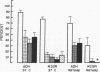Abstract
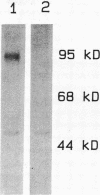
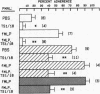
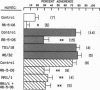
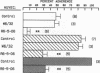
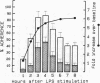
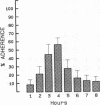
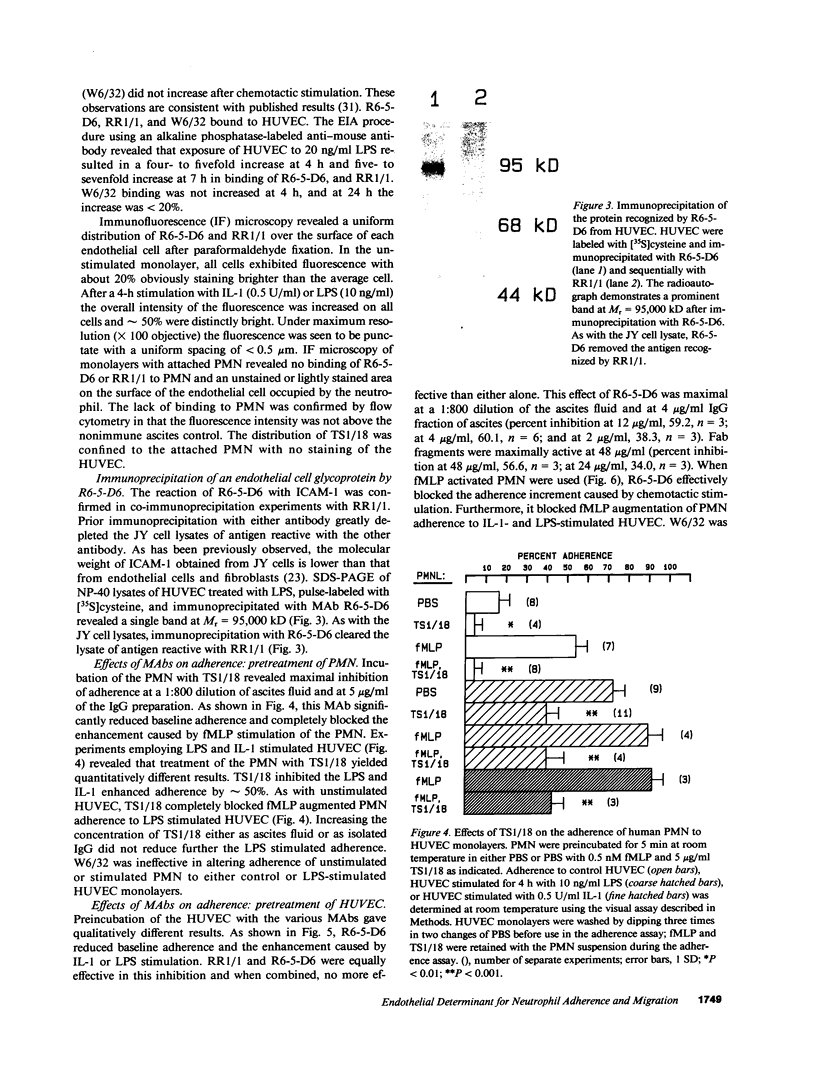
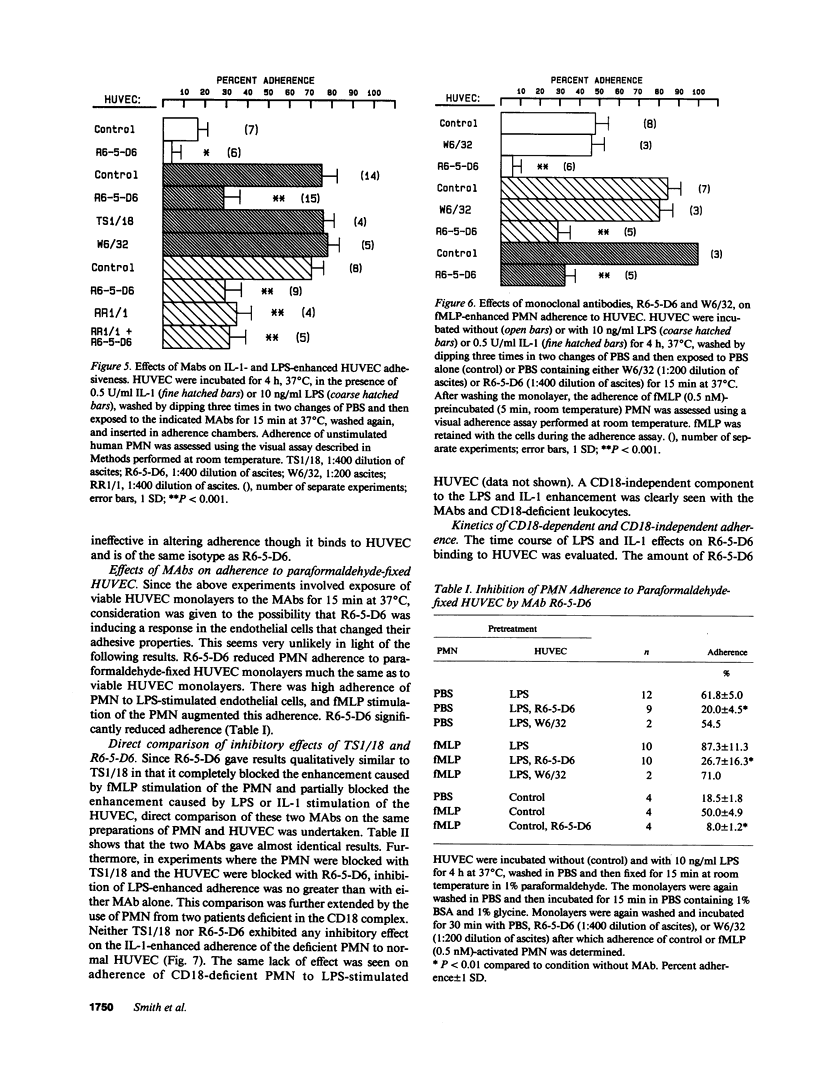
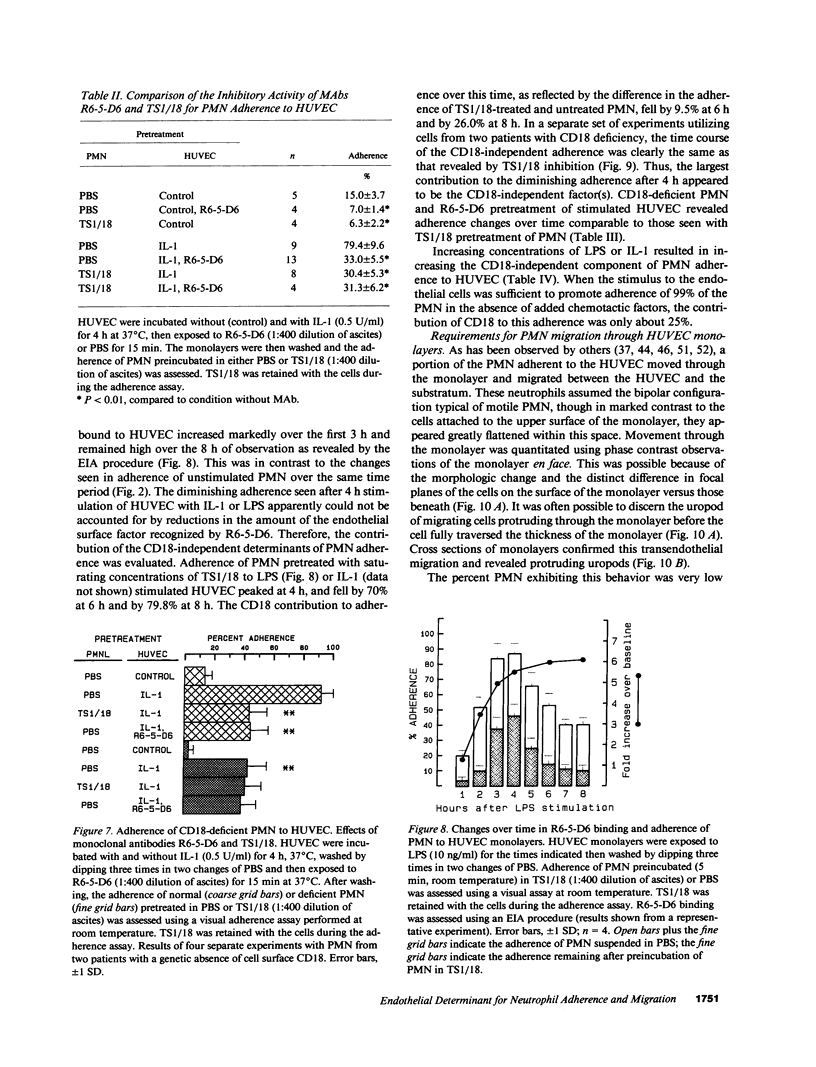
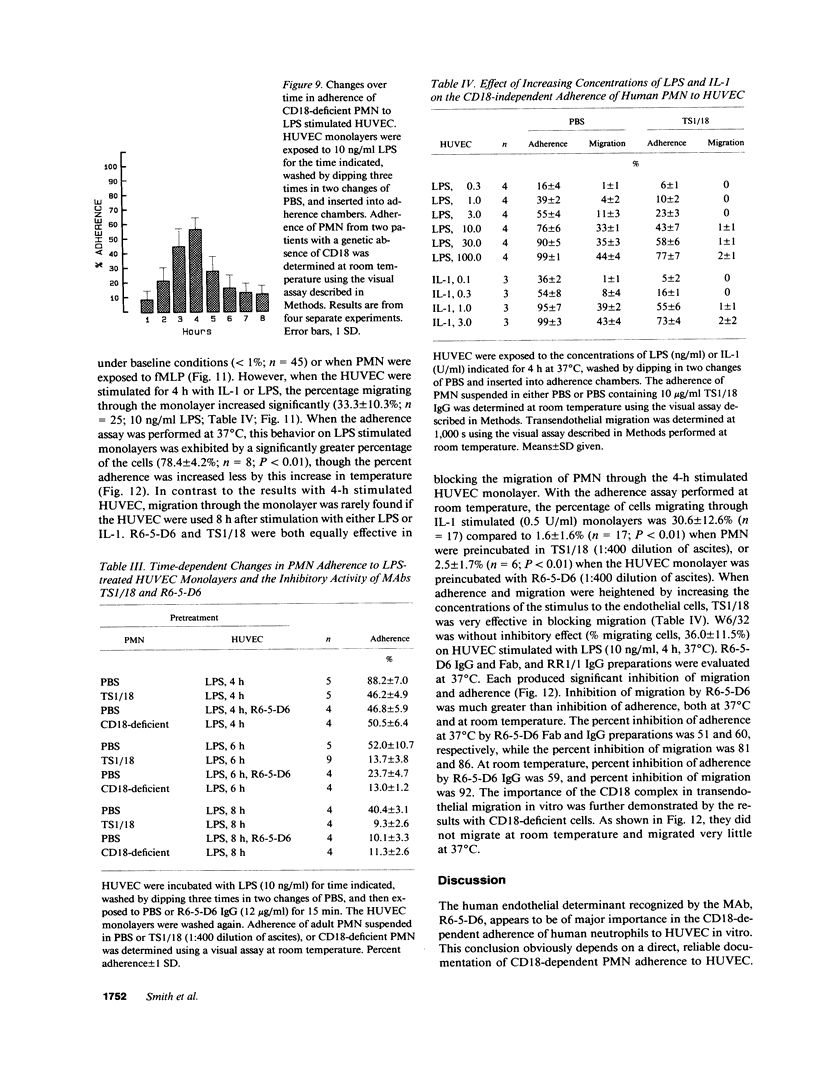
Human neutrophil (PMN) attachment to human umbilical vein endothelial cells (HUVEC) was evaluated in vitro using two MAbs, R6-5-D6 and RR1/1, that recognize intercellular