Abstract
Natural killer (NK) cells are essential components of the immune system, and due to their rapid response potential, can have a great impact during early anti-viral immune responses. We have previously shown that interleukin-2-dependent NK and CD4+ T-cell co-operative immune responses exist in long-term simian immunodeficiency virus (SIV) -infected controlling macaques and can be rescued in SIV-infected non-controlling macaques by a short course of antiretroviral therapy (ART). Given that co-operative responses may play an important role in disease prevention and therapeutic treatment, in the present study we sought to determine if these responses can be enhanced in chronically SIV-infected macaques by vaccination with a single-dose of envelope protein given during ART. To this end, we treated 14 chronically SIV-infected macaques with ART for 11 weeks and gave 10 of these macaques a single intramuscular dose of SIV gp120 at week 9 of treatment. ART significantly decreased plasma and mucosal viral loads, increased the numbers of circulating CD4+ T cells in all macaques, and increased T-cell-dependent envelope- and gag-specific interferon-γ and tumour necrosis factor-α production by circulatory CD56+ NK cells. The therapeutic envelope immunization resulted in higher envelope-specific responses compared with those in macaques that received ART only. Functional T-cell responses restored by ART and therapeutic Env immunization were correlated with transiently reduced plasma viraemia levels following ART release. Collectively our results indicate that SIV-specific T-cell-dependent NK cell responses can be efficiently rescued by ART in chronically SIV-infected macaques and that therapeutic immunization may be beneficial in previously vaccinated individuals.
Keywords: antiretroviral therapy, envelope immunization, natural killer cells, T cells
Introduction
Natural killer (NK) cells are specialized innate lymphoid cells that bridge innate and adaptive immunity.1 Given their widespread circulatory and tissue distributions, NK cells play both immunoregulatory (production of inflammatory and regulatory cytokines) and cytotoxic (elimination of pathogenic and neoplastic cells) roles.2 Circulatory NK cells in rhesus macaques are phenotypically characterized as CD3− CD8α+ CD159a+, and can be further subdivided into three distinct subsets based on their expression patterns for CD16 and CD56: CD16+ CD56dim (CD16+ NK cells), CD16− CD56+ (CD56+ NK cells) and CD16− CD56− [double-negative (DN) NK cells].3,4 The proportional distribution of these NK cell subsets greatly varies depending on the tissues being studied. A fourth subset (CD16+ CD56+) exists in tissue-resident NK cells.5,6
Recent studies have identified several potentially protective functions of NK cells in response to HIV/simian immunodeficiency virus (SIV) exposures. For example, certain killer cell IgG-like receptor phenotypes have been associated both with reduced risk of HIV infection and slower disease progression in individuals who become infected and have been shown to influence viral loads, independently of MHC background, in SIV-infected rhesus macaques.7,8 Furthermore, NK depletion during acute SIV infection has been associated with increased viral loads in the chronic phase.9 NK cells can also mediate effector functions in collaboration with SIV-specific humoral immune responses through mechanisms such as antibody-dependent cell-mediated cytotoxicity and antibody-dependent cell-mediated virus inhibition, which have been previously correlated with protection against SIV and SHIV infection.10–15
Recently, murine NK cells have been shown to possess memory-like function, which can be induced through viral infection,16 cytokine activation17 or hapten sensitization.18 Despite this, the identification of memory-like functions in humans and non-human primate models has proved difficult. Nevertheless, studies using non-human primate and human peripheral blood mononuclear cells (PBMCs) have shown that an antigen-specific collaborative response involving memory-experienced CD4+ T cells and circulatory, as well as, tissue-resident NK cells exists in response to viral exposures as disparate as rabies,19 influenza20 and SIV5 as well as protozoan21 pathogens. Further, a recent study showed that a therapeutic HIV-1 gp120/Nef/Tat subunit protein vaccination of chronically infected individuals aided recovery of HIV-specific interleukin-2 (IL-2) production by CD4+ T cells and subsequently improved HIV-specific interferon-γ (IFN-γ) production by NK cells.22 With regard to SIV infection, we previously reported that SIV-specific T-cell-dependent NK cell effector responses were present in a group of SIV-infected (SIV+) controller rhesus macaques able to maintain low levels of chronic viraemia, but were absent in SIV+ non-controlling macaques. In SIV+ controlling animals, IL-2-dependent co-operative responses were exhibited by all NK cell subsets in the circulation and in various tissues as measured by cytokine production [IFN-γ and tumour necrosis factor-α (TNF-α)] as well as by expression of the de-granulation surrogate marker CD107a.5 Furthermore, a short course of antiretroviral therapy (ART) was able to partially rescue SIV-specific T-cell-dependent NK cell effector responses in SIV+ non-controlling macaques.
To pursue these observations, in the present study we evaluated whether T-cell-dependent NK cell effector responses in SIV+ non-controlling macaques could be enhanced through a combined approach of ART plus a single intramuscular SIVmac251 gp120 immunization and lead to control of viraemia rebound upon ART cessation. We observed that although ART significantly decreased plasma and rectal tissue viral loads, it only improved SIV-specific T-cell-dependent NK cell responses transiently. The addition of a single Env immunization had a modest effect, most evident in macaques that had previously been vaccinated with SIV Env. The combination of ART and Env immunization impacted not only T-cell function and associated NK responses, but also plasma viral loads following ART release. Our results suggest that although therapeutic modulation of SIV-specific T-cell-dependent NK cell responses is possible, longer or more effective ART regimens will be required to fully overcome the chronic effects of SIV infection. The combined approach of ART plus therapeutic immunization may be most effective in previously vaccinated individuals that subsequently become infected.
