Abstract
Background
125I seed implantation is a new method in treatment of nasopharyngeal carcinoma (NPC), and it is worthwhile to evaluate its feasibility. In this study, we performed brachytherapy with computed tomography (CT)-guided permanent implantation of 125I seeds in the treatment of patients with the recurrence of NPC.
Methods
A total 30 patients (20 male and ten female) at the median age of 55 (range 25–80) years were diagnosed with recurrent nonkeratin NPC, with a total 38 lesions and a short disease-free interval (mediañ11 months) after primary radiotherapy alone or combined with chemotherapy. Patients received CT scan, starting from 2 months after the treatment. Follow-up was conducted for ~2–38 months to observe the local control rate and overall survival rate. We also analyzed the possible correlation between survival periods and the status of recurrent tumors.
Results
The local control rates at 6, 12, 24, 30, and 36 months after the procedure of 125I seed implantation were 86.8%, 73.7%, 26.3%, 15.8%, and 5.3%, respectively. The overall 1-, 2-, and 3-year survival rates were 80.0% (24/30), 30.0% (9/30), and 6.7% (2/30), respectively, with a median survival period of 18 months (17.6±8.6 months). Interestingly, the survival periods of the patients who had primary radiotherapy with or without chemotherapy were 15.8±7.9 and 24.3±7.9 months, respectively. Kaplan–Meier survival analysis demonstrated that χ2 (log rank) was 7.555, with very significant difference (P<0.01). The survival periods of patients in tumor stages I, II, III, and IV were 25.4±8.7, 19.8±9.4, 16.1±4.5, and 12.8±7.8 months, respectively, with significant differences (P<0.05).
Conclusion
Our data suggest that the survival period of recurrent NPC patients after 125I seed implantation is inversely related to the tumor stages of the recurrence but not to chemotherapy after the primary radiotherapy. Therefore, CT-guided 125I seed implantation can be set for treatment of recurrent NPC, for better survival rate with minimal damage.
Keywords: CT-guided 125I seed, radiochemotherapy, NPC
Introduction
Nasopharyngeal carcinoma (NPC) caused 65,000 deaths worldwide in 2010.1 Although it is a relatively uncommon disease in Western countries (0.5–2/100,000/year), nasopharyngeal carcinoma is a common type of head and neck cancers in southern China (30–80/100,000/year).2 The World Health Organization (WHO) has classified NPC into three histologic types: type I (keratinizing), type II (differentiated nonkeratinizing), and type III (undifferentiated nonkeratinizing). In southern China, 2% of NPC is type I, 3% type II, and 95% type III.3 Because of their anatomic situation at the base of skull, most nasopharyngeal carcinomas spread early to cervical nodes and are difficult to treat with surgical operation. The primary treatment of NPC is radiotherapy, usually combined with chemotherapy.4 The improvement of therapeutic strategies increased the 5-year survival rate of NPC from ~50% in the 1980s to ~70% in the 1990s and is currently ~80%.5,6 However, the recurrence rate of NPC following the initial radiotherapy is 15%–58%.7–10 The recurrence of tumors reduces their sensitivity to radiotherapy and leads to a poor prognosis. Therefore, recurrence becomes a major modality of failure in NPC patients and a challenge to clinicians.
Current retreatment methods of the locally recurrent NPC include external radiotherapy, stereotactic radiosurgery, and brachytherapy, alone or in combination.4 Permanent implantation of 125I seeds has a major advantage of delivering a high dose of irradiation to cancers of the nasopharynx with a very sharp fall off outside the implanted volume.11–13 Computed tomography (CT)-guided permanent brachytherapy was initially used for treating liver malignancies14,15 and now is also widely applied in treatment of NPC.16 The 125I seeds have an average energy of 27.4–31.4 keV. After implantation of 125I seeds, the gamma rays are concentrated in the immediate surroundings of the target tissues (valid radius ~1.7 cm).17 This novel technique delivers high-dose radiation that ensures protracted cancer cell killing over a period of several months.
