Abstract
Progranulin (PGRN) is a multi-functional protein known to be involved in inflammation. Recent studies have found that PGRN has dual roles in inflammation and exerts anti-inflammatory and pro-inflammatory function in different diseases. However, the role of PGRN in psoriasis has not been fully elucidated. Here, we detected preferential expression of PGRN in human psoriatic lesions and serum. Moreover, serum PGRN/tumour necrosis factor-α ratio was negatively correlated with disease severity. To investigate the role of PGRN in the pathogenesis of psoriasis, we used wild-type (WT) and PGRN−/− mice in a model of 12-O-tetradecanoylphorbol 13-acetate (TPA) -induced psoriasis-like inflammation. We demonstrated that PGRN expression was dramatically enhanced in the psoriasis-like lesions of TPA-treated WT mice, in accordance with human psoriatic lesions. Surprisingly, PGRN−/− mice were more sensitive to the development of TPA-induced psoriasis-like inflammation. The mechanism underlying this increased sensitivity of PGRN−/− mice to TPA-induced psoriasis-like inflammation was impaired differentiation of regulatory T cells in lymph nodes and decreased recruitment of these cells in the affected skin, which results in more severe inflammation. Hence, in WT mice, PGRN promotes differentiation and recruitment of regulatory T cells at the site of inflammation, which protects the skin from an exaggerated psoriasis-like inflammatory response.
Keywords: inflammation, progranulin, psoriasis, regulatory T cells
Introduction
Psoriasis is a common chronic inflammatory skin disease characterized by marked thickening of the epidermis, tortuous and dilated dermal blood vessels, and characteristic inflammatory cell infiltrates. Although the pathogenesis of psoriasis has not been fully elucidated, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are over-expressed in psoriasis and are believed to have central roles in its processes. In particular, biological agents targeting TNF-α signalling are highly effective in the treatment of patients with psoriasis.1
Progranulin (PGRN) – also known as granulin epithelin precursor, PC-cell-derived growth factor, pro-epithelin and acrogranin – is a 593-amino-acid autocrine growth factor with multiple physiological and pathological functions.2 PGRN plays a critical role in early embryogenesis,3 wound healing,4 tumorigenesis,5,6 maintenance of neuronal survival,7 host defence and inflammation.8 Recently, PGRN was discovered as a novel ligand of TNF receptors (TNFR). PGRN blocks TNF-α-mediated signalling pathways by competing with TNF-α binding to TNFR1/2 and exhibits anti-inflammatory function in inflammatory arthritis models.9 Furthermore, the administration of recombinant human PGRN or a recombinant PGRN derivative named Atsttrin consisting of three modified granulin motifs and their accompanying linker regions, had strong anti-inflammatory effects comparable with, or even stronger than, the administration of Etanercept.9 PGRN and its derivative Atsttrin may represent a promising therapeutic agent in the treatment of rheumatoid arthritis and contact dermatitis.10,11 Some studies speculate potential applications of PGRN and Atsttrin in other autoimmune diseases, such as psoriasis and inflammatory bowel disease, on the grounds that TNF inhibitors have been used for treating such diseases.11,12 PGRN has been increasingly the focus of research on autoimmune diseases because of it anti-inflammatory effect. In contrast to its anti-inflammatory role, PGRN also has pro-inflammatory effects in obesity and insulin-resistant diabetes through the production of IL-6.13 Hence, the exact function of PGRN may vary depending on the pathophysiological context of the disease. At present, little information is available about the effect of PGRN protein in psoriasis vulgaris. In this study, we examined the serum PGRN levels and tissue PGRN expression in patients with psoriasis vulgaris, evaluated the relationship between serum PGRN level and serum TNF-α and IL-6 levels, and investigated the biological significance of PGRN in the pathogenesis of psoriasis vulgaris.
Materials and methods
Patients and sample collection
A total of 34 patients with psoriasis vulgaris together with 20 age- and sex-matched controls, were included in the study (demographic characteristics of subjects are provided in Table1). The subjects were enrolled in the Department of Dermatology, the First Affiliated Hospital of Chongqing Medical University, China. Ethical approval was obtained from the hospital ethics committee before the beginning of the study and all participants signed an informed consent form. The diagnosis of psoriasis vulgaris was made on the basis of typical clinical manifestation and histopathology. The patients had not received any treatment for at least 3 months before the first collection of peripheral blood and tissue samples. All patients were given systemic treatment including methotrexate, cyclosporin, acitretin and phototherapy, either alone or in combination, and topical treatment including corticosteroid and vitamin D3 derivates. The severity of disease in patients was evaluated using the Psoriasis Area and Severity Index (PASI) before and after treatment. Decreased PASI score for skin lesions = [(pre-treatment PASI score – post-treatment PASI score)/pre-treatment PASI score]. Effective treatment (defined as > 75% decreased index of PASI score) was achieved in 18 patients at the end of 8–10 weeks. Peripheral blood samples were obtained again from these 18 patients after effective treatment.