Materials and methods
Animals, immunization regimens and sample collection
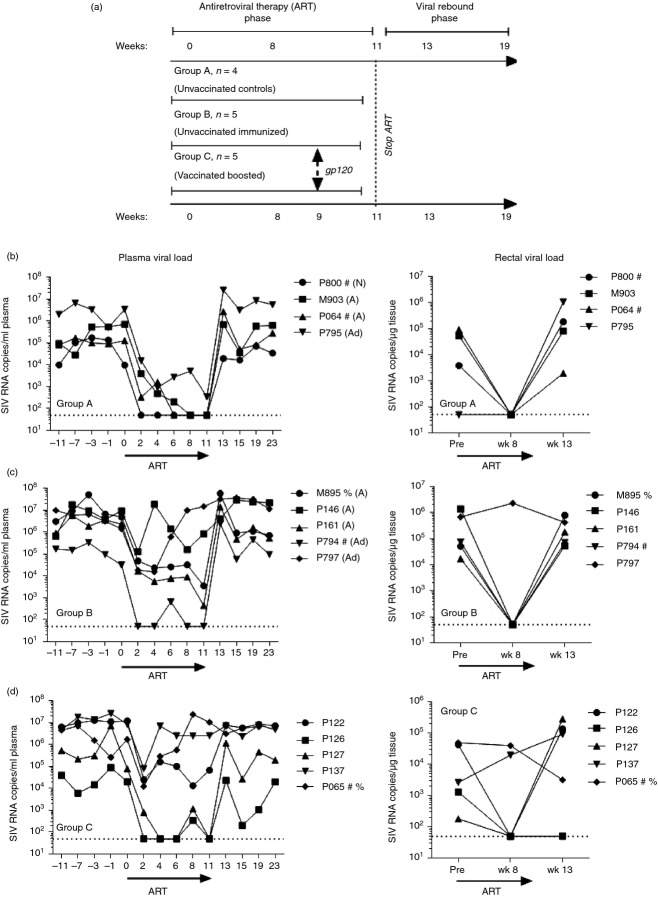
Therapeutic modulation of CD4+-dependent NK cell responses was studied in 14 chronically SIV+ outbred Indian rhesus macaques housed at Advanced BioScience Laboratories, Inc. (ABL, Rockville, MD) and maintained according to Institutional Animal Care and Use Committee guidelines and the NIH Guide for the Care and Use of Laboratory Animals. The chronically infected macaques were recycled from two previous vaccine studies.23,24 Ten had been infected for 27–32 weeks and four had been infected for 60–65 weeks before the start of the study. They were treated with an ART cocktail of three reverse transcriptase inhibitors [intravenous didanosine, oral stavudine, and subcutaneous 9-(2-phosphonylmethoxypropyl) adenine] as previously described in a concurrent study that evaluated B-cell dynamics.25 Group A macaques (ART alone controls) also served as controls for another concurrent study that evaluated the immune targeting of programmed cell death-1.26 Animals were divided into three treatment groups (Fig.1a) based on their previous immunization status (either unvaccinated or vaccinated) and balanced to achieve similar plasma viral load levels and CD4+ T-cell counts before treatment initiation. Unvaccinated macaques used in Groups A and B (n = 9) had previously been used as controls and were either naive or had received empty adenoviral vector followed by monophosphoryl lipid A-stable emulsion (MPL-SE) adjuvant only or alum adjuvant alone as indicated (Fig.1b,c). Vaccinated macaques in Group C had all previously been immunized only with 200 µg of SIV gp120 protein in alum. Group A contained two Mamu A*01 positive macaques; Group B contained one A*01 and one B*17 positive macaques and Group C contained an A*01/B*17 positive macaque as noted (Fig.1b–d). ART therapy was given for 11 weeks and at week 9 animals in Groups B and C received 100 μg of SIVmac251 gp120 adjuvanted with 50 μg of MPL-SE intramuscularly.
Figure 1.
Study design and plasma and tissue viral loads for individual animals throughout the study. (a) Schematic design of the study. (b–d) Plasma and rectal tissue viral loads were obtained at the indicated time-points by nucleic acid sequence-based amplification (NASBA). Dotted lines indicate the lower limit of viraemia detection (50 copies) by NASBA. Viral loads in Group A [antiretroviral therapy (ART) only controls] were previously reported.26 #, Mamu A*01 positive; %, Mamu B*17 positive; N, naive; A, received alum only; Ad, received empty Ad vector and MPL-SE adjuvant only.
Blood samples were collected by venepuncture of anaesthetized animals into EDTA-treated collection tubes. PBMCs were obtained by centrifugation on Ficoll-Paque PLUS gradients (GE Healthcare, Piscataway, NJ). Cells were washed and resuspended in R-10 medium (RPMI-1640 containing 10% fetal bovine serum, 2 mm l-glutamine, 1% non-essential amino acids, 1% sodium pyruvate and antibiotics). Rectal biopsies were digested for 60 min on an orbital shaker in R-10 medium containing 1 mg/ml of collagenase II (Sigma; St Louis, MO).Viral loads in plasma and rectal tissue biopsies were determined by nucleic acid sequence-based amplification assay as previously described.27
Flow cytometry and absolute cell counts
In the present study we used anti-human fluorochrome-conjugated monoclonal antibodies known to cross-react with rhesus macaque antigens. These included: V450 anti-IFN-γ (B27), phycoerythrin (PE) -Cy5 anti-CD95 (DX2), PE-Cy7 anti-CD56 (B159), Alexa Fluor 700 anti-CD3 (SP34-2), allophycocyanin (APC) -Cy7 anti-IL-2 (MQ1-17H12) and APC-Cy7 anti-CD16 (3G8), all from BD Biosciences (San Jose, CA); PE-Cy7 anti-CD28 (CD28.2), and eFluor 650NC anti-CD8α (RPA-T8), all from eBioscience (San Diego, CA); QDot605 anti-CD8α (3B5) and PE-Texas Red anti-Granzyme B (GB11) from Invitrogen (Carlsbad, CA); PE anti-NKG2A (Z199) from Beckman Coulter (Fullerton, CA); QDot655 anti-CD4 (T4/19Thy5D7) and APC anti-α4β7 (IgG1 rhesus recombinant) from the NIH Nonhuman Primate Reagent Resource (Boston, MA); FITC anti-Perforin (Pf-344) from MabTech (Mariemont, OH); and Pacific Blue anti-CD197 (TG8/CCR7) from Biolegend (San Diego, CA). Yellow and aqua LIVE/DEAD viability dyes (Invitrogen) were used to exclude dead cells. For circulatory and rectal NK cell phenotyping, PBMCs and rectal lymphocytes were used fresh. For measurement of T-cell-dependent NK cell function, fresh PBMCs were stimulated with SIV-specific peptides (see below) before surface and intracellular staining. In both cases cells were stained for specific surface molecules, fixed and permeabilized with a Cytofix/Cytoperm Kit (BD Biosciences), and then stained for specific intracellular molecules. At least 500 000 singlet events were acquired on an SORP LSR II (BD Biosciences) and analysed using FlowJo Software (Tree Star Inc., Ashland, OR). For all samples, gating was established using a combination of isotype and fluorescence-minus-one controls.
