Abstract
Over one million newborns die annually from sepsis with the highest mortality in premature and low-birthweight infants. The inflammasome plays a central role in the regulation of innate immunity and inflammation, and is presumed to be involved in protective immunity, in large part through the caspase-1-dependent activation of interleukin-1β (IL-1β) and IL-18. Studies in endotoxic shock, however, suggest that endogenous caspase-1 activity and the inflammasome contribute to mortality primarily by promoting excessive systemic inflammatory responses. We examined whether caspase-1 and the inflammasome also regulate neonatal inflammation, host protective immunity and myelopoiesis during polymicrobial sepsis. Neonatal (5–7 days) C57BL/6 and caspase-1/11−/− mice underwent a low-lethality caecal slurry model of intra-abdominal sepsis (LD25–45). Ablation of caspase-1/11, but not apoptosis-associated speck-like protein containing a CARD domain or nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), improved neonatal survival following septic challenge compared with wild-type mice (P < 0·001), with decreased concentrations of inflammatory cytokines in the serum and peritoneum. Surprisingly, caspase-1/11−/− neonates also exhibited increased bone marrow and splenic haematopoietic stem cell expansion (P < 0·001), and increased concentrations of granulocyte and macrophage colony-stimulating factors in the peritoneum (P < 0·001) after sepsis. Ablation of caspase-1/11 signalling was also associated with increased recruitment of peritoneal macrophages and neutrophils (P < 0·001), increased phagocytosis by neutrophils (P = 0·003), and decreased bacterial colonization (P = 0·02) in the peritoneum. These findings suggest that endogenous caspase-1/11 activity, independent of the NLRP3 inflammasome, not only promotes the magnitude of the inflammatory response, but also suppresses protective immunity in the neonate, so contributing to innate immune dysfunction and poor survival in neonatal sepsis.
Keywords: caspase-1/11, emergency myelopoiesis, inflammation, mouse, neonates
Introduction
Despite continual advancements in neonatal intensive care and tailored antibiotic therapy, mortality from neonatal sepsis remains high.1 Neonatal sepsis is the leading cause of mortality in infants, with more than one million deaths per year worldwide.2 The economic burden of sepsis in the neonatal population is significant with costs in the USA alone estimated to be $700 million annually.2 Although sepsis occurs throughout the paediatric population, premature and very-low-birthweight neonates are the most susceptible, and the incidence is highest in the first year of life.1,2
Neonates exhibit deficits in both their innate and adaptive immune systems.1,3 It is known that neonates respond to polymicrobial and Escherichia coli sepsis distinctly from adults.4–7 These unique, and often inadequate or under-developed immune responses lead to increased mortality from sepsis when compared with adults.8–10 Additionally, we have previously demonstrated that in neonatal mice, innate immunity can be enhanced by pre-treating mice with specific toll-like receptor agonists with the net result being improved survival, even in the absence of adaptive immunity.7 Therefore, one can surmise that notwithstanding the functional deficits in the neonatal adaptive immune system, the neonatal innate immune response can be modulated to improve survival.7
Recent studies have revealed the importance of various recognition and signalling pathways within the innate immune system that confer a survival advantage to neonates with sepsis, including the toll-like receptor 4 and CXCL10/CXCR3 pathways.10,11 The inflammasome is a group of large multi-protein complexes assembled around nucleotide-binding oligomerization domain-like receptor proteins (NLRPs) that detect microbial pathogen-associated molecular patterns and damage-associated molecular patterns in intracellular compartments, similar to the role of toll-like receptors on the cell surface.12 Ultimately, the assembly of the inflammasome complex results in both increased pyroptosis, as well as the activation of caspase-1 through apoptosis-associated speck-like protein containing a CARD domain (ASC), which catalyses the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 in macrophages, dendritic cells and epithelial cells.13 Surprisingly, despite its role in the recognition of both endogenous and external alarmins, excessive activation of the inflammasome and pro-inflammatory cytokines during sepsis can lead to inflammation-dependent multi-organ injury and ultimately mortality. Neonatal mice, however, do not appear to die from an exaggerated inflammatory response in response to E. coli or polymicrobial sepsis, but rather from a failure in protective immunity.7 The role of caspase-1 ablation and the inflammasome on other components of innate immunity in the response to neonatal sepsis has not been explored.
We demonstrate here that in neonatal sepsis, ablation of caspase-1/11, but not the NLRP3 inflammasome, improves survival. Although ablation of caspase-1/11 is associated with a reduced inflammatory response, it is also associated with increased protective immunity, as documented by increased numbers and function of innate immune effector cells at the site of infection. Increased myelopoiesis and concentrations of peritoneal growth factors were also observed following inhibition of caspase-1/11 signalling. Together, these results strongly suggest that caspase-1/11 contributes paradoxically to neonatal innate immune dysfunction during polymicrobial sepsis independent of the NLRP3 inflammasome.
Materials and methods
Murine models
All studies were approved by the Institutional Animal Care and Use Committee at the University of Florida before their initiation. Six- to eight-week-old male and female pathogen-free C57BL/6J (B6 wild-type) mice and caspase-1/11 knockout B6N.129S2-Casp1tm1Flv/J (on a B6 background) mice were purchased from Jackson Laboratory (Bar Harbor, ME). NLRP3−/− and ASC−/− mice (on a B6 background) were a generous gift from Dr Vishva Dixit, at Genentech (San Francisco, CA). Mice were allowed a minimum of 7 days to equilibrate to their environment before experimental use or breeding. To generate neonatal mice, harem-breeding schemes were established with separation of pregnant females once pregnancy was visually identified. Mixed gender litters aged 5–7 days were used in experiments and defined as neonates. Juveniles used in experiments were females between 6 and 8 weeks of age. Caspase-1/11−/−, NLRP3−/− and ASC−/− mice are born viable and show no gross abnormalities in appearance, bodyweight or organ size for at least the first 16 weeks of life. Polymicrobial intra-abdominal neonatal sepsis was induced using the caecal slurry model as previously described.6 Briefly, fresh caecal contents were harvested from killed 8-week-old adult female C57BL/6J ‘donor’ mice (Jackson Laboratory) and used within 4 weeks of their arrival at the facility. Caecal contents were suspended in 5% dextrose at a concentration of 80 mg/ml, and 1·1 mg/g bodyweight was injected intraperitoneally within 15 min of harvest to achieve the desired lethal dose (LD) as determined previously.6 Neonatal and adult mice were followed for 7 days to assess for absolute survival differences between the two strains. All animals were monitored every 6–8 hr for signs of distress, inability to right themselves or signs of poor feeding or dehydration. Neonates were also monitored for neglect by mother and the absence of milk in the stomach by external visual examination. Severely septic animals meeting the above criteria were humanely killed and were considered non-survivors.