adhesion molecule-1 (ICAM-1), and one MAb, TS1/18, that recognizes CD18. Pretreatment of the HUVEC with anti-ICAM-1 MAbs produced greater than 50% inhibition of attachment to HUVEC, and IL-1 (0.5 U/ml)- or lipopolysaccharide (LPS) (10 ng/ml)-stimulated HUVEC, and greater than 99% inhibition of f-Met-Leu-Phe (0.5 nM) enhanced adherence. Anti-ICAM-1 MAbs also inhibited by greater than 85% the transendothelial migration induced by 4-h IL-1 (0.5 U/ml) and LPS (10 ng/ml) activation of the HUVEC. That these effects involved a CD18-dependent mechanism is supported by the following results: pretreatment of PMN with TS1/18 produced the same degree of inhibition of attachment and migration as seen with R6-5-D6. In addition, the use of both MAbs together did not further increase the inhibition of cell attachment to stimulated HUVEC. The attachment of PMN from patients with CD18 deficiency to stimulated HUVEC was not reduced by R6-5-D6, and both R6-5-D6 and TS1/18 revealed the same time course for appearance and disappearance of an adherence component on stimulated HUVEC not blocked by either MAb. These results demonstrate that attachment and transendothelial migration of PMN in vitro depend substantially on both CD18 on the PMN and ICAM-1 on the endothelial cell.