Table 1.
Demographic and clinical characteristics of the study groups
| Variable | Psoriasis patients | Control groups | |
|---|---|---|---|
| Pre-treatment | Post-treatment | ||
| n (male/female) | 34 (19/15) | 18 (11/8) | 20 (10/10) |
| Age (years) | 43·7 ± 11 | 41·8 ± 11·4 | 40·7 ± 10·5 |
| Disease duration (years) | 17·1 ± 12·4 | 15·7 ± 12 | NA |
| PASI score | 19·1 ± 5·6 | 2·8 ± 1·5 | NA |
| PGRN level (ng/ml) | 60·81 ± 9·47a | 43·03 ± 5·01 | 41·45 ± 8·50 |
| TNF-α level (pg/ml) | 16·01 ± 3·91a | ND | 4·14 ± 1·58 |
| Interleukin-6 level (pg/ml) | 5·86 ± 3·10b | ND | 4·19 ± 2·24 |
| PGRN/TNF-α ratio | 3·93 ± 0·76 | ND | NA |
Abbreviations: NA, not applicable; ND, not determined; PASI, Psoriasis Area and Severity Index; PGRN, progranulin; TNF-α, tumour necrosis factor-α.
Data are presented as n or means ± SD.
P < 0·01 compared with normal controls.
P < 0·05 compared with normal controls.
The tissue samples were excised under local anaesthesia. Each sample was divided into two parts: one was fixed in 4% paraformaldehyde and embedded in paraffin wax, the other was snap-frozen and stored at −80° and used in quantitative RT-PCR. The blood samples were centrifuged and the serum was stored at −80° and used for ELISA.
Mice
All animal studies were performed in accordance with institutional guidelines and approval by the Institutional Animal Care and Use Committee of Chongqing Medical University. The C57BL/6 background PGRN-deficient (PGRN−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME), and generation and genotyping of PGRN−/− mice was based on the Jackson Laboratoy's protocol for these experiments. Ten- to twelve-week-old male wild-type (WT) and PGRN−/− mice were used for these experiments.
TPA-induced psoriasis-like inflammation model and sample collection
The ears of mice were treated with 0·01% 12-O-tetradecanoyl-phorbol 13-acetate (TPA; Sigma-Aldrich, St Louis, MO), as described previously.14 Ears of each mouse were divided into two parts: one was fixed in 4% paraformaldehyde and embedded in paraffin wax, the other was snap-frozen and stored at -80°c and used in quantitative RT-PCR.
Histology
At least four consecutive 5-μm-thick paraffin-embedded ear sections were obtained, and stained using haematoxylin & eosin for routine morphological analysis.
Immunohistochemical staining and assessment
Immunohistochemical staining was performed as previously described.6 Briefly, immunostaining was performed using rabbit anti-PGRN polyclonal antibody (1 : 100; Proteintech Group, Inc., Chicago, IL) followed by incubation with horseradish peroxidase-labelled polymer anti-rabbit secondary antibody (Boster Biological Technology, Ltd., Wuhan, China).
To evaluate the expression of PGRN, three independent observers without knowledge of any clinicopathological data examined the immunostaining. The numbers of PGRN-positive cells in the cell cytoplasm were counted in 10 representative microscope fields, and the percentages of positive cells were calculated. The criteria used for assessment were previously reported15 as follows: < 5% of cell staining, negative; > 5% of cell staining, positive; positive staining was graded from weak/focal to moderate/focal or diffuse to strong diffuse.