Harvest of peritoneal cells
To isolate peritoneal cells, 500 µl of cold PBS (Cellgro, Manassas, VA) was instilled into the peritoneal cavity, and then collected over a sterile Petri dish. Peritoneal cells were isolated from peritoneal lavage specimens as previously described and approximately two million cells were obtained per peritoneal wash sample after sepsis.7 Erythrocytes in peritoneal lavage samples were lysed with ammonium chloride lysis buffer and washed with PBS. Four to five peritoneal wash samples were collected per experiment.
Determination of serum and peritoneal cytokine and chemokine response
Mouse whole blood and peritoneal wash samples were collected at two, six, 18 and 24 hours following sepsis and cytokine concentrations were measured by multiplex Luminex™ assay (EMD Millipore, Billerica, MA) for 12 analytes [granulocyte (G-CSF), granulocyte–macrophage (GM-CSF) and macrophage (M-CSF) colony-stimulating factors, interferon-γ (IFN-γ), IL-1α, IL-1β, IL-6, IL-12(p70), IFN-γ inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and tumour necrosis factor-α]. Based on our previous studies using neonatal mice, we have found that healthy neonatal mice (both wild-type and caspase-1/11−/−) have negligible levels of these cytokines, so baseline levels in healthy animals were not performed.
Bacterial count determination
Peritoneal bacterial counts were determined by culturing 100 μl of serially diluted peritoneal lavage sample on sheep's blood agar plates (Thermo Fisher Scientific, Waltham, MA) at 37° as previously described.11,14–16 Whole blood was collected and plated undiluted on sheep's blood agar plates for bacterial count determination 18, 24 and 48 hr after sepsis. Plates were counted after 24 hr of incubation at 37°. Taking into consideration constraints on litter size, experiments at different time-points were performed using different caecal donors; as a result, evaluation of bacterial counts over time was not possible.
Flow cytometry for characterization of cell phenotype
Single-cell suspensions were stained with anti-Ly6G-allophycocyanin (-APC) [Becton-Dickinson (BD) Franklin Lakes, NJ], anti-CD11b-phycoerythrin-Cy7 (BD) and anti-F4/80-APC (Ebioscience, San Diego, CA). Sytox Blue (Invitrogen, Carlsbad, CA) was used for cell viability analysis. Samples were acquired and analysed on an LSR II flow cytometer with FACSDiva™ software (BD). A minimum of 1 × 104 live cells were collected for analysis. The absolute numbers of innate immune effector cells were determined by multiplying the percentage of neutrophils (CD11b+ Ly6G+) and macrophages (CD11b+ Ly6G− F4/80+) within the total sample population by the total sample cell number.
Determination of pyroptosis
Pyroptosis was determined by staining a minimum of one million single cells in peritoneal wash samples with anti-Ly6G-APC (BD), anti-CD11b-PECy7 (BD) and anti-F4/80-APC (Ebioscience) to characterize neutrophils (CD11b+ Ly6G+) and macrophages (CD11b+ Ly6G− F4/80+). Next, the cells were stained with Annexin V (A5) (FITC) and 5 µl of Vital Dye (propidium iodide) (BD Pharmingen, Franklin Lakes, NJ) and flow cytometry was performed within 1 hr.17 As published, cells that were propidium iodide- and A5-positive were considered to have undergone pyroptosis whereas cells that had undergone early apoptosis were propidium iodide-negative.17 Additionally, we measured pyroptosis in the peritoneum by assaying for released lactate dehydrogenase activity, using a lactate dehydrogenase cytotoxicity kit (ThermoScientific, Rockford, IL), on peritoneal wash samples, correcting for cell counts.18
Functional analysis of peritoneal macrophages or neutrophils
To assay for phagocytosis, peritoneal cell suspensions containing 1 × 105 cells were stained for surface markers, washed with PBS, and then incubated with pHrodo Green E. coli BioParticles™ (Invitrogen) in a 37° water bath for 30 min. Cells were washed with PBS, and then analysed by flow cytometry. To assay for reactive oxygen species (ROS) production, cell suspensions containing 2 × 106 cells were stained for cell surface markers, washed with PBS and labelled with dihydrorhodamine 123 (Invitrogen) to determine ROS production. Cells were stimulated with 1 μm of PMA (Sigma-Aldrich, St Louis, MO) at 37° and aliquots were evaluated by flow cytometry every 10 min for 30 min. A minimum of 1 × 104 live, non-debris cells were collected for all analyses.
Isolation of bone marrow and splenocytes for lineage− sca-1+ c-kit+ (LSK) progenitor cell identification
Spleen and bone marrow were harvested and single cell suspensions were created by dissociating cells through a 70-μm pore-size sterile cell strainer (BD Falcon, Durham, NC). Erythrocytes were lysed using ammonium chloride lysis buffer and washed with PBS. To obtain lineage− sca-1+ c-kit+ (LSK) cells, the cells were stained with mouse biotin lineage antibody cocktail (anti-Gr1, anti-B220, anti-Ter119, anti-CD3e and anti-CD11b; Invitrogen) and labelled with anti-peridinin chlorophyll protein Cy5.5 streptavidin, anti-C-kit and anti-sca-1. All antibodies were purchased from Becton-Dickinson unless otherwise indicated. A minimum of 1 × 104 non-debris live cells were used and analysis was performed by flow cytometry.
Additional statistical analysis
Continuous variables were tested for normality and equality of variances. Differences in 7-day survival were calculated using log-rank (Mantel–Cox) test. Differences among groups were evaluated by either two-way analysis of variance with Bonferroni multiple comparisons post hoc or Student's t-test. Significance was determined at the 95% confidence level. Values are presented as means with standard error of the mean (SEM).