NK and T-cell activation assays
T-cell recall responses as well as T-cell-dependent NK cell responses were assayed in PBMCs as previously described.5 Briefly, T-cell-dependant NK cell responses were assayed by stimulating 2 × 106 PBMCs with 1 μg/ml SIV239 Gag or Env peptide pools (complete sets of 15-mer peptides, overlapping by 11 and spanning the entire protein; NIH AIDS Research & Reference Reagent Program, Germantown, MD) for 24 hr. Stimulation was performed in the presence of 2 μg/ml unconjugated anti-CD49d and anti-CD28 (BD Biosciences). BD GolgiPlug, BD GolgiStop and Alexa Fluor 647 anti-CD107a were added for the last 5 hr of culture at the manufacturer's recommended concentrations. T-cell recall responses were assayed by stimulating 2 × 106 PBMCs with 1 μg/ml SIVmac239 Gag or Env peptide pools for 6 hr. Stimulation was performed in the presence of 10 μg/ml Brefeldin A, 2 μg/ml anti-CD49d and 0·375 μg/ml PE-Cy7 anti-CD28 (all from BD Biosciences). For both T-cell and NK cell activation assays, non-stimulated and SEB-treated (5 μg/ml; Sigma) tubes were used as negative and positive controls, respectively.
Statistical analysis
Data were analysed using Prism (v5.03, GraphPad Software, Inc., La Jolla, CA). A P value of ≤ 0·05 was considered statistically significant for each test.
Results
Impact of ART and Env immunization on plasma and rectal tissue viral loads and CD4+ T cell counts in chronically SIV-infected macaques
Given that we had previously shown that 8 weeks of ART partially restores SIV-specific T-cell-dependent NK cell effector responses in SIV+ non-controlling macaques,5 we sought to determine whether a single intramuscular immunization with SIVmac251 gp120 protein given during ART would boost Env-specific CD4+ T-cell immune responses and therefore improve T-cell-dependent NK cell effector function. For this purpose 14 chronically SIV+ rhesus macaques were divided into three treatment groups (Fig.1a). Group A received 11 weeks of daily ART; Groups B and C also received 11 weeks of daily ART plus a single 100 μg dose of SIVmac251 gp120 at week 9. Animals in Groups A and B received no SIV immunogens before SIV infection, whereas animals in Group C had been vaccinated twice with SIV gp120 in alum before SIV infection.24 Fig.1(b–d) shows the effect of ART therapy on plasma and rectal tissue viral loads in each individual macaque by group. Group A macaques responded well to ART and rectal tissue viral loads were below the limit of detection at week 8 (Fig.1b). On the other hand, ART was less successful in Group B and C macaques, which displayed incomplete plasma and rectal tissue viral load reductions (Fig.1c,d), although both parameters were reduced at least two logs in all macaques. Some discrepancies in viral load reductions were seen between plasma and rectal tissue viral loads in individual macaques, attributable to tissue sampling, as rectal viral loads of each macaque were determined on single rectal pinch biopsies. Despite the variable response to ART, there were no significant differences in geometric mean plasma and rectal tissue viral loads between treatment groups over the course of the study (Fig.2a,b). Further, all groups of animals showed a comparable and statistically significant recovery in their absolute number of circulating CD4+ T cells following the 11 weeks of ART (Fig.2c).25 No significant changes in the absolute number of circulating CD8+ T cells were observed (Fig.2d). Upon ART cessation, all animals displayed a rebound in their plasma and tissue viral loads and a slow decrease in their circulating CD4+ T cells (Fig.2a–c).
Figure 2.
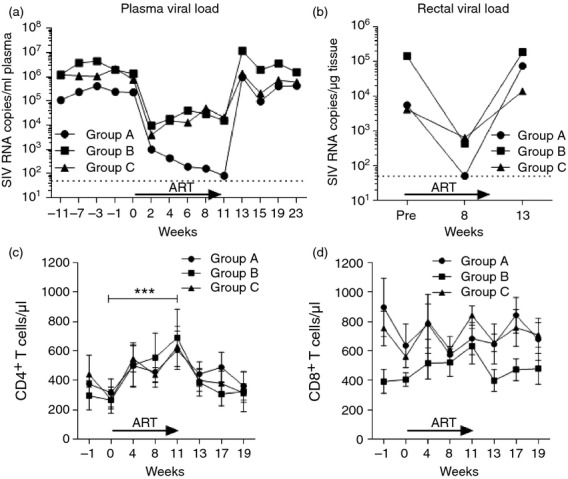
Impact of antiretroviral therapy (ART) and Env immunization on viral load rebound and circulatory CD4+ T-cell counts. Geometric mean plasma (a) and rectal (b) viraemia for each group of macaques as measured by nucleic acid sequence-based amplification (NASBA). (c, d) Absolute counts of circulatory CD4+ (c) and CD8+ (d) T cells throughout the study. CD4+ T-cell counts from these macaques have been previously reported as part of concurrent B-cell25 study. ***P < 0·001 indicates statistically significant differences between the indicated time-points by the Wilcoxon signed rank test.