Quantitative real-time reverse transcription PCR
Total RNA was extracted from skin or ear tissue using TRIzol reagent (Invitrogen, Carlsbad, CA). The cDNA was obtained from total RNA using a PrimeScript™ RT reagent Kit (Perfect Real Time; Takara Bio Inc., Shiga, Japan) according to the manufacturer's protocol. Real-time PCR was run using the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) kit (TaKaRa Bio Inc., Shiga, Japan). Sequence-specific PCR primers in the present study were synthesized as follows: 5′-GTTCCCTGCACAAAAGACCAA-3′ and 5′-GGGTCTTAGCATCAGGGCAC-3′ for mouse PGRN; 5′-AATGTGTCCGTCGTGGATCTGA-3′ and 5′-AGTGTAGCCCAAGATGCCCTTC-3′ for mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH); 5′-TAGCCAATGGGAGCGGGTA-3′ and 5′-GTAGCGCTCAGACTACAGACC-3′ for human PGRN; 5′-CTATAAATTGAGCCCGCAGCC-3′and 5′-GCGCCCAATACGACCAAATC-3′ for human GAPDH. All samples were analysed in parallel for GAPDH gene expression as an internal control.
ELISA
Serum levels of PGRN, IL-6 and TNF-α were measured using a commercial ELISA kit (PGRN: R&D Systems, Inc., Minneapolis, MN; IL-6 and TNF-α: BioLegend, San Diego, CA) according to the manufacturer's instructions.
Cell isolation and flow cytometry
Single-cell suspension of mouse ear skin and cervical lymph node were prepared as previously reported.14 For regulatory T (Treg) cells, 1 × 106 cells were stained with CD4-FITC, CD25-allophycocyanin and Foxp3-phycoerythrin (PE) (eBioscience, San Diego, CA) antibodies according to eBioscience protocols. For T helper type 1 (Th1), Th2 and Th17, cells were activated with TPA (100 ng/ml) and ionomycin (1 μg/ml) (both from Liankebio, Hangzhou, China) in the presence of brefeldin A (5 μg/ml; Biolegend, San Diego, CA) for 5 hr at 37° in 5% CO2. Then, 1 × 106 cells were stained with CD4-FITC antibody. CD4-stained cells were then stained for cytokines interferon-γ-PE, IL-4-PE and IL-17-PE with antibodies from Biolegend (San Diego, CA), according to Biolegend protocols. Cells were analysed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA). All plots were gated on CD4+.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). SPSS software (version 13·0; SPSS Inc., Chicago, IL) was used for statistical analysis. The differences between groups were analysed using Mann–Whitney U-test or Student's t-test. The relations between PGRN levels and other continuous variables were analysed by using Spearman rank correlation. The PGRN levels before and after treatment were compared using a paired t-test. Statistical significance was set at a level of P < 0·05.
Results
PGRN expression was increased in skin lesions of psoriasis vulgaris
We applied immunohistochemical staining to examine PGRN expression in skin specimens derived from psoriatic patients (n = 34) and in control samples derived from normal individuals (n = 20). Of the 20 healthy skin specimens, 19 (95%) were negative for PGRN (Fig.1a), the remaining specimens exhibited light positive staining in the cytoplasm of some keratinocytes in the basal layer of the epidermis. In the psoriatic lesions a marked over-expression of PGRN in epidermal keratinocytes was detected in all specimens (Fig.1b). To further verify this alteration of PGRN expression, we performed quantitative RT-PCR to evaluate the mRNA levels of PGRN. As shown in Fig.1(c), mRNA expression levels of PGRN in psoriatic lesions were significantly higher than those in normal skin.
Figure 1.
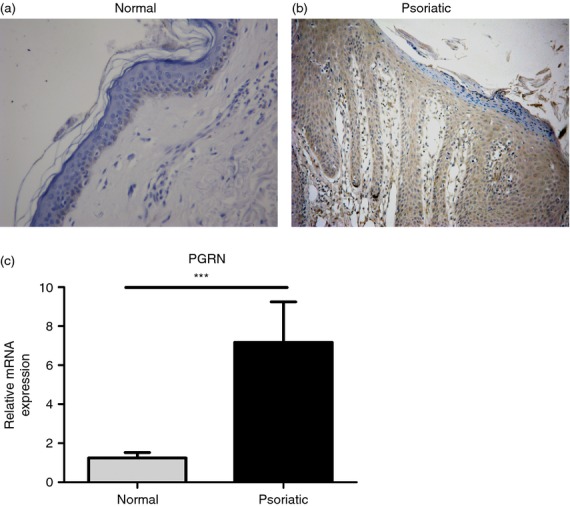
Progranulin (PGRN) expression was increased in lesional skin of psoriasis vulgaris. (a) Immunohistochemistry for PGRN showed negative staining in normal skin. (b) PGRN was over-expressed in psoriatic lesions. Original magnification × 200. (c) Relative mRNA levels of PGRN were higher in psoriatic lesions than those in normal skin, measured by quantitative RT-PCR. ***P < 0·005.