Full text
PDF

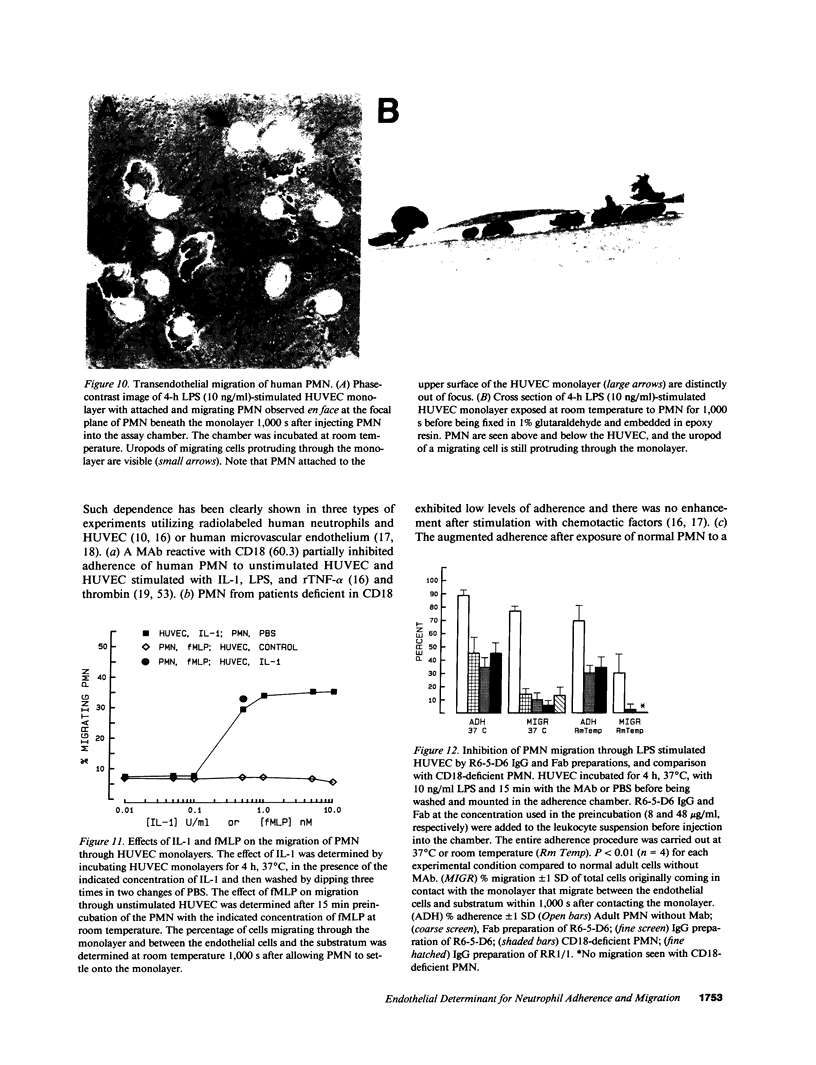



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Krishna G. S., Hughes B. J., Mace M. L., Mintz A. A., Smith C. W., Nichols B. L. Impaired polymorphonuclear leukocyte motility in malnourished infants: relationship to functional abnormalities of cell adherence. J Lab Clin Med. 1983 Jun;101(6):881–895. [PubMed] [Google Scholar]
- Anderson D. C., Mace M. L., Brinkley B. R., Martin R. R., Smith C. W. Recurrent infection in glycogenosis type Ib: abnormal neutrophil motility related to impaired redistribution of adhesion sites. J Infect Dis. 1981 Mar;143(3):447–459. doi: 10.1093/infdis/143.3.447. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Armstrong P. B., Lackie J. M. Studies of intercellular invasion in vitro using rabbit peritoneal neutrophil granulocytes (PMNS). I. Role of contact inhibition of locomotion. J Cell Biol. 1975 May;65(2):439–462. doi: 10.1083/jcb.65.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beesley J. E., Pearson J. D., Hutchings A., Carleton J. S., Gordon J. L. Granulocyte migration through endothelium in culture. J Cell Sci. 1979 Aug;38:237–248. doi: 10.1242/jcs.38.1.237. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Bizios R., Lai L. C., Cooper J. A., Del Vecchio P. J., Malik A. B. Thrombin-induced adherence of neutrophils to cultured endothelial monolayers: increased endothelial adhesiveness. J Cell Physiol. 1988 Feb;134(2):275–280. doi: 10.1002/jcp.1041340214. [DOI] [PubMed] [Google Scholar]
- Buchanan M. R., Crowley C. A., Rosin R. E., Gimbrone M. A., Jr, Babior B. M. Studies on the interaction between GP-18-0-deficient neutrophils and vascular endothelium. Blood. 1982 Jul;60(1):160–165. [PubMed] [Google Scholar]
- Charo I. F., Yuen C., Perez H. D., Goldstein I. M. Chemotactic peptides modulate adherence of human polymorphonuclear leukocytes to monolayers of cultured endothelial cells. J Immunol. 1986 May 1;136(9):3412–3419. [PubMed] [Google Scholar]
- Colditz I. G. Margination and emigration of leucocytes. Surv Synth Pathol Res. 1985;4(1):44–68. doi: 10.1159/000156964. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Forrester J. V., Lackie J. M. Adhesion of neutrophil leucocytes under conditions of flow. J Cell Sci. 1984 Aug;70:93–110. doi: 10.1242/jcs.70.1.93. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Naprstek B. L., Silverstein S. C. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J Cell Sci. 1987 Sep;88(Pt 2):161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Brock A. F., Schafer A. I. Leukotriene B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest. 1984 Oct;74(4):1552–1555. doi: 10.1172/JCI111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Hoover R. L., Robinson J. M., Karnovsky M. J. Adhesion of polymorphonuclear leukocytes to endothelium enhances the efficiency of detoxification of oxygen-free radicals. Am J Pathol. 1987 Feb;126(2):258–268. [PMC free article] [PubMed] [Google Scholar]