Phenotypic and functional changes in NK cells of SIV-infected macaques undergoing ART with or without SIV gp120 immunization
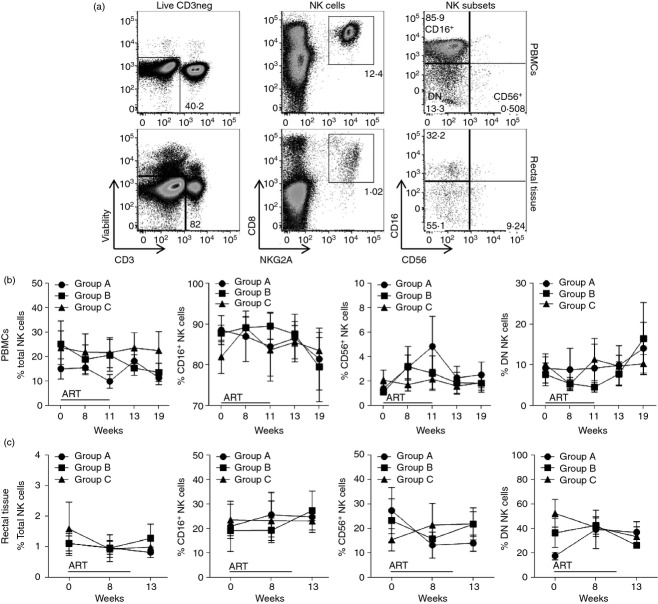
The objective of the present study was to monitor phenotypic and functional changes in circulatory and rectal NK cells (CD3− CD8α+ CD159a+) associated with ART and gp120 immunization. Initially, we measured the proportional abundance of NK cells (total and subset populations) in PBMCs and rectal tissue over the course of the study. The NK gating strategy is shown in Fig.3(a). There were no significant differences in the proportion of total NK cells or subsets at any time-point in any group either in PBMCs (Fig.3b) or rectal tissue (Fig.3c).
Figure 3.
Changes in total and subset-specific natural killer (NK) cells in peripheral blood mononuclear cells (PBMCs) and rectal tissue during antiretroviral therapy (ART), with or without Env immunization. Freshly isolated PBMCs and rectal tissue-derived lymphocytes were stained to determine the percentage of total (CD3− CD8+ NKG2A+) NK cells as well as CD16+, CD56+ and double-negative (DN) NK subsets. (a) Gating strategy used to identify NK cells in PBMC (top) and rectal (bottom) samples. (b, c) Total and subset-specific distribution of NK cells in PBMCs (b) and rectal tissue (c) samples. Data reported are means ± SEM.
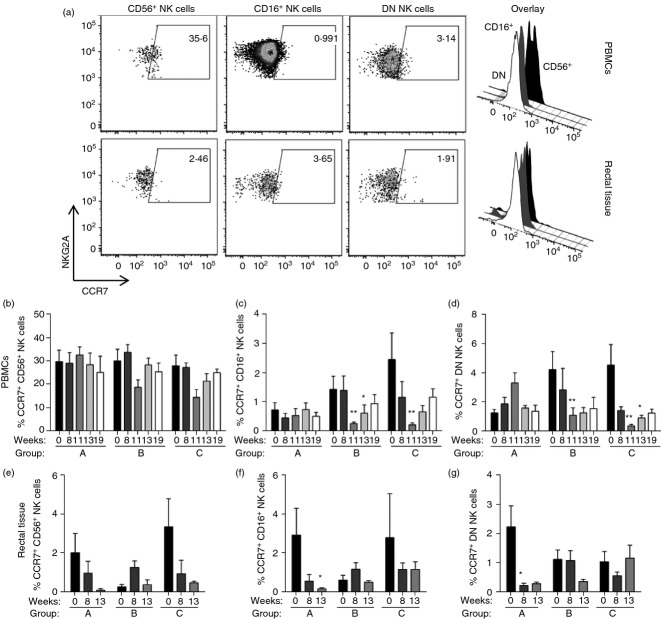
Next, we evaluated the changes in surface expression levels of the homing markers CCR7 and α4β7, as well as the cytotoxic intracellular proteins granzyme B and perforin. Throughout the study, no changes were detected in expression levels of the gut homing marker α4β7 or the cytotoxic proteins granzyme B and perforin in any subset of NK cells (data not shown). However, we detected a subset-specific change in expression of the lymph node homing marker CCR7 both in PBMC and in rectal tissue-resident NK cells (see representative dot plots, Fig.4a). Within PBMCs, no changes in CCR7 expression were observed in CD56+ NK cells (Fig.4b). On the other hand, we observed a reduction in CCR7 expression in CD16+ and DN NK cells at week 11, but only in the two groups of animals (Groups B and C) that received the Env therapeutic immunization at week 9 (Fig.4c,d; week 11). At week 13 (2 weeks post-ART release), CCR7 expression was still significantly reduced in CD16+ NK cells in Group B macaques (Fig.4c) and DN NK cells in Group C macaques (Fig.4d). It should be mentioned that while rhesus macaque CD56+ and DN NK cells have previously been shown to express CCR7,4,6 CD16+ NK cells have been reported not to express CCR7.28 Here we observed that before any treatment CCR7 was expressed at low and variable levels, similar between groups except for CCR7+ DN NK cells, where pre-ART levels differed between Groups A and C (P < 0·05). Mean levels of CCR7+ cells before treatment were 1·5 ± 0·4% of CD16+ NK cells and 3·5 ± 0·7% of DN NK cells in all groups compared with 29·2 ± 2·6% within CD56+ NK cells.
Figure 4.
Antiretroviral therapy- and Env immunization-associated changes in CCR7 expression in circulatory and rectal tissue-resident natural killer (NK) cells. Freshly isolated peripheral blood mononuclear cells (PBMCs) and rectal tissue-derived lymphocytes were analysed for their CCR7 surface expression levels on CD56+, CD16+ and double negative (DN) NK cell subsets. (a) Representative dot plots and overlaying histograms showing CCR7 expression patterns in different NK cell subsets in PBMCs (top) and rectal (bottom) samples. (b–g) Percentage of CCR7+ NK cells in each subset in PBMCs (top, b–d) and rectal (bottom, e, f) samples. Data reported are means ± SEM. *P < 0·05 and **P < 0·01 indicate statistically significant differences in each group when compared with Pre (week 0) by repetitive one-way analysis of variance followed by Dunn's multiple comparisons test.