Many lines of evidence have shown that the implantation of 125I radioactive seeds into tissues of NPC significantly increases therapeutic doses of radiation in the target area of tumors and as well, probably reduces the unnecessary damage in surrounding heathy tissues. In this study, we have analyzed and evaluated the efficacy of CT-guided 125I seed implantation in the treatment of recurrent nonkeratin nasopharyngeal carcinoma at Shenzhen People’s Hospital. Our results suggest that this therapeutic method can be used for treating the recurrence of NPC after radiotherapy with or without chemotherapy.
Patients and methods
Patient selection
Between 2008 and 2014, 30 patients were diagnosed with the recurrence of nonkeratin NPC (Table 1) and selected for the treatment of brachytherapy with permanent implantation of 125I seeds. The diagnoses were made by needle biopsy. The lesions were located in the pharyngeal recess, parapharyngeal space, nasopharynx side, retropharyngeal lymph nodes, and neck, distinct from the skull base and not associated with any distant metastasis. The enrolled patients were informed of the potential risks of the proposed CT-guided 125I seed implantation therapy, and provided written informed consent before beginning the treatment. The protocol was approved by the ethics committee of Jinan University.
Table 1.
Patient characteristics (n=30)
| Characteristics | Number (%) |
|---|---|
| Sex | |
| Male | 20 (66.7) |
| Female | 10 (33.3) |
| Age | 51.2±13.9 (years) |
| Range | 25–80 |
| Recurrence | 30 (100) |
| DFI | 11.0±3.4 (months) |
| Histology | |
| WHO I | 0 (0) |
| WHO II | 23 (77.7) |
| WHO III | 7 (23.3) |
| Tumor stage | |
| I | 5 (16.7) |
| II | 8 (26.7) |
| III | 8 (26.7) |
| IV | 9 (30.0) |
| Previous radiotherapy | 30 (100) |
| Previous chemotherapy | 20 (66.7) |
Notes: WHO type I, keratinizing; type II, differentiated nonkeratinizing; type III, undifferentiated nonkeratinizing. Tumor stage according to TNM staging: stage I, T1 (confined to nasopharynx, or extension to oropharynx/nasal cavity without posterolateral spread), NO (no regional metastasis), M0 (no metastasis); stage II, T2 (extension into posterolateral pharyngeal soft tissues), N0, M0, or T2, N1 (unilateral metastasis to cervical nodes, <6 cm size, not level IV/Vb, and/or unilateral or bilateral involvement of retropharyngeal nodes, <6 cm size), M0; stage III, T3 (invasion of bony structures, skull base, and/or sinuses), N0 to N2 (bilateral metastasis to cervical nodes, <6 cm size, not level IV/Vb), M0, or T2, N2, M0; and stage IV, any T (T1, T2, T3, or T4: intracranial extension, cranial nerve involvement, spread to hypopharynx/orbit/infratemporal fossa), any N (N1, N2, or N3: involvement of nodes in levels IV/Vb, supraclavicular fossa), M0.27
Abbreviations: DFI, disease-free interval; M, metastasis; N, node; T, tumor; WHO, World Health Organization.
Patient information
A total of 30 patients (20 male and ten female) at the age of 51.2±13.9 years (median 55, range 25–80 years) were treated with 125I seed implantation. All tumors were recurrent nonkeratin NPC. The interval time from the end of the first course of radiotherapy to diagnosis of recurrence (disease-free interval [DFI]) was 11.0±3.4 months (median 11, range 6–20 months). The tumor diameters were 2.5±1.2 cm and varied from 1.0 to 5.9 cm (16 lesions ≤2 cm, 20 lesions 2–5 cm, and two lesions ≥ 5 cm). Patients had previously been treated with radiotherapy alone or followed with chemotherapy (Table 1). The doses of the first radiotherapy were 70 Gy, 35 times, 7 weeks (64~88 Gy, 32~44 times, 6.5~8.5 weeks). After radiotherapy, 20 patients were further treated with the chemotherapy regimens, which included cisplatin (100–120 mg·m−2, intravenous drip) and 5-fluorouracil (800–1,000 mg·m−2, for 5 days, intravenous drip) at 3-week intervals. No patients received chemotherapy after 125I seed implantation.