- Ismail G., Morganroth M. L., Todd R. F., 3rd, Boxer L. A. Prevention of pulmonary injury in isolated perfused rat lungs by activated human neutrophils preincubated with anti-Mo1 monoclonal antibody. Blood. 1987 Apr;69(4):1167–1174. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Wu N., Bargatze R. F., Butcher E. C. Human lymphocyte and lymphoma homing receptors. Annu Rev Med. 1987;38:467–476. doi: 10.1146/annurev.me.38.020187.002343. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Burakoff S. J., Faller D. V. Adhesion of T lymphocytes to human endothelial cells is regulated by the LFA-1 membrane molecule. J Cell Physiol. 1986 Feb;126(2):285–290. doi: 10.1002/jcp.1041260219. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Crimmins M. A., Burakoff S. J., Faller D. V. Alpha and beta subunits of the LFA-1 membrane molecule are involved in human monocyte-endothelial cell adhesion. J Cell Physiol. 1987 Mar;130(3):410–415. doi: 10.1002/jcp.1041300314. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstieg F. C., Rudloff H. E., Anderson D. C. Binding of the adhesive protein complex (LFA-1/Mac-1/p150,95) to concanavalin A. J Leukoc Biol. 1986 Feb;39(2):193–203. doi: 10.1002/jlb.39.2.193. [DOI] [PubMed] [Google Scholar]
- Schmid-Schoenbein G. W., Fung Y. C., Zweifach B. W. Vascular endothelium-leukocyte interaction; sticking shear force in venules. Circ Res. 1975 Jan;36(1):173–184. doi: 10.1161/01.res.36.1.173. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Usami S., Skalak R., Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980 Jan;19(1):45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- Smart Y. C., Cox J., Roberts T. K., Brinsmead M. W., Burton R. C. Differential effect of cigarette smoking on recirculating T lymphocyte subsets in pregnant women. J Immunol. 1986 Jul 1;137(1):1–3. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. Motility and adhesiveness in human neutrophils. Redistribution of chemotactic factor-induced adhesion sites. J Clin Invest. 1980 Apr;65(4):804–812. doi: 10.1172/JCI109731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Beatty P. G., Ochs H. D., Harlan J. M. Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol. 1985 Oct;135(4):2323–2330. [PubMed] [Google Scholar]
- Woodruff J. J., Clarke L. M., Chin Y. H. Specific cell-adhesion mechanisms determining migration pathways of recirculating lymphocytes. Annu Rev Immunol. 1987;5:201–222. doi: 10.1146/annurev.iy.05.040187.001221. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Butcher E. C., Stoolman L. M., Rosen S. D. Receptors involved in lymphocyte homing: relationship between a carbohydrate-binding receptor and the MEL-14 antigen. J Cell Biol. 1987 Mar;104(3):725–731. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M. Neutrophil adherence to human endothelium in vitro occurs by CDw18 (Mo1, MAC-1/LFA-1/GP 150,95) glycoprotein-dependent and -independent mechanisms. J Clin Invest. 1988 Feb;81(2):531–537. doi: 10.1172/JCI113351. [DOI] [PMC free article] [PubMed] [Google Scholar]