In rectal tissue-resident NK cells we observed an ART-associated trend, especially in Group A macaques, towards a decrease in CCR7 expression in all NK cell subsets (Fig.4e–g). These decreases in CCR7 expression were only statistically significant at week 13 (Fig.4f) and week 8 (Fig.4g) for CD16+ and DN NK cells, respectively. Importantly, the proportions of CCR7+ NK subsets in rectal tissue of all macaques before treatment were also low and variable, but not different across NK cell subsets and macaque groups: 2·2 ± 0·9% for CD16+ NK cells, 2·0 ± 0·6% for CD56+ NK cells, and 1·4 ± 0·3% for DN NK cells. The biological significance of the changes seen in the minor populations of CCR7+ CD16+ and DN NK cells is unknown. CCR7+ CD56+ NK cells represent the major subset of NK cells present in lymph node T-cell areas where they participate in bidirectional T-cell–NK cell interactions.29 Changes in CCR7 expression, particularly in CD16+ NK cells, may reflect the immunomodulatory properties of the Toll-like receptor 4 (TLR4) -agonist (MPL-SE) used to adjuvant the Env therapeutic immunization. It has been previously reported that dendritic cells activated through TLR4 recruit NK cells and induce CCR7 expression in CD56dim CD16+ NK cells.30 Whether the changes seen here would be confirmed using larger numbers of macaques requires further study.
We previously reported that Gag-specific and Env-specific T-cell-dependent NK cell effector responses were observed in a group of SIV+ macaques that maintained low levels of chronic viraemia (SIV+ controllers). Furthermore, although these responses were not observed in SIV+ non-controlling animals, they were partially rescued in these animals by short-term ART.5 Here we assessed if ART combined with an Env immunization would further enhance these NK effector responses in SIV+ non-controlling macaques. As shown in Fig.5, Gag-specific and Env-specific T-cell-dependent NK cell effector responses, measured as IFN-γ production by NK cells in a 24-hr peptide stimulation assay, were significantly increased in CD56+ (Fig.5a) and DN (Fig.5b) NK cells of all treatment groups after 11 weeks of ART. Interestingly, although these responses were mostly Gag-specific in the first weeks, Env-specific CD56+ IFN-γ responses were observed at week 11 in the groups that received an Env immunization at week 9 (Fig.5a, week 11, Groups B and C). ART therapy also minimally rescued Gag-specific IFN-γ production by CD16+ NK cells (Fig.5c) but only in macaques from Group A, which were the ones that best responded to ART.
Figure 5.
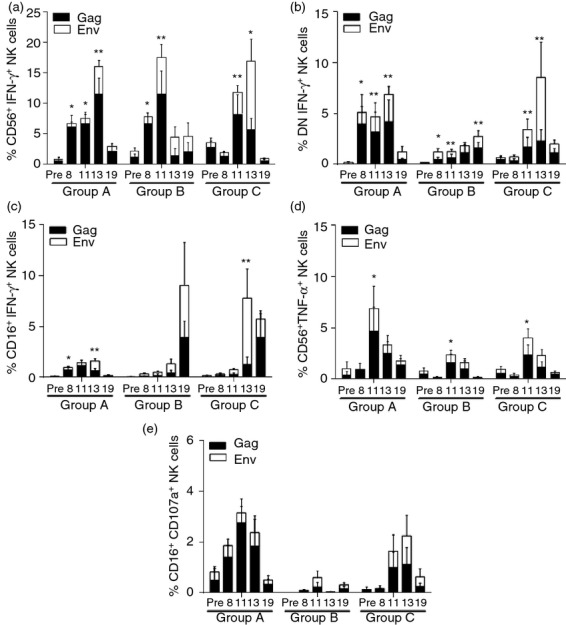
Antiretroviral therapy (ART) -mediated and Env immunization-mediated improvement of simian immunodeficiency virus (SIV) -specific T-cell-dependent natural killer (NK) cell effector function. Peripheral blood mononuclear cells (PBMCs) were stimulated with SIV-specific Gag and Env peptides for 24 hr to evaluate T-cell-dependent production of interferon-γ (IFN-γ) by CD56+ (a), double-negative (DN) (b) and CD16+ (c) NK cells. Similarly, tumour necrosis factor-α (TNF-α) production by CD56+ (d) and CD107a expression by CD16+ (e) NK cells was also measured. Data reported are means ± SEM. *P < 0·05 and **P < 0·01 indicate statistically significant differences at each time-point in combined Gag and Env responses when compared with Pre-ART values by the Wilcoxon matched-pairs signed rank test.
Previously, 8-week ART-mediated recovery of T-cell-dependent NK cell effector responses in SIV+ non-controlling macaques efficiently rescued only IFN-γ production.5 In the present study, 11 weeks of ART therapy successfully, although transiently, recovered TNF-α production by CD56+ NK cells, and this effect appeared to be independent of the therapeutic Env immunization (Fig.5d). Unlike cytokine-producing functions, cytotoxic potential in NK cells could not be rescued by ART plus therapeutic Env immunization as shown in Fig.5(e) by the lack of significant enhancement of CD107a expression. Although 11 weeks of ART plus a single gp120 boost significantly increased T-cell-dependent production of IFN-γ and TNF-α by CD56+ and DN NK cells, upon ART release these responses were progressively decreased and returned to pre-ART levels by week 19 (Fig.5a,b,d).
