Abstract
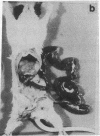
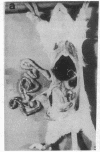
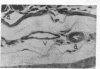
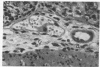
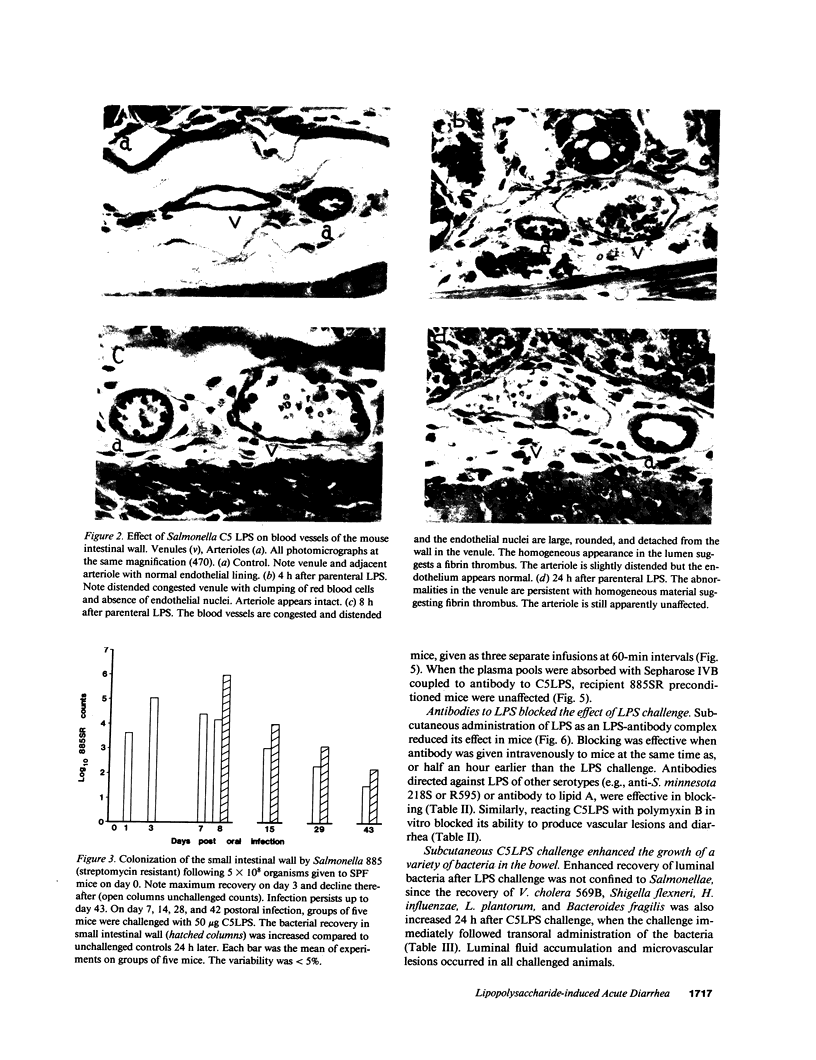
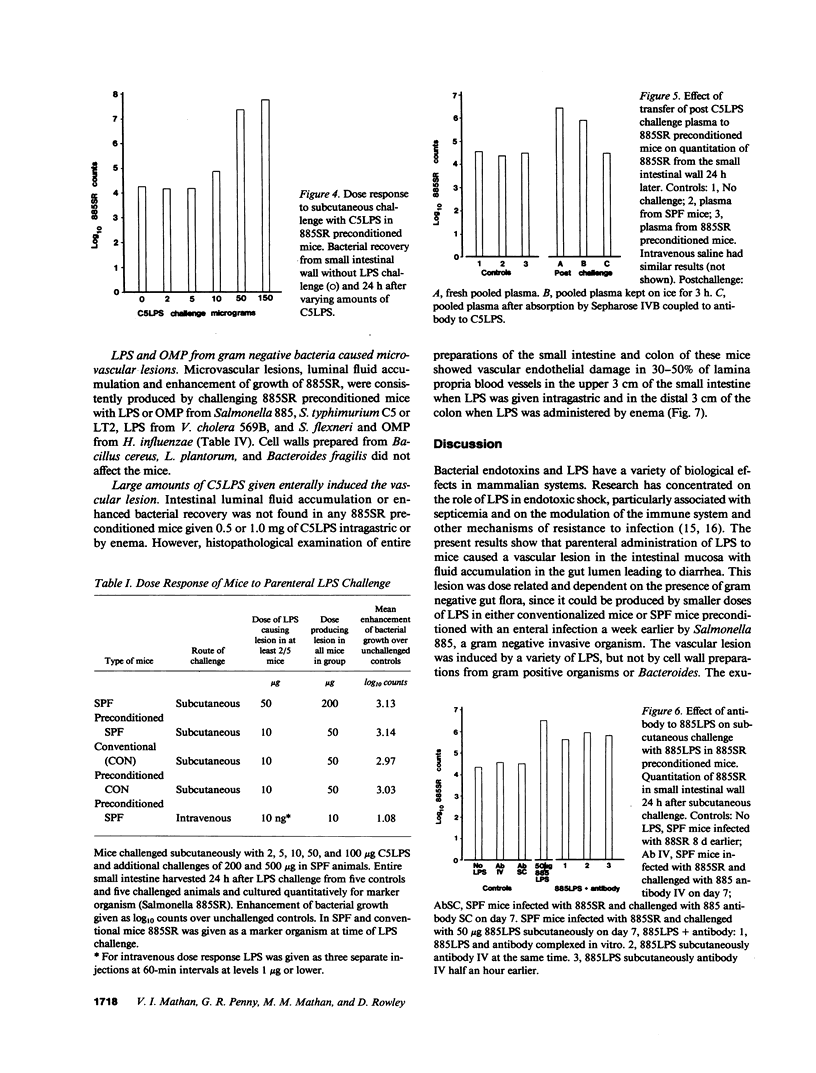
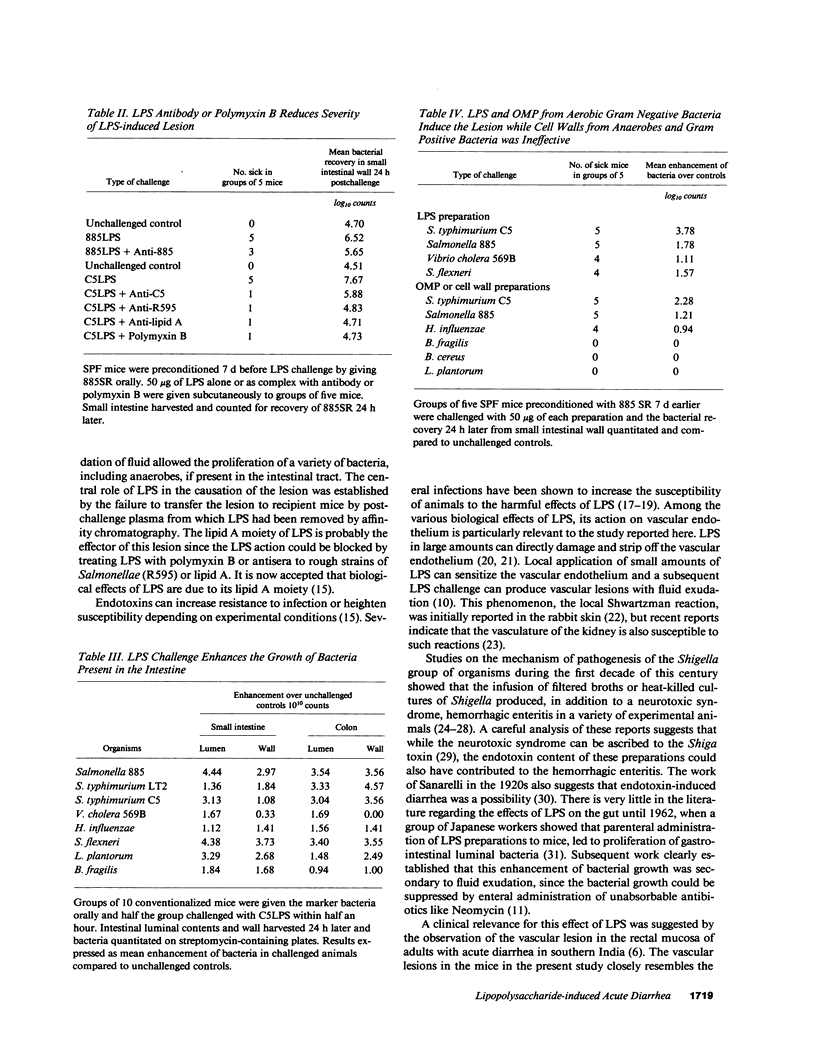
Subcutaneous challenge of mice with lipopolysaccharide (LPS) from gram negative bacteria, produced an intestinal microvascular lesion causing fluid exudation into the lumen of the intestine and diarrhea. The microvascular lesion was characterized by endothelial cell damage and microthrombi in the venules and capillaries of the intestinal lamina propria. Marker organisms, given orally to challenged mice, grew in the exuded fluid and could invade the mucosa. Intravenous transfer of postchallenge plasma produced the lesion in normal mice and absorption of such plasma by Sepharose coupled to LPS-antibody abolished this effect. Instillation of large quantities of LPS into the lumen of the intestine produced scattered microvascular lesions, although none of these animals developed diarrhea. Since a similar microvascular lesion has been described in the rectal mucosal lamina propria of adults with acute diarrhea, it is suggested that LPS-induced vascular damage may be a novel mechanism in the pathogenesis of acute diarrhea.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choudari C. P., Mathan M., Rajan D. P., Raghavan R., Mathan V. I. A correlative study of etiology, clinical features and rectal mucosal pathology in adults with acute infectious diarrhea in southern India. Pathology. 