Abstract
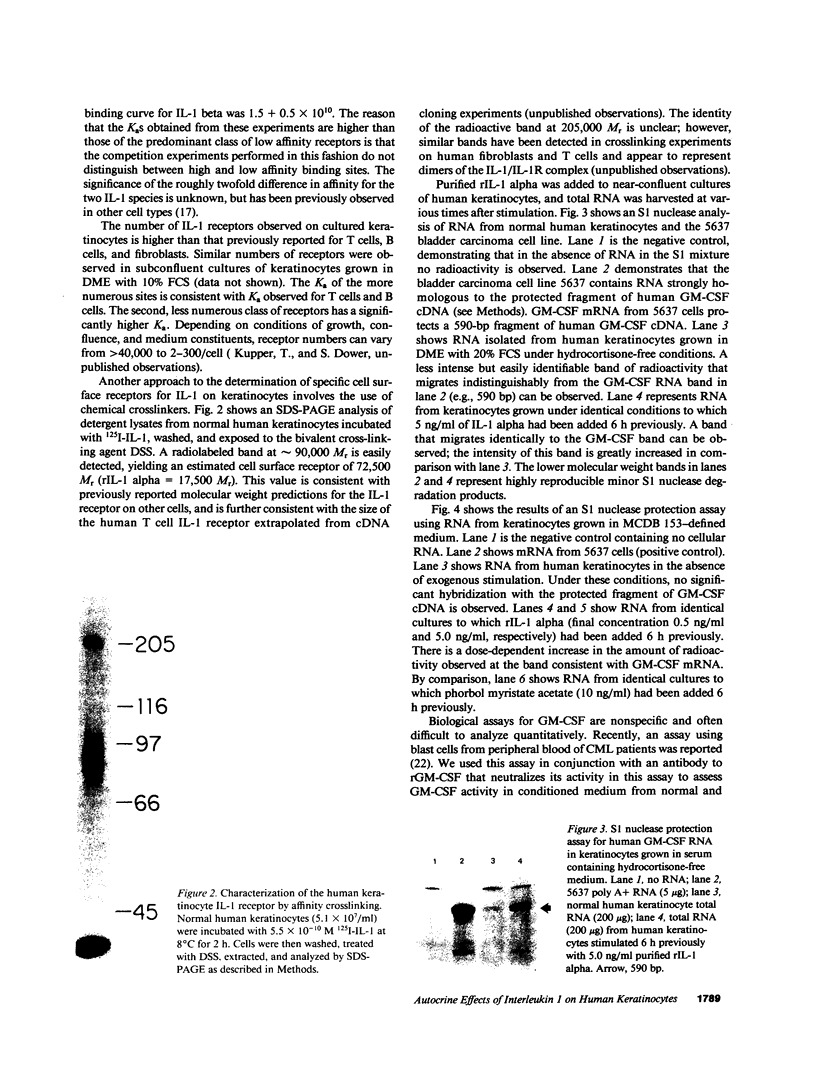
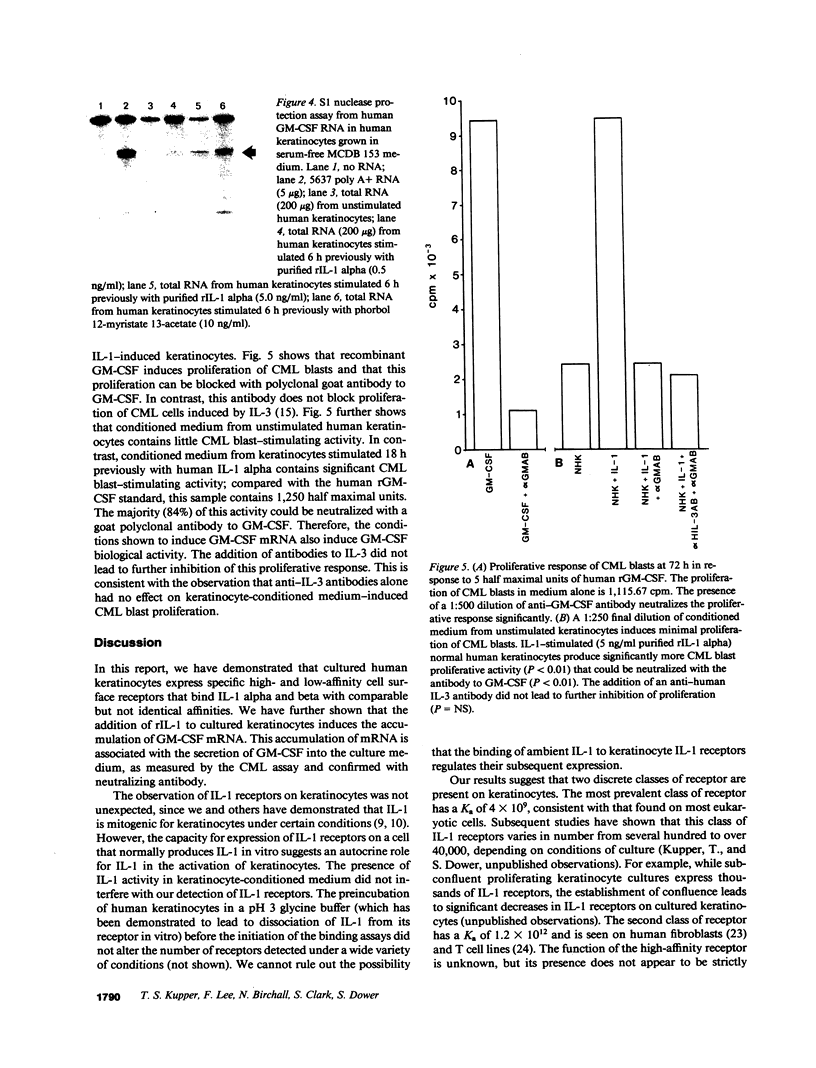
Cultured human keratinocytes have been shown to produce IL-1 alpha and beta mRNA and protein. IL-1 biological activity has been identified in normal human epidermis; in vitro, most biologically active IL-1 resides in a cell-associated compartment. The potential for autocrine effects of IL-1 on human keratinocytes was assessed by measurement of keratinocyte IL-1 receptors. Both high- and low-affinity cell surface receptors that bound recombinant (r) IL-1 alpha and beta with comparable affinities could be identified on cultured human keratinocytes, using 125I-labeled rIL-1. Chemical crosslinking experiments identified a cell surface molecule of roughly 72,500 Mr that bound 125I-labeled IL-1, similar to the molecular weight of previously described IL-1 receptors on fibroblasts, B cells, and T cells. To assess the biological consequences of keratinocyte IL-1 binding, granulocyte-macrophage colony-stimulating factor (GM-CSF) gene expression was measured. The addition of exogenous rIL-1 alpha led to a dose-dependent increase in the accumulation of GM-CSF mRNA, as measured by a sensitive and specific S1 nuclease assay. This increase in mRNA was reflected in a marked increase in GM-CSF biological activity as measured by proliferation of blast cells from chronic myelogenous leukemia patients. The biological activity was completely inhibitable by an antibody to human rGM-CSF. GM-CSF activates mature neutrophils and macrophages and appears to enhance the efficiency of Langerhans cell antigen presentation to T cells. Release of IL-1 from injured or activated keratinocytes may lead to enhanced epidermal GM-CSF gene expression via an autocrine mechanism, thus enhancing local host defense.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird T. A., Saklatvala J. Identification of a common class of high affinity receptors for both types of porcine interleukin-1 on connective tissue cells. Nature. 1986 Nov 20;324(6094):263–266. doi: 10.1038/324263a0. [DOI] [PubMed] [Google Scholar]