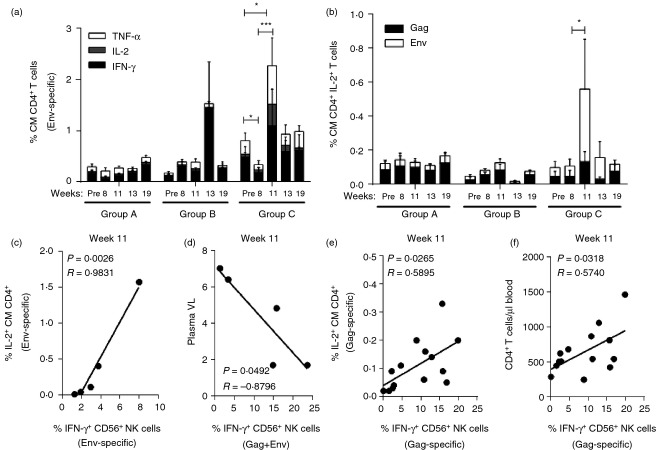
ART plus gp120 immunization impacts SIV-specific IL-2 production by central memory CD4+ T cells and viraemia rebound post-ART
Given that SIV-specific NK cell effector responses are dependent at least in part on IL-2 production by central memory (CM) CD4+ T cells,5 we evaluated the effect of ART plus Env immunization on the function of CD4+ T cells in SIV+ non-controlling macaques. As shown in Fig.6(a), 11 weeks of ART had a minimal effect on the Env-specific production of IFN-γ, IL-2 and TNF-α by CM CD4+ T cells (Group A macaques). On the other hand, gp120 immunization given during ART significantly improved Env-specific production of these three cytokines at week 11 in previously immunized macaques (Fig.6a, Group C), and also enhanced the Env-specific anamnestic response in Group B macaques at week 13 following ART release (Fig.6a). Important for T-cell-dependent NK cell effector function, SIV-specific IL-2 production by CM CD4+ T cells was significantly rescued only in Group C macaques after the therapeutic Env immunization, suggesting a contribution of the previous SIV Env vaccination (Fig.6b). This rescue was lost post-ART. Further validation of the therapeutic effect of the ART plus Env immunization was seen in Group C macaques where Env-specific NK cell responses were strongly correlated with Env-specific IL-2 production at week 11 (Fig.6c) and inversely correlated with plasma viral loads (Fig.6d). In contrast to the Env-specific NK cell responses, we observed a global positive correlation in macaques of all three groups between Gag-specific IL-2-producing CM CD4+ T cells with Gag-specific IFN-γ secreted by CD56+ NK cells at week 11 (P = 0·0265, Fig.6e), reflecting an overall effect of ART. Similarly, the absolute number of circulatory CD4+ T cells at week 11 also correlated with Gag-specific IFN-γ production by CD56+ NK cells (P = 0·0318, Fig.6f).
Figure 6.
Effect of antiretroviral therapy (ART) plus Env therapeutic immunization on simian immunodeficiency virus (SIV) -specific interleukin-2 (IL-2) production by circulatory central memory CD4+ T cells. Freshly isolated peripheral blood mononuclear cells (PBMCs) were stimulated with SIV-specific peptides for 6 hr to measure intracellular cytokine production by central memory (CD28+ CD95+, CM) CD4+ T cells. (a) Env-specific production of interferon-γ (IFN-γ), IL-2 and tumour necrosis factor-α (TNF-α) by CM CD4+ T cells. (b) Gag- and Env-specific production of IL-2 by CM CD4+ T cells. *P < 0·05 and ***P < 0·001 indicate statistically significant differences of combined Env-specific cytokine responses (a) and combined Gag- and Env-specific responses (b) between the compared time-points by Wilcoxon matched-pairs signed rank test. (c, d) Correlation in Group C macaques between Env-specific IFN-γ-producing CD56+ NK cells with Env-specific IL-2 production by CM CD4+ T cells (c) and week 11 plasma viral loads (d). (e, f) Correlation in all macaques between Gag-specific IFN-γ-producing CD56+ NK cells with Gag-specific IL-2 production by CM CD4+ T cells (e) and absolute number of circulatory CD4+ T cells (f). Spearman's correlation analysis was used to determine statistical significance in c–f.
Although all animals in the present study displayed increasing levels of plasma viral loads post-ART, we observed a positive impact of the improved SIV-specific functionality of CM CD4+ T cells at the end of 11 weeks of ART in reducing the amount of circulatory virus observed 2 weeks following ART release (Fig.7). As shown in Fig.7(a) and (b), the extent of both Gag- and Env-specific T-cell functionality recovered at the end of ART, as seen by the percentage of IL-2+ CM CD4+ T cells at week 11, had a positive effect on reducing rebound viraemia at week 13 in all three groups of macaques. This effect of ART was further improved by Env immunization as observed in Group B and C macaques (Fig.7d). In these immunized macaques, Env-specific T-cell responses displayed a much stronger negative correlation (Fig.7d, P = 0·0008) compared with all the macaques (Fig.7b, P = 0·025). In contrast, Gag-specific T-cell responses in the same Group B and C macaques did not exhibit a similarly enhanced negative correlation (Fig.7c, P = 0·031 versus Fig.7a, P = 0·018).
Figure 7.
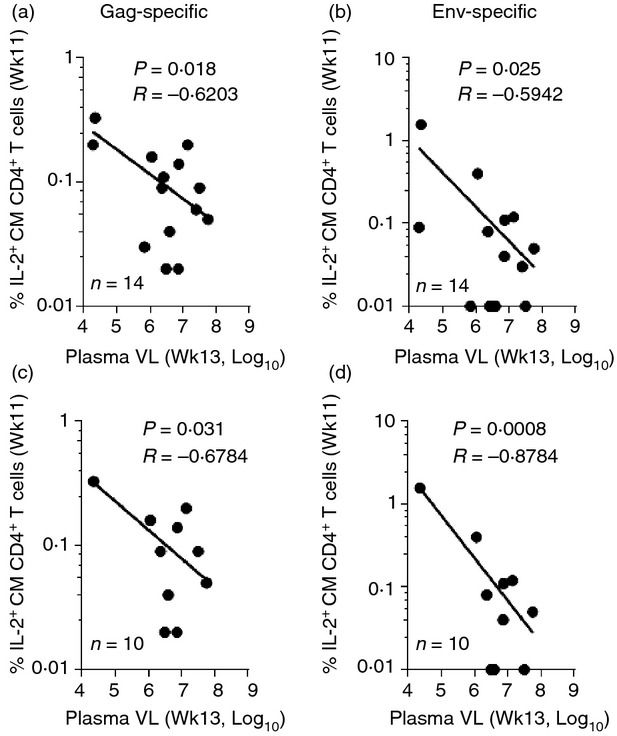
Impact of antiretroviral therapy (ART) plus Env therapeutic immunization on plasma viral load rebound post-ART. Correlations between Gag (a and c) and Env-specific (b and d) interleukin-2 (IL-2) production by central memory (CM) CD4+ T cells at week 11 with plasma viral load levels at week 13. Graphs on top (a and b, n = 14) include all macaques in the study, whereas graphs on the bottom (c and d, n = 10) include Env-immunized macaques only. Spearman's correlation analysis was used to determine statistical significance.