Instruments
For 125I seed implantation, we used a HiSpeed Advantage Genesis CT scanner (GE Healthcare, Little Chalfont, UK) and a treatment-planning system (Beijing University of Aeronautics and Astronautics, Beijing, People’s Republic of China), and the instruments of seed implantation included 18G implantation needles and a turntable implantation gun. The diameter and length of each 125I seed (Beijing Atom and High Technique Industries Inc. Pharmaceuticals Co Ltd, Ningbo, People’s Republic of China) was 0.8 mm and 4.5 mm, respectively. The thickness of the wall of the titanium capsule was 0.05 mm. The 125I emits gamma rays (5% of 35.5 keV and 95% of 28 keV) with a half-life of 59.4 days, half-value thickness of 0.025 mm of lead, penetration of 17 mm, and incipient rate of 7 cGy/hour. All 125I seeds (6,711/BT-125I) were delivered in a type-A pack that had passed leak detection and activity series tests.
Patient preparation
At 1–2 weeks prior to the procedure, patients were examined, using CT scans with a thickness of 5 mm, for the detailed tumor volumes. The planning target volume included the entire gross tumor volume and 0.5–1.0 cm margins, and was prescribed the minimal peripheral dose of 125I seeds (median 130 Gy, range 90–160 Gy).
125I seed implantation
Before 125I seed implantation, patients fasted for 1 to 3 hours. In a specially designated operating room patients were given sedatives and local anesthesia. The procedure included making a 3 mm incision on the skin and inserting a seed implantation applicator into each tumor under the guidance of CT with caution in avoiding puncture of large blood vessels or important organs. The 125I seeds (0.8 mCi) were implanted into each tumor, and then, the catheters were retracted and incisions were bound and compressed. Medical personal were protected by wearing lead gloves, hats, and other protective clothing. A special measurement of the ray dosage was performed to detect any missing radioactive seeds after the procedure.
Patient assessment and follow-up
After the implantation of 125I seeds was completed, vital signs were monitored and recorded for 24 hours. Patients were hospitalized for at least 3 days. A clinical examination and CT scan were performed every 2–3 months until death. To evaluate the therapeutic feasibility of 125I seed implantation, we used criteria according to the Response Evaluation Criteria in Solid Tumors (RECIST), which include: complete response (CR), defined as the disappearance of all target lesions; partial response (PR), defined as a decrease of at least 30% in the sum of the largest diameter of target lesions; stable disease (SD), defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD); PD, defined as at least a 20% increase in the sum of the longest diameter of target lesions; and total treatment response rate (RR), defined as CR cases + PR cases/case number (Table 2).
Table 2.
Clinical efficacy of recurrent NPC with 125I seed implantation therapy
| Tumor Dia (cm) | Local control efficacy (%)
|
RR | |||
|---|---|---|---|---|---|
| CR | PR | SD | PD | ||
| ≤2 | 13 (81.3) | 3 (18.8) | 0 (0.0) | 0 (0.0) | 16/16 (100.0) |
| 2–5 | 14 (70.0) | 4 (20.0) | 2 (10.0) | 0 (0.0) | 16/20 (80.0) |
| >5 | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 1/2 (50.0) |
| Total | 35/38 (92.1) | ||||
Notes: CR, disappearance of all target lesions; PR, a decrease of at least 30% in the sum of the largest diameter of target lesions; SD, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD; PD, at least a 20% increase in the sum of the longest diameter of target lesions; RR, (cases of CR + cases of PR)/number of NPC cases.
Abbreviations: CR, complete response; Dia, diameter; NPC, nasopharyngeal carcinoma; PD, progressive disease; PR, partial response; RR, total treatment response rate; SD, stable disease.