Results
Neonatal caspase-1/11−/− mice have improved survival in both juvenile and neonatal mice, and an attenuated inflammatory response following intra-abdominal sepsis
Previous studies have shown that adult mice deficient in caspase-1 are resistant to endotoxic shock and intraperitoneal E. coli injection.19,20 The prevailing thought is that the survival advantage in caspase-1-deficient adult mice is secondary to reduced production of pro-inflammatory cytokines with attenuated tissue and organ injury,21 and similar to other groups, we observed here that caspase-1/11−/− juvenile (6–8 weeks) mice had improved survival compared with wild-type juveniles (6–8 weeks) following intra-abdominal polymicrobial sepsis (Fig.1a). To date, the role of caspase-1/11 in neonatal sepsis has not been examined. Using our established model of neonatal sepsis, we next evaluated survival in caspase-1/11−/− and wild-type neonates (5–7 days). Caspase-1/11−/− neonates also had markedly improved survival following sepsis compared with wild-type neonates (P = 0·0026) (Fig.1b). The improvements in survival in caspase-1/11−/− neonates were greater than the improvements seen in survival in caspase-1/11−/− juveniles when compared with wild-type mice. Additionally, when comparing the survival of all four groups simultaneously, the caspase-1/11−/− neonatal mice had the greatest survival of all four groups (Fig.1a).
Figure 1.
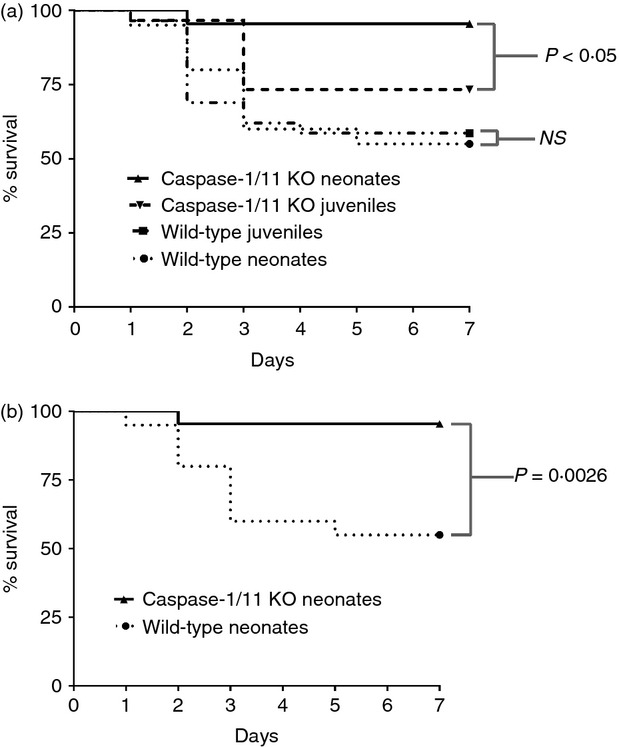
Caspase-1/11−/− neonatal mice have superior survival following intra-abdominal polymicrobial sepsis compared with wild-type (WT) mice. Caspase-1/11−/− neonates (5–7 days) and juveniles (6–8 week) and WT neonates (5–7 days) and juveniles (6–8 weeks) were challenged via intraperitoneal injection of polymicrobial caecal slurry (1·1 mg/g body weight) to achieve an LD25–45. Mice were followed for 7 days to assess survival. (a) Caspase-1/11−/− (Casp-1/11 KO) neonates (5–7 days) and juveniles (6–8 week) and WT (WT B6) neonates (5–7 days) and juveniles (6–8 weeks) were challenged with caecal slurry simultaneously. Caspase-1/11−/− neonatal mice had the greatest survival compared with juvenile caspase-1/11−/− and WT mice (neonates and juveniles) (P < 0·05, log-rank Mantel–Cox Test) (Caspase-1/11 juveniles n = 30, WT juveniles n = 30). (b) Caspase-1/11−/− (Casp-1/11 KO) neonates (5–7 days) had improved survival to caecal slurry induced sepsis compared with wild-type neonates (Caspase-1/11−/− n = 40, WT n = 42) (P < 0·001, Log Rank test).
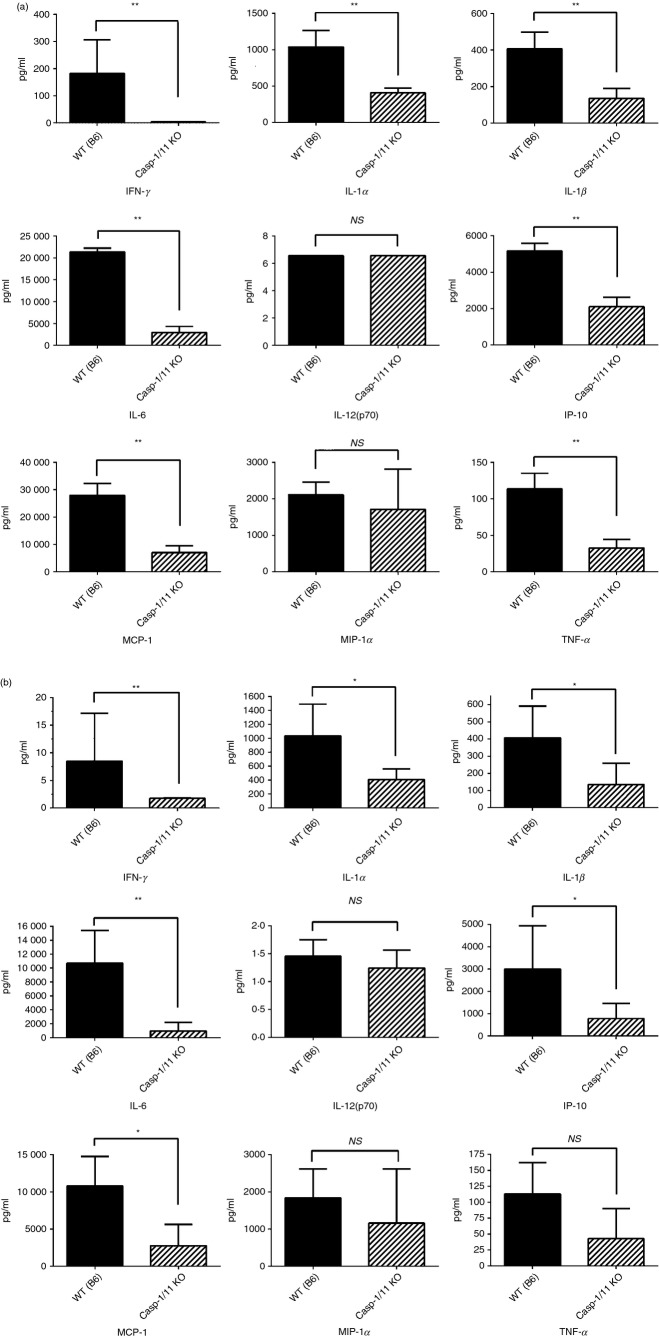
We next evaluated cytokine concentrations in both the serum and the peritoneum 2, 6, 18 and 24 hr following polymicrobial sepsis in neontal wild-type and caspase-1/11−/− mice. As shown in Fig.2(a), neonatal caspase-1/11−/− mice had an attenuated serum cytokine response with decreased concentrations of inflammatory cytokines including IFN-γ, IL-1α, IL-1β, IL-6, IFN-γ inducible protein-10, macrophage inflammatory protein-1 and tumour necrosis factor-α at 18 hr. Likewise there were decreased concentrations of IFN-γ, IL-1α, IL-1β, IL-6, IFN-γ inducible protein-10, macrophage inflammatory protein-1 and tumour necrosis factor-α in the peritoneum of caspase-1/11−/− neonates compared with wild-type mice at 18 hr (Fig.2b). As expected, caspase-1/11−/− mice also had decreased levels of IL-1β and IL-18 across all time-points compared with wild-type mice (data not shown).
Figure 2.