Discussion
In recent years, more attention has been given to NK cells as mediators of antigen-specific effector responses and their potential role in conferring protection against several diseases that greatly impact public health.19,21,31 The role played by NK cells during SIV and HIV infection has been revisited, and it is now recognized that NK cells are more than just effectors of innate immunity. They also interact with the adaptive immune system in contributing to disease control, and perhaps also assist in the generation of long-term memory through the production of the Th1-polarizing cytokine IFN-γ.32–35 ART has been shown to impact NK cells during SIV infection. A short course of ART (11 weeks) was recently shown to partially restore the normal distribution of systemic and mucosal NK cell subsets altered by SIV infection.36 We have previously reported a novel T-cell-dependent effector function of NK cells associated with strong and durable control of viraemia in SIV+ controlling macaques,5 that could be partially restored in SIV+ non-controlling macaques by 8 weeks of ART. In that study, only CD69 and IFN-γ production by NK cells was recovered,5 whereas here 11 weeks of ART was sufficient to rescue TNF-α production as well (Fig.5). The result suggests that longer periods of ART or a more effective ART regimen might result in more long-lasting effects.
Whereas our previous study documented recovery of T-cell-dependent Gag-specific NK responses, here we extended our results to show recovery of Env-specific NK cell responses, resulting from the effects of ART combined with Env immunization. Notably, the Env immunization was administered in MPL-SE, a TLR4 agonist known to stimulate type 1 IFN, leading to maturation of dendritic cells and NK cells, and IL-12, enabling cross-activation of both cell types.37 Figure5(a) shows that an Env-specific response by CD56+ IFN-γ+ NK cells occurred at week 11 only in Groups B and C after the gp120 immunization. This effect was maintained 2 weeks post-ART release in the previously immunized Group C macaques, suggesting that further boosting of the Env-specific response in these macaques enhanced the anamnestic response following the subsequent viral rebound upon ART release. In this regard, Group A macaques exhibited a delayed response to both Gag and Env at week 13 (Fig.5a), also attributable to increased viraemia upon ART release.
Restoration of Env-specific CD4+ T-cell function was also seen, most strikingly in Group C macaques that were previously vaccinated with SIV gp120 and subsequently boosted here with SIV gp120 at week 9 (Fig.6a,b). The result was a strongly positive correlation between Env-specific IFN-γ+ CD56+ NK cells and Env-specific IL-2+ CM CD4+ T cells (Fig.6c). However, consistent with previous results, all macaques showed positive correlations between Gag-specific IFN-γ+ CD56+ NK cells and both Gag-specific IL-2+ CM CD4+ T cells (Fig.6e) and absolute numbers of CD4+ T cells in peripheral blood (Fig.6f), again implicating ART in contributing to the overall response. The overall impact of ART was reduced viraemia, which correlated with improved T-cell function. This was seen in all 14 macaques in the three groups for Gag-specific IL-2+ CM CD4+ T-cell responses (Fig.7a). The gp120 immunization had little effect on this Gag-specific response, as seen by the similar correlation coefficient and P-value when only the 10 macaques of Groups B and C were analysed (Fig.7c). This was not the case when Env-specific responses were examined. While Env-specific IL-2+ CM CD4+ T-cell responses of all 14 macaques were significantly correlated with reduced viraemia (Fig.7b), the correlation coefficient and P-value for the 10 macaques of Groups B and C were much stronger (Fig.7d). This result occurred in spite of the lack of a significant correlation of Env-specific NK responses of Group B macaques with IL-2+ CM CD4+ T cells. The lack of functional correlation seen in Group B macaques is most likely because NK cells can respond to picomolar amounts of IL-2,38,39 although a secondary contribution from other NK-cell-activating cytokines, such as IL-12 and IL-15 produced by antigen-presenting cells, is also feasible.19 Hence, both ART and gp120 immunization contributed to viraemia reduction. Although the various NK responses did not by themselves correlate with reduced viraemia, our data showing strong correlations of co-operative NK responses with functional T cells suggest that they contributed to the protective outcome. The significant, albeit transient, recovery in SIV-specific T-cell and NK cell responses observed in the present study is similar to previously described transient recoveries in HIV- and SIV-specific immune functions following cytokine-based immunotherapies.40–43 Whether a combination of gp120 immunization with cytokine-based NK cell targeted immunotherapy during ART would further benefit T-cell-dependent NK cell effector functions will require further study.
Recently, Jost et al.22 reported that therapeutic immunization of HIV-infected individuals on ART could restore CD4+ T-cell function, thereby enhancing NK cell activity. Here, we confirmed these results and extended the findings to the SIV-rhesus macaque model. Our study differs from that of Jost et al., in that we treated chronically infected animals de novo, whereas viraemia was already well controlled in the HIV+ individuals. Moreover, Jost et al. continued ART following immunization, perhaps leading to greater enhancement of NK cell function, whereas in our study ART was discontinued 2 weeks post-immunization. Nevertheless, we clearly show that therapeutic modulation of SIV-specific T-cell-dependent NK cell responses is possible through ART and Env immunization. The greatest benefit of such combined treatment may be in previously vaccinated individuals who subsequently become infected, as shown by our results in previously vaccinated macaques.