The serum PGRN levels were elevated in the patients with psoriasis vulgaris
We detected serum levels of PGRN in 34 patients with psoriasis vulgaris and 20 normal individuals by using ELISA. Serum PGRN levels in patients with psoriasis vulgaris were significantly higher than those in healthy controls (P < 0·01, Table1). Then, we tested serum PGRN levels in 18 patients with psoriasis vulgaris before and after treatment. The high serum PGRN levels at pre-treatment were significant down-regulated after treatment (P < 0·005, Fig.2a, Table1).
Figure 2.
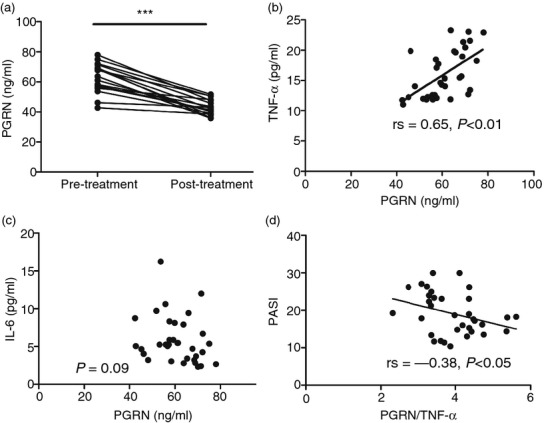
The change of serum progranulin (PGRN) levels and correlation analysis. (a) The serum PGRN levels decreased significantly after treatment (***P < 0·005). (b) The serum PGRN levels were positively correlated with the levels of tumour necrosis factor-α (TNF-α) (rs = 0·65, P < 0·01). (c) The serum PGRN levels were not correlated with the levels of interleukin-6 (IL-6) (P > 0·05). (d) The serum PGRN/TNF-α ratio was negatively correlated with Psoriasis Area and Severity Index (PASI) scores (rs = −0·38, P < 0·05).
Correlations of serum PGRN levels with inflammatory factors in the patients with psoriasis vulgaris before treatment
To examine the relationship between serum PGRN level and inflammatory factors related with psoriasis vulgaris, serum levels of TNF-α and IL-6 were detected by ELISA before treatment. The levels of TNF-α and IL-6 in psoriasis vulgaris patients were up-regulated significantly compared with levels in normal controls (P < 0·05, Table1). And there was a significant and positive correlation between serum PGRN and TNF-α levels in pre-treatment psoriasis vulgaris patients (rs = 0·65, P < 0·01, Fig.2b). However, there was no significant correlation between serum levels of PGRN and IL-6 (P = 0·09, Fig.2c). Furthermore, the serum PGRN/TNF-α ratio was negatively correlated with PASI scores (rs = −0·38, P < 0·05, Fig.2d, Table1).
Expression of PGRN was increased in psoriasis-like mouse skin lesions
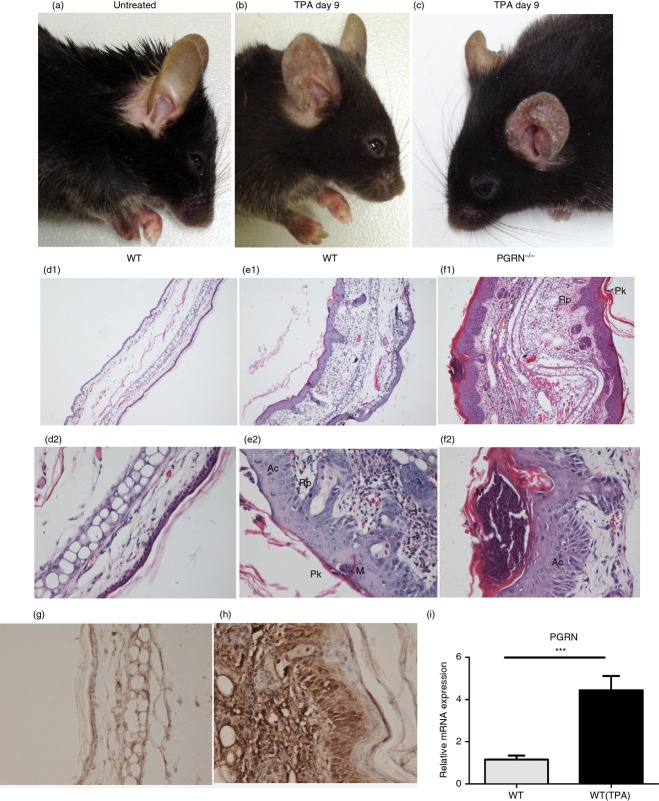
Psoriasis is unique to humans, but multiple topical applications of TPA to mouse skin has traditionally been used to model some aspects of psoriasis.16 Repeated application of TPA for 9 days (every other day) to the ears of WT C57BL/6 mice induced progressive moderate psoriasis-like inflammation manifested by redness, scaling and thickening (Fig.3b). Analysis of haematoxylin & eosin-stained sections from the ear in untreated WT mice showed one- to two-cell thick epidermis and few-cell thick sub-epidermal layers with blood vessels, sebaceous glands, hair follicles, muscle bundles and central fat and connective tissue layer (Fig.3d1, d2). Histology of TPA-treated ears in WT mice revealed acanthosis (thickening of the epidermis due to proliferation of keratinocytes), formation of rete pegs (downward papillary projections of epidermis), parakeratosis (retention of keratinocyte nuclei in stratum corneum), formation of epidermal microabscesses and thickening of the sub-epidermal layer with dense inflammatory cellular infiltrates (Fig.3e1, e2). All of these changes are highly characteristic of psoriasis lesions.