Caspase-1/11−/− neonatal mice have decreased production of inflammatory cytokines and chemokines 18 hr after sepsis compared with wild-type (WT) mice. Neonatal (5–7 day) caspase-1/11−/− (Casp-1/11 KO) and WT (WT B6) mice were challenged with intraperitoneal polymicrobial caecal slurry (1·1 mg/g body weight; LD25–45). Serum and peritoneal washes were collected at 18 and 24 hr following sepsis and analysed by Luminex™ assay for cytokine/chemokine concentrations. (a) Caspase-1/11−/− neonatal mice (hashed bars) had decreased concentrations of interferon-γ (IFN-γ), IL-1α, IL-1β, IL-6, IFN-γ inducible protein-10 (IP-10), macrophage inflammatory protein-1 (MCP-1) and tumour necrosis factor-α (TNF-α) in the serum compared with WT neonates (solid bars). (b) Caspase-1/11−/− neonatal mice (hashed bars) have decreased concentrations of IFN-γ, IL-1α, IL-1β, IL-6, IP-10, MCP-1, and TNF-α in the peritoneum compared with WT mice (solid bars). Symbols placed within the graph show all significant differences between groups for specific cytokine. NS = non-significant, *P < 0·01, **P ≤ 0·001, Student's t-test. (n = 4 or 5 neonates per group). Data in (a) and (b) is representative of three independent experiments.
Although caspase-1/11−/− neonates had an attenuated inflammatory response compared with wild-type mice, the concentrations of inflammatory cytokines in the neonates are generally lower than those seen in juvenile animals, and not fully consistent with death secondary to an exaggerated inflammatory response.6 To examine other components of innate immunity dependent upon caspase-1/11 that could explain the differential survival, we examined individual components of protective immunity, and both myelopoiesis and myeloid cell functions.
The absence of caspase-1/11 leads to increased medullary and extramedullary haematopoeisis via increased production of splenic and bone marrow lineage− sca-1+ c-kit+ (LSK) progenitor cells in neonatal mice following sepsis
Although caspase-1/11 does not appear to play a regulatory role in apoptosis, its activation mediates a type of pro-inflammatory cell death, termed pyroptosis in selected cell populations.17 More recently, it has been demonstrated that the NLRP1 inflammasome activation is responsible for pyroptosis of haematopoietic stem cells in the bone marrow following infection in a caspase-1/11-dependent manner, so preventing their proliferation and differentiation.22 Our laboratory has previously demonstrated that neonatal mice have impaired emergency myelopoiesis and impaired reconstitution of innate immne effector cells following polymicrobial sepsis.23 To determine whether caspase-1/11 inhibition affected haematopoiesis, we next examined the percentage of LSKs (primitive haematopoietic stem cells) at baseline and 36 hr following sepsis in neonatal mice. Although both groups of neonatal mice demonstrated expansion of bone marrow LSKs 36 hr following sepsis, caspase-1/11−/− neonates had a significantly greater expansion compared with wild-type mice (57·4% versus 24·5%; P = 0·01, Student's t-test) (Fig.3a). As the spleen is a haematopoietic organ in neonates, we also examined whether caspase-1/11 inhibition impacted extra-meduallary haematopoiesis; hence, we examined the percentage of LSKs in the spleen at baseline and 36 hr following sepsis. Similar to LSK expansion in the bone marrow, both wild-type and caspase-1/11−/− neonates had increased percentages of LSKs compared with baseline following sepsis (Fig.3b). However, caspase-1/11−/− neonates, again demonstrated more than a twofold increased expansion of splenic LSKs (43% versus 18%; P < 0·0001) 36 hr following sepsis compared with wild-type mice (Fig.3b).
Figure 3.
