1039–1046. doi: 10.1515/bchm2.1983.364.2.1039. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Peltzman C. G., Morrow J. F. The sequence and structure of a new serum amyloid A gene. Nucleic Acids Res. 1986 Jan 24;14(2):797–809. doi: 10.1093/nar/14.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P., Sipe J., Dinarello C. A., Colten H. R. Structure of a human serum amyloid A gene and modulation of its expression in transfected L cells. J Biol Chem. 1987 Nov 15;262(32):15790–15795. [PubMed] [Google Scholar]
- Yamamoto K., Goto N., Kosaka J., Shiroo M., Yeul Y. D., Migita S. Structural diversity of murine serum amyloid A genes. Evolutionary implications. J Immunol. 1987 Sep 1;139(5):1683–1688. [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]