Figure 3.
Progranulin (PGRN) expression was increased in psoriasis-like mouse skin lesions and PGRN−/− mice have enhanced response in the skin following multiple 12-O-tetradecanoylphorbol 13-acetate (TPA) application. (a) Untreated wild-type (WT) mice showed normal appearance. TPA application to ears on days 0, 2, 4, 6 and 8 induced mild psoriasis-like inflammation in WT mice (b) and severe psoriasis-like inflammation in PGRN−/− mice with increased redness, swelling and extensive scaling (c) (n = 5 for each group). (d1, d2) Ear histology of untreated WT mice revealed one- to two-cell thick epidermis and few-cell thick sub-epidermal layer. Ear histology of TPA-treated ears in WT mice (e1, e2) revealed acanthosis (Ac), formation of rete pegs (Rp), parakeratosis (Pk), formation of epidermal microabscesses (M), and thickening of the sub-epidermal layer with dense inflammatory cellular infiltrates, that were all highly prominent in PGRN−/− mice (f1, f2). Original magnification: (d1–f1) × 100; (d2–f2) × 400; (n = 5 for each group). Immunohistochemical staining showed that PGRN expression was dramatically enhanced in the ear of TPA-treated WT mice (h) compared with untreated WT mice (g). (Original magnification × 400; n = 5 for each group). (i) Relative mRNA levels of PGRN were higher in the ear of TPA-treated WT mice than those of untreated WT mice, measured by quantitative RT-PCR (n = 5 for each group). ***P < 0·005.
We next studied the expression of PGRN in the ears of untreated and TPA-treated WT mice. As shown in Fig.3(g, h), PGRN expression was dramatically enhanced in the ears of TPA-treated WT mice, and high-resolution analysis showed that PGRN was mainly expressed in epidermis and infiltrating inflammatory cells. Moreover, we assessed the expression of PGRN by quantitative RT-PCR. As indicated in Fig.3(i), markedly increased levels of PGRN mRNA were detected in the ears of TPA-treated WT mice.
PGRN−/− mice have an enhanced inflammatory response to TPA in the skin
To investigate the role of PGRN in psoriasis, we used PGRN−/− mice, along with their WT counterparts, in a multiple TPA topical application model. The skin of PGRN−/− mice has normal appearance and pathology (not shown). Applying multiple topical TPA challenges in both genotypes, we found a significantly stronger inflammatory response, manifested by more redness, thickening and scaling, in PGRN−/− mice compared with WT mice (Fig.3c and b, respectively). The ear samples were also assessed via histology and results showed that PGRN−/− mice exhibited thicker epithelium, larger epidermal microabscesses, and increased dermal angiogenesis and infiltration of inflammatory cells (Fig.3f1, f2) than WT mice (Fig.3e1, e2).