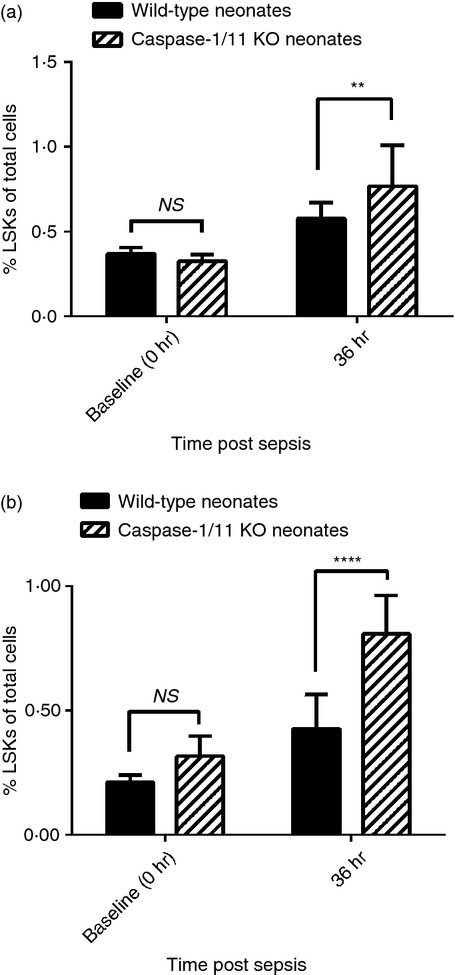
Caspase-1/11−/− mice have increased expansion of splenic and bone marrow lineage− sca-1+ c-kit+ (LSK) progenitor cells in the bone marrow 36 hr after sepsis. Caspase-1/11−/− (Casp-1/11 KO) and wild-type (WT B6) neonates (5–7 days) were challenged with polymicrobial caecal slurry (1·1 mg/g body weight) via intraperitoneal injection. Bone marrow and splenocytes were collected at 36 hr and analysed by flow cytometry for the presence of lineage− sca-1+ c-kit+ (LSKs). Bone marrow and splenocytes were collected from untreated neonates to establish baseline (0 hr) LSK levels. A minimum of 1 × 104 non-debris live cells were collected for analysis. Both neonatal WT and caspase-1/11−/− mice had increased splenic and bone marrow LSKs 36 hr after sepsis (P < 0·0001, two-way analysis of variance with Bonferroni multiple comparison's post hoc test). (a) Caspase-1/11−/− neonates (hashed bars) had a significantly greater expansion of bone marrow LSKs compared with WT (solid bars) neonates (57·4% versus 24·5%; **P < 0·01, Student's t-test, n = 13 or 14/group). (b) Caspase-1/11−/− neonates (hashed bars) demonstrated more than a twofold increased expansion of splenic LSKs 36 hr following sepsis compared with WT (solid bars) mice (43% versus 18%; ****P < 0·0001, Student's t-test, n = 13 or 14/group). Data shown are from three or more independent experiments. Values are expressed as per cent total of all viable cells.
In caspase-1/11-deficient neonatal mice, increased haematopoeisis leads to increased emergency myelopoeisis associated with increased production of growth factors as well as increased recruitment and activation of innate immune effector cells
Whereas caspase-1/11−/− mice had decreased production of inflammatory chemokines and cytokines in the serum and peritoneum following sepsis (Fig.2a, b), we observed increased production of local growth factors (G-CSF, M-CSF) in the peritoneum 24 hr after sepsis compared with wild-type mice (P < 0·001, Fig.4). There were no significant differences in the measured levels of growth factors in the serum (data not shown). In addition to the increased production of local growth factors, we found that caspase-1/11−/− mice had increased numbers of innate immune effector cells 18 hr after sepsis, with increased absolute numbers of neutrophils (CD11b+ Ly6G+) and macrophages (CD11b+ Ly6G− F4/80+) in the peritoneum (P < 0·001) despite decreased levels of inflammatory cytokines in the peritoneum (Fig.5a, b). Although no differences were detected in ROS production of neutrophils and macrophages (data not shown), neutrophils (CD11b+ Ly6G+) from caspase-1/11−/− neonates had significantly increased phagocytosis compared with wild-type neutrophils (P = 0·003, Fig.5c). There was no difference in phagocytosis in peritoneal macrophages (CD11b+ Ly6G− F4/80+) between the groups (Fig.5d).
Figure 4.
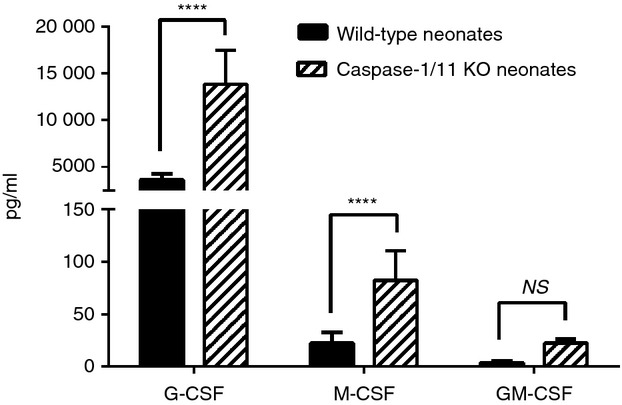
Caspase-1/11−/− mice have increased concentrations of local growth factors 24 hr after sepsis. Caspase-1/11−/− (Casp-1/11 KO) and wild-type (WT B6) neonates (5–7 days) were challenged with intraperitoneal polymicrobial caecal slurry as described (LD25–45). Peritoneal washes were collected at 24 hr and analysed by Luminex™ assay for the presence of granulocyte (G-CSF), macrophage (M-CSF) and granulocyte–macrophage (GM-CSF) colony-stimulating factors. Caspase-1/11−/− (hashed bars) neonates had increased production of local growth factors (G-CSF, M-CSF) in the peritoneum 24 hr after sepsis compared with WT (solid bars) mice (****P < 0·0001, NS = no statistical significance, two-way analysis of variance with Bonferonni's multiple comparisons test) (n = 5 to n = 8 neonates/group). Data shown are from three or more independent experiments.
Figure 5.