Statistical analyses
All values are presented as mean ± standard deviation. Student’s t-test, analysis of variance (ANOVA) test, and Kaplan–Meier survival analysis were used to evaluate the significance of differences. P<0.05 was considered significant and P<0.01, very significant.
Results
Treatment and follow-up
The 125I seed implantation procedure required 60 to 180 minutes and was guided by CT scan imaging according to the treatment-planning system established (Figure 1). The 125I seeds implanted were distributed with an interval of 1 cm between any two seeds, and the radioactive activity per seed was 29.6 MBq to obtain a peripheral dose of 90–160 Gy. The median number of seeds implanted per patient was 42 (range 4–112), and patients underwent one to four 125I seed implantation sessions (median of two). All patients were successfully implanted. The range of follow-up period was from 2 to 38 months for monitoring the local control rate, overall survival rate, and any clinical complications.
Figure 1.
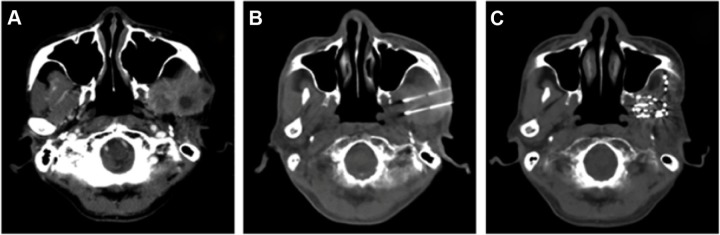
CT images of a recurrent NPC patient.
Notes: (A) A recurrent enhancing mass with NPC following initial radiochemotherapy. (B) CT-guided 125I seed implantation. (C) Resolution of the tumor mass at 2 months post–125I brachytherapy.
Abbreviations: CT, computed tomography; NPC, nasopharyngeal carcinoma.
CT imaging performed prior to 125I seed implantation revealed images of all the tumors. When the same imaging was performed 2 months after 125I seed implantation, CT showed resolution of the tumor mass, which exhibited the size and shape of the inactivated tumor (Figure 1 and 2).
Figure 2.
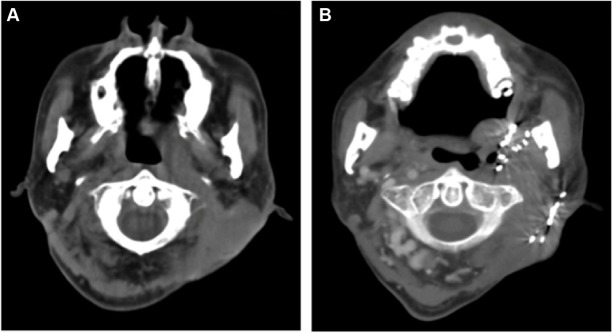
Metastasis of nasopharyngeal carcinoma.
Notes: (A) Lymph node metastasis of NPC behind the ear. (B) CT-image of resolution of tumor mass at 6 months post–125I brachytherapy.
Abbreviations: CT, computed tomography; NPC, nasopharyngeal carcinoma.
Local control
Local control rates for the recurrent NPC treated with 125I seed implantation at 3, 6, 12, 18, 24, 30, and 36 months after the procedure were 92.1% (35/38), 86.8% (33/38), 73.7% (28/38), 44.7% (17/38), 26.3% (10/38), 15.8% (6/38), and 5.3% (2/38), respectively. For tumors ≤2 cm in diameter, 125I seed implantation treatment achieved a CR rate of 81.3% (13/16), a PR rate of 18.8% (3/16), and a RR of 100% (16/16); for tumors of 2–5 cm in diameter, the CR rate was 70.0% (12/20), PR was 20.0% (4/20), SD rate was 20.0% (2/20), and RR was 80% (16/20); for tumors > 5 cm in diameter, the PR was 50.0% (1/2), SD was 50.0% (1/2), and the RR was 50% (1/2). The overall RR was 92.1% (35/38) in all cases (Table 2).