PGRN−/− mice have decreased percentages of Treg cells in the skin and cervical lymph nodes
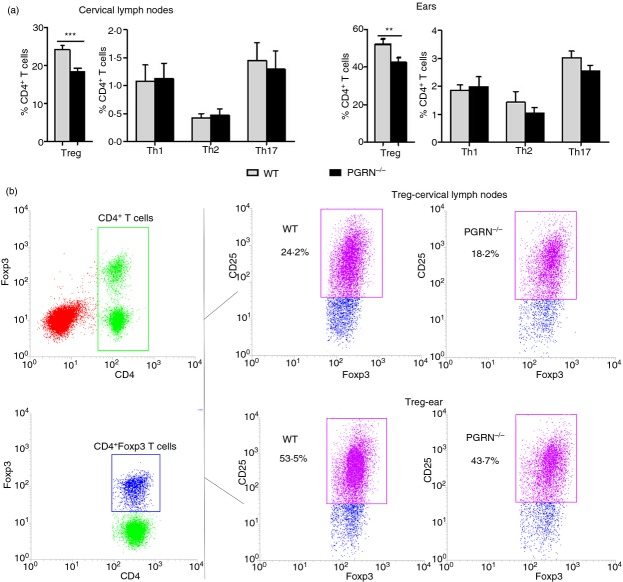
We next studied the CD4+ T-cell subsets in the ears and cervical lymph nodes by flow cytometry to determine the cellular basis for the differences in the inflammatory response between PGRN−/− mice and WT mice. TPA-treated PGRN−/− mice had significantly lower percentages of Treg cells (CD4+ CD25+ FoxP3+) in the affected ears and draining cervical lymph nodes compared with TPA-treated WT mice (Fig.4). In contrast, there was no significant difference in the percentages of Th1 (CD4+ IFN-γ+), Th2 (CD4+ IL-4+) and Th17 (CD4+ IL-17+) cells in the ears and cervical lymph nodes between WT and PGRN−/− mice (Fig.4).
Figure 4.
12-O-Tetradecanoylphorbol 13-acetate (TPA) -treated progranulin-deficient (PGRN−/−) mice have decreased percentages of regulatory T (Treg) cells in the affected skin and cervical lymph nodes. (a) Percentages of T helper type 1 (Th1), Th2, Th17 and Treg cells in the ears and cervical lymph nodes in WT and PGRN−/− mice on day 9 after application of TPA to the ears on days 0, 2, 4, 6 and 8 measured by flow cytometry are shown as means ± SEM of four mice/group (***P < 0·001; **P < 0·005). (b) Representative dot plots for Treg cells in the ears and cervical lymph nodes in WT and PGRN−/− mice are shown; numbers beside boxes indicate the percentages of Treg cells among CD4+ T cells.
Discussion
Progranulin is an autocrine growth factor that is mainly expressed in epithelial cells, immune cells, neurons5 and chondrocytes.17 Studies have confirmed the anti-inflammatory role of PGRN in mouse models of inflammatory diseases, including inflammatory arthritis,9 cerebral ischaemic injury,18 toxin-induced brain injury,19 atherosclerosis20and contact dermatitis.11 But the expression and role of PGRN in psoriasis have not been fully investigated. In the present study, we demonstrated that PGRN levels were significantly higher in both lesions and serum of patients with psoriasis vulgaris than in those of control subjects. In addition, serum PGRN concentrations in psoriasis vulgaris patients were significantly decreased after effective treatment. These results indicate that PGRN is related to the pathogenesis of psoriasis.
To elucidate the role of PGRN in psoriasis, we studied the mouse model of TPA-induced psoriasis-like skin inflammation. In accordance with human psoriatic lesions, PGRN expression was dramatically enhanced in the ears of TPA-treated WT mice. These results suggest that PGRN over-expression is involved in the development of the TPA-induced mouse model of psoriasis. Surprisingly, analysis of TPA-treated skin in PGRN−/− mice suggested that PGRN deficiency aggravates the psoriasis-like inflammation in both lesional appearance and histological characteristics. This finding reveals that PGRN plays both protective and anti-inflammatory roles in the development of psoriasis. In fact, several mouse models (including topical application of imiquimod) are considered superior to the TPA model in replicating human psoriatic lesions,21 because TPA application in WT mice simulates part of the human psoriatic phenotype. Hence, induction of more typical psoriasiform lesions in PGRN−/− mice following TPA treatment convincingly implicates the important protective role of PGRN in pathogenesis of psoriasis vulgaris.