1985 Jul;17(3):443–450. doi: 10.3109/00313028509105498. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Doenhoff M. J., Allison A. C. Increased hepatotoxicity of bacterial lipopolysaccharide in mice infected with Schistosoma mansoni. Parasite Immunol. 1979 Winter;1(4):289–294. doi: 10.1111/j.1365-3024.1979.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Carter R. F., Ferluga J., Allison A. C. Lipopolysaccharide hyperreactivity of animals infected with Trypanosoma lewisi or Trypanosoma musculi. Infect Immun. 1984 Nov;46(2):501–506. doi: 10.1128/iai.46.2.501-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. Increased mitotic activity in rabbit endothelium after endotoxin. An autoradiographic study. Lab Invest. 1971 Apr;24(4):318–320. [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M. Shigella toxin(s): description and role in diarrhea and dysentery. Pharmacol Ther. 1981;15(3):403–438. doi: 10.1016/0163-7258(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977 Dec;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. L., Rowley D. Intestinal absorption of bacterial antigens in normal adult mice. II. A comparative study of techniques. Int Arch Allergy Appl Immunol. 1985;76(1):30–36. doi: 10.1159/000233657. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Local Shwartzman reaction in the rectal mucosa in acute diarrhoea. J Pathol. 1985 Jul;146(3):179–187. doi: 10.1002/path.1711460304. [DOI] [PubMed] [Google Scholar]