Survival rates
After the 125I seed implantation, the overall 1-, 2-, and 3-year survival rates were 80.0% (24/30), 30.0% (9/30), and 6.7% (2/30), respectively, with a median survival period of 18 months (17.6±8.6 months). Furthermore, the 1-, 2-, and 3-year survival rates of 125I seed implantation-treated patients with or without previous chemotherapy after radiotherapy were 70.0% (14/20) and 100% (10/10), 20% (4/20) and 50% (5/10), and 5% (1/20) and 10% (1/10), respectively. After 125I seed implantation, the survival period of patients with primary radiotherapy alone was 24.3±7.9 months, longer than that of patients with primary radio- and chemotherapy, 15.8±7.9 months. Kaplan–Meier survival analysis demonstrated that χ2 (log rank) was 7.555 (P<0.001), with very significant difference (Figure 3).
Figure 3.
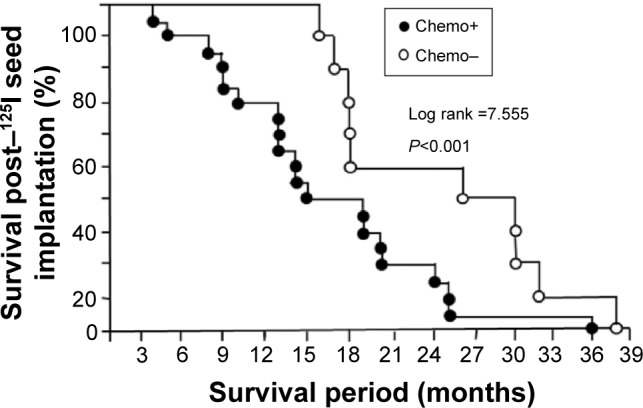
Kaplan–Meier survival curves of patients with the recurrent NPC post–125I seed implantation therapy.
Notes: Survival rates post–125I seed implantation therapy, for NPC patients who received primary radiotherapy with or without chemotherapy. The 1-, 2-, and 3-year survival rates of 125I seed implantation-treated patients with or without previous chemotherapy, after radiotherapy were 70.0% and 100%, 20% and 50%, and 5% and 10%, respectively. The survival periods of patients with and without chemotherapy were 15.8±7.9 and 24.3±7.9 months, respectively. Kaplan–Meier survival analysis demonstrated that χ2 (log rank) was 7.555, with very significant difference (P<0.001). After 125I seed implantation therapy, 1-, 2-, and 3-year survival rates of all 30 recurrent NPC patients were 80.0%, 30.0%, and 6.7%, respectively, with a median survival period of 18 months.
Abbreviations: chemo, chemotherapy; NPC, nasopharyngeal carcinoma.
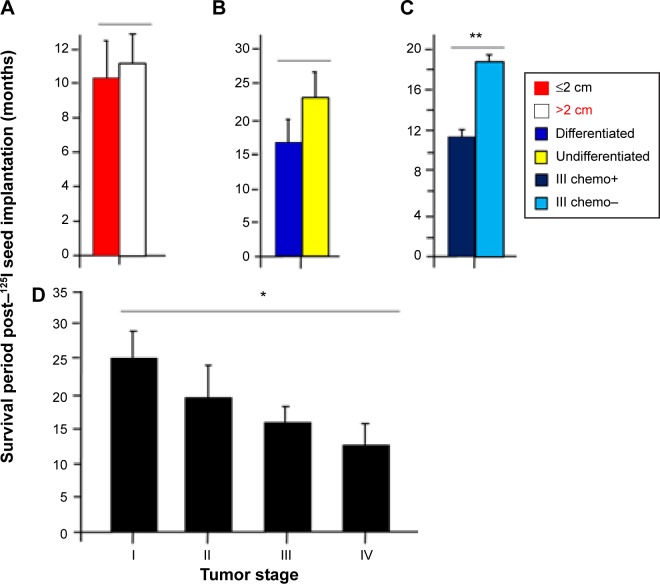
Survival periods and status of tumors
The survival period of all 30 patients after 125I seed implantation was 17.6±8.6 months (n=30) (Figure 3). The survival periods of patients with the tumor size ≤ 2 cm and >2 cm were 10.1±2.9 (n=7) and 11.3±3.6 months (n=23), respectively, without significant difference (P>0.05) (Figure 4A). The survival periods of patients with differentiated and undifferentiated nonkeratin NPC were 16.9±8.4 (n=23) and 24.1±8.1 months (n=7), respectively, without significant difference (P >0.05) (Figure 4B). In contrast, the survival periods of patients with the tumor stages I, II, III, and IV were 25.4±8.7 (n=5), 19.8±9.4 (n=8), 16.1±4.5 (n=8), and 12.8±7.8 (n=9) months, respectively, with significant differences (P<0.05, ANOVA tests) (Figure 4D). These data suggest that the survival period of NPC patients after 125I seed implantation is inversely related to the tumor stage. We also analyzed survival periods of 13 patients with stage III recurrent NPC after 125I seed implantation therapy. The survival period of five patients with the primary radiotherapy alone was 18.6±4.7 months, longer than that of eight patients with the primary combined radiochemotherapy, 12.0±1.9 months, with very significant difference (P<0.01) (Figure 4C). The interesting results question the effect of radio- and chemotherapy in primary NPC.
Figure 4.
Analyses of survival periods post–125I seed implantation therapy and status of NPC.