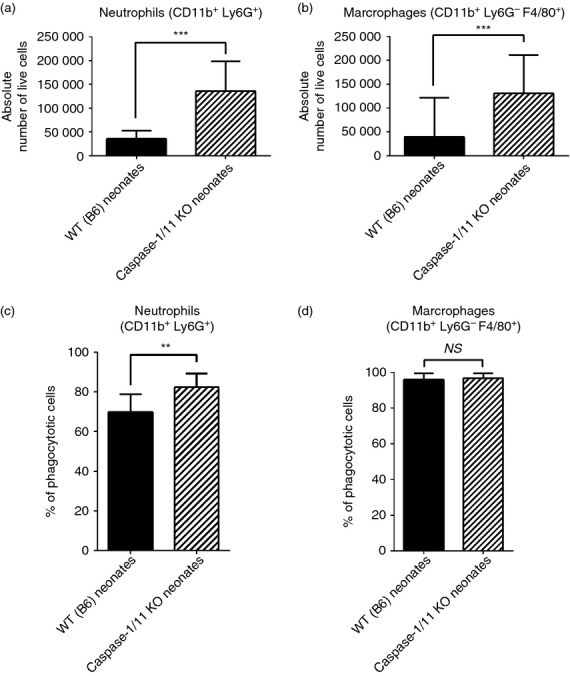
Caspase-1/11-deficient neonates have increased recruitment and function of innate immune effector cells after sepsis. Caspase-1/11 KO neonates (Casp-1/11 KO) and wild-type (WT B6) neonates (5–7 days) were challenged with intraperitoneal caecal slurry as described (LD25–45). Peritoneal washes were collected at 18 and 24 hr following sepsis, stained and analysed by flow cytometery to determine cell phenotype. Erythrocytes were lysed with ammonium chloride lysis buffer. (a) Caspase-1/11−/− neonates (hashed bars) had increased absolute numbers of neutrophils (CD11b+ Ly6G+) and (b) macrophages (CD11b+ Ly6G− F4/80+) in the peritoneum 18 hr after sepsis compared with WT (solid bars) neonates (***P < 0·001, Student's t-test) (n = 4 or 5 neonates/group (a,b)). (c) Caspase-1/11−/− (hashed bars) had significantly increased neutrophil (CD11b+ Ly6G+) phagocytosis compared with WT (solid bars) mice (**P = 0·003, Student's t-test) (n = 5 to n = 8 neonates/group). (d) There was no difference in macrophage (CD11b+ Ly6G− F4/80+) between the groups (NS = no statistical difference) (n = 5 to n = 8 neonates/group). A minimum of 1 × 104 live cells were collected per peritoneal wash for analysis. Absolute numbers of innate immune effector cells were determined by multiplying the percentage of neutrophils and macrophages within the total sample population by the total sample cell number. Data shown are from three or more independent experiments.
Caspase-1/11−/− neonates have decreased extracellular peritoneal bacteria following sepsis compared with wild-type mice
It has been reported that caspase-1/11 plays an important role in the innate immune defence against cytosolic bacteria by regulating pyroptosis.24 Cells undergoing pyroptosis expel pathogens into the extracellular space, so allowing uptake and destruction by neutrophils.24 One can therefore propose that inhibition of caspase-1/11 would negatively impact bacterial clearance at the site of infection. To investigate this phenomenon, we collected blood and peritoneal lavage samples from neonatal mice 18 and 24 hr following sepsis. Samples were plated on blood agar plates and colony-forming units (CFUs) were determined. We observed that caspase-1/11−/− mice had a 50-fold reduction in extracellular peritoneal bacteria recovered from the wash fluid compared with wild-type neonates at 18 hr (P = 0·02, Fig.6a). Similarly, blood collected at 18 hr in caspase-1/11−/− neonates had a fivefold reduction in extracellular CFUs compared with wild-type neonates (P = 0·0012) (Fig.6b). Twenty-four hours following sepsis, caspase-1/11−/− mice had nearly 3·5 times fewer extracellular bacteria present in the peritoneal cavity; however, this result was not statistically different compared with wild-type neonates (Fig.6c). Blood CFUs were not statistically different at 24 hr (Fig.6d).
Figure 6.

Caspsase-1/11−/− neonates have decreased extracellular peritoneal bacteria after sepsis compared with wild-type (WT) mice. Caspase-1/11 KO neonates (Casp-1/11 KO) and WT (WT B6) neonates (5–7 days) were challenged with intraperitoneal caecal slurry (LD25–45). Peritoneal washes were collected at 18, 24 and 48 hr following sepsis. Bacterial counts were determined by culturing 100 μl of serially diluted peritoneal lavage sample on sheep's blood agar plates. Whole blood was collected 18, 24 and 48 hr following sepsis and plated undiluted on sheep's blood agar plates. Plates were counted after 24 hr of incubation at 37°. Colony-forming units (CFUs) were then counted a minimum of three times. (a) Caspase-1/11−/− neonates (hashed bars) had a 50-fold reduction of extracellular bacteria in the peritoneum compared with WT (solid bars) neonates 18 hr following sepsis (****P < 0·05, Student's t-test) (n = 5 or 6 neonates/group). (b) Caspase-1/11−/− neonates (hashed bars) had a fivefold reduction in CFUs in the blood compared with WT (solid bars) neonates 18 hr after sepsis (**P = 0·0012, Student's t-test) (n = 5 or 6 neonates/group). (c) Twenty-four hours after sepsis, caspase-1/11−/− (hashed bars) mice had 3·5 times fewer extracellular bacteria present in the peritoneal cavity; however, this result was not statistically different compared with wild-type (solid bars) neonates (n = 5 or 6 neonates/group) and (d) no difference in blood CFUs (NS = no statistical difference) (n = 5 or 6 neonates/group). Data shown are from three or more independent experiments.
Inhibition of the NLRP3 inflammasome does not improve neonatal survival following intra-abdominal sepsis
Cytosolic NLRs are pattern recognition receptors similar to toll-like receptors that function to regulate the innate immune system.12 Following pathogen recognition, NLRPs associate with the adaptor protein ASC and pro-caspase-1 to form the inflammasome. There are at least three families of inflammasomes, including NLRP1, NLRP3 and NLRC4.25 Activation of NLRC4 and NLRP1 require bacterial products, and although ASC is required for maximal caspase-1 activation, controversy exists whether low levels of caspase-1 can be activated by these inflammasomes in the absence of ASC.26 To distinguish whether improved survival was inflammasome-dependent or caspase-1/11-dependent, we next performed survival studies using neonatal NLRP3−/− and ASC−/− knockout mice. Following polymicrobial sepsis, we observed no difference in NLRP3−/− or ASC−/− survival compared with wild-type neonates (Fig.7a, b, respectively). These results strongly suggest a critical role for caspase-1/11 in neonatal sepsis outcomes independent of the NLRP3 inflammasome.