Given that PGRN acts as a strong anti-inflammatory mediator by being an antagonist of TNF-α signalling,9 we further investigated the relationship between serum PGRN levels and TNF-α levels in patients with psoriasis vulgaris. We found that serum PGRN levels were positively correlated with TNF-α levels. In other words, the serum TNF-α levels increased with rising PGRN levels. Based on these results, we speculated that PGRN, as a competitive molecule of TNF-α, is highly expressed in psoriasis vulgaris secondary to the increased inflammatory cytokines, in particular TNF-α. It is well known that TNF-α promotes an inflammatory response in psoriasis vulgaris,1 whereas PGRN prevented inflammation in this study. Therefore, we speculated that the ratio of PGRN/TNF-α in serum is an important factor to evaluate the inflammatory microenvironment of a patient. To confirm this speculation, we studied the relationship between serum PGRN/TNF-α ratio and disease severity. We demonstrated that serum PGRN/TNF-α ratio was negatively correlated with PASI scores. The result suggested that the lower the PGRN/TNF-α ratio, the more severe the disease. Further increasing the levels of PGRN by applications of exogenous PGRN, or its derived engineered protein Atsttrin, may be useful as therapies for psoriasis. Matsubara et al. found that PGRN played a pro-inflammatory role in obesity and insulin-resistant diabetes through the production of IL-6.13 In our study, we evaluated the relationship between PGRN and IL-6 levels in serum and showed that there was no significant correlation between them, which did not support the pro-inflammatory role of PGRN in psoriasis vulgaris.
To further elucidate the mechanisms for the anti-inflammatory role of PGRN in psoriasis, we detected the CD4+ T-cell subsets in the ears and cervical lymph nodes by flow cytometry. A difference of Treg cells, not Th1, Th2 and Th17 cells, in the ears and cervical lymph nodes was observed between TPA-treated WT and PGRN−/− mice. It is well-established that Treg cells are able to inhibit the immunological response and to maintain cutaneous immunological homeostasis.22 Human skin is enriched in Treg cells – up to 80% of CD4+ T cells could be Treg cells in skin of healthy individuals.23 In contrast, patients with psoriasis have lower percentages of Treg cells in affected skin, for example 33%,23 45%24 or 50%25 of total CD4+ cells. Moreover, studies of Treg cells in patients or mouse models treated with infliximab,26 vitamin D327 or 8-methoxypsoralen plus ultraviolet A28 showed that numbers of Treg cells were increased. Hence, deficient generation or recruitment of Treg cells may be a significant factor contributing to the development of psoriasis, although this aspect has not been sufficiently studied. In addition, it has been reported that the number of Treg cells in lymph nodes was reduced in PGRN−/− mice upon induction of contact dermatitis11 and that PGRN promoted the differentiation of Treg cells from naive T cells in vitro.9 Furthermore, Hu et al. reported that PGRN enhanced proliferation of Treg cells in the presence of TNF29 in vitro. In the present study, our results support these views and extend these findings by showing that TPA-treated PGRN−/− mice have decreased numbers of Treg cells not only in draining cervical lymph nodes but also in the affected ears compared with WT mice. These results suggest that PGRN−/− mice are less efficient than WT mice in generating Treg cells in lymph nodes and recruiting these cells to the inflamed skin. Hence, the mechanism underlying increased sensitivity of PGRN−/− mice to TPA-induced psoriasiform inflammation is impaired differentiation of Treg cells and decreased recruitment of these cells in the affected skin, which results in more severe inflammation. In WT mice, PGRN promotes differentiation and recruitment of Treg cells, which protects the skin from an exaggerated psoriasis-like inflammatory phenotype.
In conclusion, our study demonstrates that PGRN plays a protective role in the pathogenesis of psoriasis vulgaris partly by increasing the numbers of Treg cells. Serum PGRN/TNF-α ratio could be a useful biomarker for disease severity. The feedback increase of PGRN was not sufficient to neutralize the development of psoriasis vulgaris. It is suggested that the applications of recombinant PGRN or its derived engineered protein, Atsttrin, may be useful as therapies for psoriasis. Further studies aimed at understanding the molecular events during the regulation of PGRN on Treg cells are important in the development of effective therapeutic strategies in psoriasis.
Acknowledgments
This study was supported by the National Natural Science Foundation grants of China (No. 81402606). We thank the patients who participated in this study.
Glossary
Abbreviations:
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL-6
interleukin-6
- PASI
Psoriasis Area and Severity Index
- PE
phycoerythrin
- PGRN
progranulin
- Th1
T helper type 1
- TNF-α
tumour necrosis factor-α
- TNFR
tumour necrosis factor receptors
- TPA
12-O-tetradecanoylphorbol 13-acetate
- Treg
regulatory T cells
- WT
wild-type
Disclosure
The authors declare no conflict of interests.