Notes: (A) The survival period for tumor size ≤ 2 cm was 10.1±2.9 months (n=7) and for tumor size >2 cm was 11.3±3.6 months (n=23), without significant difference (P>0.05). (B) The survival period for differentiated tumor was 16.9±8.4 months (n=23) and for undifferentiated tumor was 24.1±8.1 months (n=7), without significant difference (P>0.05). (C) The survival period among 13 patients with stage III recurrent NPC was18.6±4.7 months for five patients treated with the primary radiotherapy alone, and was 12.0±1.9 months for eight treated with combined radio- and chemotherapy (**P<0.01). (D) The survival period for stage I was 25.4±8.7 months (n=5), for stage II was 19.8±9.4 months (n=8), for stage III was 16.1±4.5 months (n=8), and for stage IV was 12.8±7.8 months (n=9), with significant differences (*P<0.05, ANOVA test).
Abbreviations: ANOVA, analysis of variance; chemo, chemotherapy; NPC, nasopharyngeal carcinoma.
Discussion
An extremely poor prognosis has been observed in patients with the local recurrence of NPC. For example, one analysis of 219 cases in the 1980s reported a 5-year survival rate was less than 1% if the patients were without any treatment.18 Another analysis of 891 patients with locally recurrent NPC showed a 5-year survival rate of 14%, although 79% (706/891) of patients had been reirradiated with various techniques and doses.19 Accordingly, the local recurrence of NPC is 8%–58% (median 34%).19 Therefore, the high frequency of the recurrence is a major cause of the mortality in NPC. To develop efficient methods for treatment of the recurrent disease is still a challenge in NPC treatment. Several techniques have been used to treat the recurrent NPC, including external radiotherapy, stereotactic radiosurgery, nasopharyngectomy, and brachytherapy, alone or combined with further chemotherapy.20–23 Among these therapies, brachytherapy with 125I seed implantation has been used widely in salvage treatment of the NPC recurrence.4
To estimate the efficacy of 125I seed implantation therapy in certain NPC types, we selected 30 patients with the recurrence of differentiated or undifferentiated nonkeratinizing cancers (WHO type II or III) (Figures 1 and 2). All patients had a short DFI, representing the interval time from the end of first course of radiotherapy to diagnosis of recurrence (6–12 months in 23 patients and 13–20 months in seven patients). The median interval of recurrence after the primary radiotherapy was only about 11 months (Table 1), much shorter than 24–26 months, the median interval of recurrence of NPC in prior reports.8,24,25 After 125I seed implantation therapy, the total treatment response rate reached 92.1%, and 1-, 2-, and 3-year survival rates were 80.0%, 30.0%, and 6.7%, respectively, with a median survival period of 18 months (Figure 3). In this study, we did not set up a control group because we could not leave recurrent NPC patients with no treatment. Our therapeutic efficacy is similar to a previous study using 125I seed implantation therapies, although recurrent NPC patients had a shorter DFI. For example, a recent report has shown that the 1-, 2-, and 3-year survival rates of a total of 19 patients with recurrent NPC after surgery and radiotherapy were 53.0%, 18.2%, and 18.2%, respectively, with a median survival period of 13 months.26
After 125I seed implantation, the RR of selected NPC patients was 92.1% (Table 2). The survival periods post–125I seed implantation were 4–38 months (Figure 3). Based on the results, we analyzed the possible factors affecting the prognosis. In 30 cases, before recurrence, ten patients had received the primary radiotherapy alone and 20 had received the primary radiotherapy combined with chemotherapy. After 125I seed implantation, the survival period of patients with primary radiotherapy alone was 24.3±7.9 months, longer than that of patients with the radio- and chemotherapy combination (15.8±7.9 months). Kaplan–Meier survival analysis demonstrated that χ2 (log rank) was 7.555 (P<0.001), with very significant