Acknowledgments
We gratefully acknowledge the animal caretakers at Advanced BioScience Laboratories and Katherine McKinnon (Vaccine Branch Flow Core, NCI/NIH) for expert advice. The following reagents were obtained through the NIH Nonhuman Primate Reagent Resource: QDot655 anti-CD4. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Gag and Env peptides (complete sets). This study was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Disclosures
The authors have no financial conflicts of interest.
References
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Vargas-Inchaustegui DA, Demberg T, Robert-Guroff M. A CD8α− subpopulation of macaque circulatory natural killer cells can mediate both antibody-dependent and antibody-independent cytotoxic activities. Immunology. 2011;134:326–40. doi: 10.1111/j.1365-2567.2011.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–14. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Inchaustegui DA, Xiao P, Tuero I, Patterson LJ, Robert-Guroff M. NK and CD4+ T cell cooperative immune responses correlate with control of disease in a macaque simian immunodeficiency virus infection model. J Immunol. 2012;189:1878–85. doi: 10.4049/jimmunol.1201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–46. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostik P, Kobkitjaroen J, Tang W, et al. Decreased NK cell frequency and function is associated with increased risk of KIR3DL allele polymorphism in simian immunodeficiency virus-infected rhesus macaques with high viral loads. J Immunol. 2009;182:3638–49. doi: 10.4049/jimmunol.0803580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaichompoo P, Bostik P, Stephenson S, Udompunturuk S, Kobkitjaroen J, Pattanapanyasat K, Ansari AA. Multiple KIR gene polymorphisms are associated with plasma viral loads in SIV-infected rhesus macaques. Cell Immunol. 2010;263:176–87. doi: 10.1016/j.cellimm.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Byrareddy SN, Albrecht C, et al. In vivo administration of a JAK3 inhibitor during acute SIV infection leads to significant increases in viral load during chronic infection. PLoS Pathog. 2014;10:e1003929. doi: 10.1371/journal.ppat.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6:515–9. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–10. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- Xiao P, Zhao J, Patterson LJ, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–73. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman VR, Florese RH, Peng B, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43:270–7. doi: 10.1097/01.qai.0000230318.40170.60. [DOI] [PubMed] [Google Scholar]
- Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Cole KS, Marthas M, Becerra JC, Van Rompay K. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol. 2006;80:9217–25. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–18. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- He XS, Draghi M, Mahmood K, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–9. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–52. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- Jost S, Tomezsko PJ, Rands K, Toth I, Lichterfeld M, Gandhi RT, Altfeld M. CD4+ T-cell help enhances NK cell function following therapeutic HIV-1 vaccination. J Virol. 2014;88:8349–54. doi: 10.1128/JVI.00924-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Patterson LJ, Kuate S, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–57. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu P, Vaccari M, Gordon S, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–19. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demberg T, Brocca-Cofano E, Xiao P, et al. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Inchaustegui DA, Xiao P, Hogg AE, et al. Immune targeting of PD-1hi expressing cells during and after antiretroviral therapy in SIV-infected rhesus macaques. Virology. 2013;447:274–84. doi: 10.1016/j.virol.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EM, Chung HK, Livesay J, et al. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods. 2010;163:287–94. doi: 10.1016/j.jviromet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Reeves RK, Evans TI, Gillis J, Johnson RP. SIV infection induces an expansion of α4β7+ and cytotoxic CD56+ NK cells. J Virol. 2010;84:8959–63. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- Van Elssen CH, Vanderlocht J, Frings PW, et al. Klebsiella pneumoniae-triggered DC recruit human NK cells in a CCR5-dependent manner leading to increased CCL19-responsiveness and activation of NK cells. Eur J Immunol. 2010;40:3138–49. doi: 10.1002/eji.201040496. [DOI] [PubMed] [Google Scholar]
- McCall MB, Roestenberg M, Ploemen I, et al. Memory-like IFN-γ response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur J Immunol. 2010;40:3472–7. doi: 10.1002/eji.201040587. [DOI] [PubMed] [Google Scholar]
- Borrow P. Innate immunity in acute HIV-1 infection. Curr Opin HIV AIDS. 2011;6:353–63. doi: 10.1097/COH.0b013e3283495996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen CT, Shalekoff S, Meddows-Taylor S, et al. Cutting Edge: unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–8. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen CT, Shalekoff S, Meddows-Taylor S, et al. Natural killer cells that respond to human immunodeficiency virus type 1 (HIV-1) peptides are associated with control of HIV-1 infection. J Infect Dis. 2010;202:1444–53. doi: 10.1086/656535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–8. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- Liyanage NP, Gordon SN, Doster MN, et al. Antiretroviral therapy partly reverses the systemic and mucosal distribution of NK cell subsets that is altered by SIVmac251 infection of macaques. Virology. 2014;450–451:359–68. doi: 10.1016/j.virol.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquilly A, Broquet A, Jacqueline C, et al. Toll-like receptor-4 agonist in post-haemorrhage pneumonia: role of dendritic and natural killer cells. Eur Respir J. 2013;42:1365–78. doi: 10.1183/09031936.00152612. [DOI] [PubMed] [Google Scholar]
- Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–32. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–26. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, Pahwa R, Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–38. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcelain G, Autran B. Immune interventions in HIV infection. Immunol Rev. 2013;254:355–71. doi: 10.1111/imr.12083. [DOI] [PubMed] [Google Scholar]
- Villinger F, Ansari AA. Role of IL-12 in HIV infection and vaccine. Eur Cytokine Netw. 2010;21:215–8. doi: 10.1684/ecn.2010.0206. [DOI] [PubMed] [Google Scholar]
- Villinger F, Bucur S, Chikkala NF, et al. In vitro and in vivo responses to interleukin 12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res Hum Retroviruses. 2000;16:751–63. doi: 10.1089/088922200308756. [DOI] [PubMed] [Google Scholar]