Figure 7.
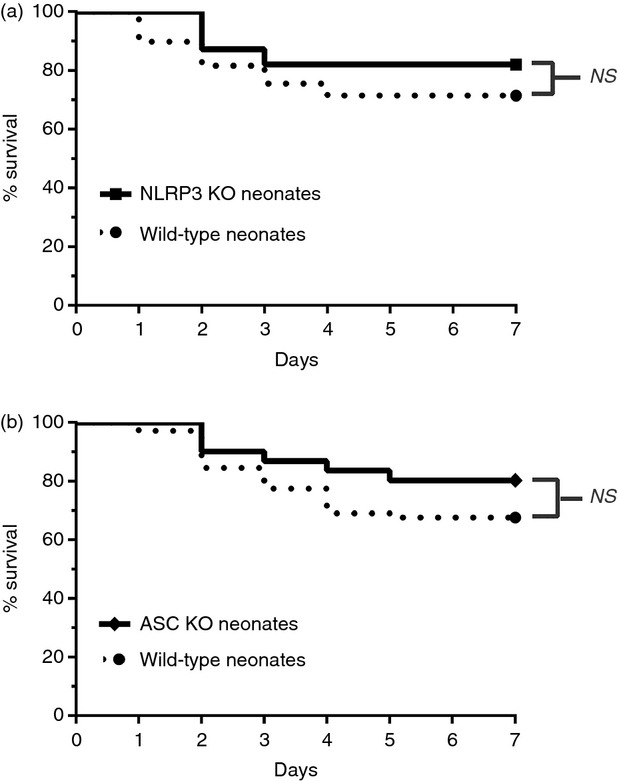
Inhibition of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome does not improve neonatal survival following intra-abdominal polymicrobial sepsis compared with wild-type (WT) mice. NLRP3−/−, apoptosis-associated speck-like protein containing a CARD domain-deficient (ASC−/−) and wild-type neonates (5–7 days) were challenged via intraperitoneal injection of polymicrobial caecal slurry (1·1 mg/g body weight) to achieve an LD25–45. Mice were followed for 7 days to assess survival. (a) NLRP3−/− (NLRP3 KO) and WT neonates (5–7 days) were challenged with caecal slurry simultaneously. NLRP3 inhibition did not improve neonatal survival compared with WT mice (NLRP3 KO n = 39, WT n = 49). (b) Similarly, inhibition of the inflammasome component ASC did not significantly improve survival compared with WT neonates following caecal slurry induced sepsis (ASC KO n = 41, WT n = 45).
Discussion
Significant differences exist between the neonate and young adult immune response to sepsis. An important difference between the groups is the neonate's greater reliance upon the innate immune response with little contribution from adaptive immunity.7 Despite the neonates' reliance on the innate immune system for defence against infection, it too remains immature and dysfunctional compared with that of adults.3 Neonates have deficiencies in their innate immune function via decreased numbers of myeloid cells migrating to the site of infection, decreased effector cell function including decreased production of ROS and neutrophil extracellular traps, decreased opsonization, and decreased phagocytic function.6,9,10 Most notably, there is a skewing toward T helper type 2-polarizing and anti-inflammatory cytokine production at the expense of impaired T helper type 1-polarizing cytokine production, so contributing to neonates being more vulnerable to bacterial infection and higher mortality.3
The inflammasome, a large multi-protein complex that detects intracellular damage-associated molecular patterns and pathogen-associated molecular patterns, is an intracellular regulator of inflammation and pyroptosis, key components of the innate immune system.12,27 Activation of the inflammasome results in the cleavage of pro-caspase-1 to active caspase-1, which in turn activates a number of regulatory proteins, including the pro-inflammatory cytokines IL-1β and IL-18.13 In adult murine models of endotoxic shock using E. coli and lipopolysaccharide, caspase-1-deficient mice have been noted to have a significant survival advantage, presumably by reducing the magnitude of the inflammatory response and multi-organ failure.19,20 In addition, Faure et al. have shown that ablation of the NLRC4 inflammasome improved outcomes of Pseudomonas pneumonia through an IL-17-dependent manner.28 Conversely, when using the caecal ligation and puncture model of polymicrobial sepsis, where death is not a result of an exaggerated inflammatory response, studies have found no difference in mortality between adult caspase-1-deficient and wild-type mice.29,30 More recently, the inflammasome has been noted to regulate pyroptosis or inflammatory cell death in a caspase-1-dependent manner. Controversy exists in the literature as to whether inflammatory cell death, either by necrosis or pyroptosis, is beneficial or harmful to the host during immune response. Some authors suggest that the release of damage-associated molecular patterns by inflammatory cell death contributes to inflammation-induced multi-organ injury whereas others argue that release of intracellular contents promotes pathogen clearance by activation of innate immunity.22,24,31,32 Hence, the role of the inflammasome and caspase-1 in both adult and neonatal sepsis remains controversial and unresolved.
In this report, we demonstrate that the absence of caspase-1/11 in neonates results in improved survival, reduced acute inflammatory cytokine response and improved protective immunity to a polymicrobial model of sepsis. The findings suggest that ablation of caspase 1/11-mediated reduction of the inflammatory response may well contribute to the improvements in outcome, but are probably not the sole explanation. Improvements in protective immunity may also contribute. For example, caspase-1/11-deficient mice have increased numbers of myeloid cells and myeloid growth factors (M-CSF and G-CSF) in the peritoneum following sepsis, potentially from increased recruitment of innate immune cells. Likewise, we show that this phenomenon may be unique to neonates, as not only did neonatal caspase-1/11-deficient mice have improved survival compared with wild-type mice, but they also had superior survival compared with even wild-type and caspase-1/11-deficient juvenile mice. Compared with adults, neonates are known to produce large quantities of IL-6, a cytokine known to inhibit myeloid cell migration to inflammatory sites in response to sepsis, which is believed to contribute to their increased suseptibility to sepsis. This phenomenon has been observed in both murine models of sepsis and human cord blood studies.6,10 We show here that wild-type neonates, with intact caspase-1 signalling, have increased production of IL-6 with reduced numbers of myeloid cells in the peritoneum compared with caspase-1/11-deficient neonates. These findings, in part, likely contribute to the survival advantage seen in neonates with caspase-1/11 inhibition.