difference (Figure 3). Among a total 13 patients with stage III recurrent NPC, after 125I seed implantation therapy, the survival period of five patients with the primary radiotherapy alone was 18.6±4.7 months, longer than that of eight patients with the primary combined radiochemotherapy, 12.0±1.9 months, with very significant difference (P<0.01) (Figure 4C). These unexpected results were probably caused by a small sample volume but may also be worth investigating for the effects of chemotherapy in the NPC treatment. Our further analyses demonstrated no significant correlation between the survival period and tumor size (Figure 4A) or differentiated and undifferentiated statues (Figure 4B). By performing ANOVA, we found there were significant differences (P<0.05) between survival periods post–125I seed implantation and stages (I, II, III, IV) of recurrent NPC (Figure 4D), suggesting that tumor stage is the most important prognostic factor, which agrees with most results of NPC treatment reports.
Because of the complex anatomy and the rich neurovascular supply in the nasopharynx region, direct implantation of radioactive particles is difficult. The 125I seed implantation brachytherapy has been extensively performed in NPC treatment in recent years. CT image guidance improves 125I seed deposition accuracy and delivers a sufficiently high dose to the tumor target, especially for the recurrent carcinoma after radiotherapy. In addition, the 125I radioactivity decreases very rapidly with distance, which reduces the risk of damage to neighboring healthy tissues, organs, or structures in proximity of the targeted tumors. Based on our results and other reports, CT-guided 125I seed implantation appears to be a feasible method for the treatment of patients with the recurrent NPC.
Acknowledgments
The authors gratefully acknowledge the financial support from the Guangdong Province Science and Technology Foundation (grant number 2013B010404028) (to YZ). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Zhang Y, Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol. 2013;37(6):793–802. doi: 10.1016/j.canep.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 4.Suárez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2010;267(12):1811–1824. doi: 10.1007/s00405-010-1385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AW, Foo W, Law SC, et al. Total biological effect on late reactive tissues following reirradiation for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;46(4):865–872. doi: 10.1016/s0360-3016(99)00512-x. [DOI] [PubMed] [Google Scholar]
- 6.Su SF, Han F, Zhao C, et al. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer. 2011;30(8):565–573. doi: 10.5732/cjc.010.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61(4):1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 8.Li JX, Lu TX, Huang Y, Han F, Chen CY, Xiao WW. Clinical features of 337 patients with recurrent nasopharyngeal carcinoma. Chin J Cancer. 2010;29(1):82–86. doi: 10.5732/cjc.009.10412. Chinese. [DOI] [PubMed] [Google Scholar]
- 9.Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 2013;20(5):e406–e419. doi: 10.3747/co.20.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65(1):161–168. doi: 10.1016/j.ijrobp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hilaris BS, Lewis JS, Henschke UK. Therapy of recurrent cancer of the nasopharynx. Value of interstitial and intracavitary radiation. Arch Otolaryngol. 1968;87(5):506–510. doi: 10.1001/archotol.1968.00760060508012. [DOI] [PubMed] [Google Scholar]
- 12.Wang CC, Busse J, Gitterman M. A simple afterloading applicator for intracavitary irradiation of carcinoma of the nasopharynx. Radiology. 1975;115(3):737–738. doi: 10.1148/15.3.737. [DOI] [PubMed] [Google Scholar]