Authors contribution
YBY, XMZ, KH and JC conceived and designed the experiments; KH, AJC, ZXS and HMX collected samples and performed the experiments; KH and XMZ analysed the data; KH, XMZ, JC and YBY wrote the paper.
References
- Lowes MA, Bowcock AM, Krueqer JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Hrabal R, Chen Z, James S, Bennett HP, Ni F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat Struct Biol. 1996;3:747–52. doi: 10.1038/nsb0996-747. [DOI] [PubMed] [Google Scholar]
- Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. J Histochem Cytochem. 2000;48:999–1009. doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. BioEssays. 2009;31:1245–54. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Huang K, Huang C, Shan K, Chen J, Li H. Significance of PC cell-derived growth factor and cyclin D1 expression in cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2012;37:411–7. doi: 10.1111/j.1365-2230.2011.04275.x. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CJ. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585:3675–80. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587:1805–10. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol Ther. 2012;133:124–32. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Mita A, Minami K, et al. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab. 2012;15:38–50. doi: 10.1016/j.cmet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Park SY, Gupta D, Hurwich R, Kim CH, Dziarski R. Peptidoglycan recognition protein pglyrp2 protects mice from psoriasis-like skin inflammation by promoting Treg and limiting Th17 responses. J Immunol. 2011;187:5813–23. doi: 10.4049/jimmunol.1101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrero G, Ioffe OB. Expression of PC cell- derived growth factor in benign and malignant human breast epithelium. Hum Pathol. 2003;34:1148–54. doi: 10.1016/s0046-8177(03)00425-8. [DOI] [PubMed] [Google Scholar]
- Petersen TK. In vivo pharmacological disease models for psoriasis and atopic dermatitis in drug discovery. Basic Clin Pharmacol Toxicol. 2006;99:104–15. doi: 10.1111/j.1742-7843.2006.pto_298.x. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Guo FJ, Jiang BC, et al. Granulin epithelin precursor: a bone morphogenic protein 2- inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J. 2010;24:1879–92. doi: 10.1096/fj.09-144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Ji F, Wang F, Liu B, Zhu Y. Neuroprotective effects of progranulin in ischemic mice. Brain Res. 2012;1436:130–6. doi: 10.1016/j.brainres.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Martens LH, Zhang J, Barmada SJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122:3955–9. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase R, Ohama T, Matsuyama A, et al. Deletion of progranulin exacerbates atherosclerosis in ApoE knockout mice. Cardiovasc Res. 2013;100:125–33. doi: 10.1093/cvr/cvt178. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Johnston A, Carbajal S, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE. 2011;6:e18266. doi: 10.1371/journal.pone.0018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Miyachi Y, Kabashima K. Regulatory T cells in cutaneous immune responses. J Dermatol Sci. 2011;63:75–82. doi: 10.1016/j.jdermsci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Antiga E, Quaglino P, Bellandi S, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. 2010;162:1056–63. doi: 10.1111/j.1365-2133.2010.09633.x. [DOI] [PubMed] [Google Scholar]
- Franz B, Fritzsching B, Riehl A, et al. Low number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosus. Arthritis Rheum. 2007;56:1910–20. doi: 10.1002/art.22699. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Okuyama R, Ito Y, Aiba S. Profiles of Foxp3+ regulatory T cells in eczematous dermatitis, psoriasis vulgaris and mycosis fungoides. Br J Dermatol. 2008;158:1256–63. doi: 10.1111/j.1365-2133.2008.08504.x. [DOI] [PubMed] [Google Scholar]
- Quaglino P, Ortoncelli M, Comessatti A, et al. Circulating CD4+ CD25bright FOXP3+ T cell are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology. 2009;219:250–8. doi: 10.1159/000238305. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, Sibiryak DS, Bakdash G, et al. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol. 2011;127:1532–40. doi: 10.1016/j.jaci.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Singh TP, Schön MP, Wallbrecht K, et al. 8-Methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder. J Immunol. 2010;184:7257–67. doi: 10.4049/jimmunol.0903719. [DOI] [PubMed] [Google Scholar]
- Hu Y, Xiao H, Shi T, Oppenheim JJ, Chen X. Progranulin promotes tumor necrosis factor-induced proliferation of suppressive mouse CD4+ Foxp3+ regulatory T cells. Immunology. 2014;142:193–201. doi: 10.1111/imm.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]