It is widely accepted that severe infection induces cytopenia in the neonatal population, with deficient numbers of myeloid cells at the site of infection contributing to their susceptibility to infection. Emergency myelopoiesis, a phenomenon by which new granulocytes and myeloid cells are generated in response to infection or sepsis is well documented in adult animal studies. We have recently demonstrated that murine neonates have impaired expansion of haematopoietic stem cells (otherwise known as LSKs) and decreased formation of myeloid progenitor cells in vitro following polymicrobial sepsis.23 We show here for the first time, that increased emergency myelopoiesis occurs in both the spleen and bone marrow of caspase-1/11-deficient neonates following sepsis, as evidenced by a significant expansion of LSKs. Recent studies suggest that caspase-1 can injure bone-marrow-derived macrophage mitochondria directly, blocking mitophagy and stimulating pyroptosis.33 Such findings are consistent with the increased medullary and extramedullary expansion of LSKs, as well as the increased expansion and recruitment of both neutrophils and macrophages to the site of infection in the absence of caspase-1/11 signalling.
Recently it was demonstrated that caspase-1 activation in adult mice leads to a loss of myeloid progenitor cells in the bone marrow and affects their differentiation into mature myeloid cells.22 This effect was attributed to caspase-1-dependent pyroptosis occurring in bone marrow LSKs and could be reversed by deletion of caspase-1.22 Although we did not specifically test for the presence of pyroptosis, our findings suggest that by deletion of caspase-1/11 in the spleen and bone marrow, neonates have a more robust mobilization of myeloid cells to the site of infection.
The second unexpected finding of this study was the observation that improvements in survival seen in caspase-1/11-deficient mice could not be replicated by ablation of ASC or NLRP3. Such findings are unexpected, especially for ASC, because ASC is thought to be obligatory for the processing of pro-caspase-1 to active caspase-1. Although this is undoubtedly true for the NLRP3 inflammasome, there is some suggestion that low levels of caspase-1 activation can occur in both the NLRC4 and NLRP1b inflammasome in the absence of ASC.25 Interestingly, both of these inflammasomes are constituted following exposure to Gram-negative pathogens through the type III secretion system (NLRC4) and muramyl dipeptide (NLRP1b), a peptidoglycan constituent of both Gram-negative and Gram-positive pathogens. In this model of polymicrobial sepsis, ablation of NLRP3 and ASC may be insufficient to completely eliminate caspase-1/11 activity. Although the focus of future research, these findings strongly suggests that the improvements in protective immunity seen with caspase-1/11 ablation may be partially independent of the inflammasome directly.
In conclusion, these results unequivocally demonstrate that in neonatal mice, endogenous caspase-1 leads to reduced survival to polymicrobial sepsis, and that the improved survival of caspase-1-deficient neonatal mice to polymicrobial sepsis can be explained only in part by an attenuated inflammatory response. Rather, caspase-1-deficient mice also have increased protective immunity with increased myeloid progenitors and effector cells leading to increased haematopoietic expansion, increased myeloid cell recruitment, and fewer local bacteria, which contributes to their improved survival. Importantly, these findings suggest a potential signalling pathway for therapeutic targeting to improve the neonate's immature immune response to sepsis, independent of the inflammasome.
Acknowledgments
Supported in part by grants R01 GM-040586-26 and R01 GM-097531-04, awarded by the National Institute of General Medical Sciences, USPHS. LFG and AGC were also supported by a Ruth Kirschstein National Research Service Award (T32 GM-008721). AGC also received an individual training grant, F32 GM-07852, also from the NIGMS.
Authorship contributions
LFG contributed to the collecting and analysis of data and interpretation, conception, design, composition, drafting and editing of the manuscript. AC contributed to collecting and analysis of data and interpretation, conception, design and editing of the manuscript. DCN performed animal experiments and data collection and contributed to the editing of the manuscript. AGC contributed to the conception and design of experiments, and editing of the manuscript. RU contributed to data collection. PAE contributed to the manuscript editing. LLM and SDL contributed to the conception and design of the manuscript, data analysis and interpretation, funding, and composition/editing/writing of the manuscript.
Conflict of interest disclosures
None of the authors have disclosed any conflict of interest.
References
- Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37:439–79. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–12. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer KL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Lopez MC, et al. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol. 2014;192:3156–65. doi: 10.4049/jimmunol.1301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Cuenca AG, Wynn JL, Kelly-Scumpia KM, et al. Critical role for CXCL10 (IP-10)/CXCR3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79:2746–54. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Joiner DN, Gentile LF, et al. TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J Immunol. 2015;194:1169–77. doi: 10.4049/jimmunol.1302676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Kelly-Scumpia KM, Thayer TC, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol. 2011;187:911–8. doi: 10.4049/jimmunol.1100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Thayer T, Gabrilovich S, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–14. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayamajhi M, Zhang Y, Miao EA. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol Biol. 2013;1040:85–90. doi: 10.1007/978-1-62703-523-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–10. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Jacob A, Wu R, et al. Novel therapeutic targets for sepsis: regulation of exaggerated inflammatory responses. J Nippon Med Sch. 2012;79:4–18. doi: 10.1272/jnms.79.4. [DOI] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–23. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Cuenca AL, Gentile LF, Efron PA, Islam S, Moldawer LL, Kays DW, Larson SD. Delayed emergency myelopoiesis following polymicrobial sepsis in neonates. Innate Immun. 2014 doi: 10.1177/1753425914542445. ; pii: 1753425914542445. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtenberg BC, Mace PD, Riedl SJ. Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol. 2014;29C:17–25. doi: 10.1016/j.sbi.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E, Mear JB, Faure K, et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- Murphey ED. Cecal ligation and puncture-induced impairment of innate immune function does not occur in the absence of caspase-1. J Immunol. 2011;187:905–10. doi: 10.4049/jimmunol.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarova M, Hesker PR, Jania L, et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol. 2012;189:2006–16. doi: 10.4049/jimmunol.1201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci USA. 2014;111:15514–9. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]