- Mathan V. I., Rajan D. P. The prevalence of bacterial intestinal pathogens in a healthy rural population in southern India. J Med Microbiol. 1986 Sep;22(2):93–96. doi: 10.1099/00222615-22-2-93. [DOI] [PubMed] [Google Scholar]
- McKay D. G., Margaretten W., Csavossy I. An electron microscope study of the effects of bacterial endotoxin on the blood-vascular system. Lab Invest. 1966 Dec;15(12):1815–1829. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Nolan J. P., Hare D. K., McDevitt J. J., Ali M. V. In vitro studies of intestinal endotoxin absorption. I. Kinetics of absorption in the isolated everted gut sac. Gastroenterology. 1977 Mar;72(3):434–439. [PubMed] [Google Scholar]
- RAVIN H. A., ROWLEY D., JENKINS C., FINE J. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J Exp Med. 1960 Nov 1;112:783–792. doi: 10.1084/jem.112.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij L., Keane W. F., Michael A. F. Unilateral Shwartzman reaction: cortical necrosis in one kidney following in vivo perfusion with endotoxin. Kidney Int. 1977 Aug;12(2):91–95. doi: 10.1038/ki.1977.85. [DOI] [PubMed] [Google Scholar]
- Rajan D. P., Mathan V. I. Prevalence of Campylobacter fetus subsp. jejuni in healthy populations in southern India. J Clin Microbiol. 1982 May;15(5):749–751. doi: 10.1128/jcm.15.5.749-751.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. HYPERREACTIVITY TO ENDOTOXIN AFTER INFECTION WITH BCG. J Immunol. 1964 Jan;92:49–54. [PubMed] [Google Scholar]