- 13.Vikram B. Permanent iodine-125 (I-125) boost after teletherapy in primary cancers of the nasopharynx is safe and highly effective: long-term results. Int J Radiat Oncol Biol Phys. 1997;38(5):1140. doi: 10.1016/s0360-3016(97)00132-6. [DOI] [PubMed] [Google Scholar]
- 14.Ricke J, Wust P, Stohlmann A, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I–II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58(5):1496–1505. doi: 10.1016/j.ijrobp.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Ricke J, Wust P, Stohlmann A, et al. CT-guided brachytherapy. A novel percutaneous technique for interstitial ablation of liver metastases. Strahlenther Onkol. 2004;180(5):274–280. doi: 10.1007/s00066-004-1179-4. German. [DOI] [PubMed] [Google Scholar]
- 16.Jiang YL, Meng N, Wang JJ, et al. Percutaneous computed tomography/ultrasonography-guided permanent iodine-125 implantation as salvage therapy for recurrent squamous cell cancers of head and neck. Cancer Biol Ther. 2010;9(12):959–966. doi: 10.4161/cbt.9.12.11700. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y, Xie Q, Tian Y, et al. Radioactive 125I seed inhibits the cell growth, migration, and invasion of nasopharyngeal carcinoma by triggering DNA damage and inactivating VEGF-A/ERK signaling. PLoS One. 2013;8(9):e74038. doi: 10.1371/journal.pone.0074038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan JH, Hu YH, Gu XZ. Radiation therapy of recurrent nasopharyngeal carcinoma. Report on 219 patients. Acta Radiol Oncol. 1983;22(1):23–28. doi: 10.3109/02841868309134335. [DOI] [PubMed] [Google Scholar]
- 19.Lee AW, Law SC, Foo W, et al. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976–1985: survival after local recurrence. Int J Radiat Oncol Biol Phys. 1993;26(5):773–782. doi: 10.1016/0360-3016(93)90491-d. [DOI] [PubMed] [Google Scholar]
- 20.Gebbia V, Zerillo G, Restivo G, et al. Chemotherapeutic treatment of recurrent and/or metastatic nasopharyngeal carcinoma: a retrospective analysis of 40 cases. Br J Cancer. 1993;68(1):191–194. doi: 10.1038/bjc.1993.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suárez C, Llorente JL, Muñoz C, García LA, Rodrigo JP. Facial translocation approach in the management of central skull base and infratemporal tumors. Laryngoscope. 2004;114(6):1047–1051. doi: 10.1097/00005537-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Teo PM, Kwan WH, Chan AT, Lee WY, King WW, Mok CO. How successful is high-dose (> or =60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 1998;40(4):897–913. doi: 10.1016/s0360-3016(97)00854-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma – treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13(7):953–956. doi: 10.1016/0360-3016(87)90030-7. [DOI] [PubMed] [Google Scholar]
- 24.Li JX, Lu TX, Huang Y, Han F. Clinical characteristics of recurrent nasopharyngeal carcinoma in high-incidence area. Scientific World Journal. 2012;2012:719754. doi: 10.1100/2012/719754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian YM, Tian YH, Zeng L, et al. Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity-modulated radiotherapy. Br J Cancer. 2014;110(2):297–303. doi: 10.1038/bjc.2013.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Jiang Y, Wang J, et al. An investigation of 125I seed permanent implantation for recurrent carcinoma in the head and neck after surgery and external beam radiotherapy. World J Surg Oncol. 2013;11:60. doi: 10.1186/1477-7819-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A III, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]