- Chin J., Cameron P. M., Rupp E., Schmidt J. A. Identification of a high-affinity receptor for native human interleukin 1 beta and interleukin 1 alpha on normal human lung fibroblasts. J Exp Med. 1987 Jan 1;165(1):70–86. doi: 10.1084/jem.165.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chodakewitz J. A., Kupper T. S., Coleman D. L. Keratinocyte-derived granulocyte/macrophage colony-stimulating factor induces DNA synthesis by peritoneal macrophages. J Immunol. 1988 Feb 1;140(3):832–836. [PubMed] [Google Scholar]
- Chu A. C., Goldstein G., Patterson J., Berger C., Takezaki S., Edelson R. Thymopoietin-like substance in human skin. Lancet. 1982 Oct 2;2(8301):766–767. doi: 10.1016/s0140-6736(82)90946-1. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Culpepper J. A., Lee F. Regulation of IL 3 expression by glucocorticoids in cloned murine T lymphocytes. J Immunol. 1985 Nov;135(5):3191–3197. [PubMed] [Google Scholar]
- Danner M., Luger T. A. Human keratinocytes and epidermoid carcinoma cell lines produce a cytokine with interleukin 3-like activity. J Invest Dermatol. 1987 Apr;88(4):353–361. doi: 10.1111/1523-1747.ep12469013. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Kurtzberg J., Le P. T., Tuck D. T., Singer K. H., Haynes B. F. Human thymic epithelial cells directly induce activation of autologous immature thymocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3125–3129. doi: 10.1073/pnas.85.9.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., Hopp T. P., Cantrell M., Deeley M., Gillis S., Henney C. S., Urdal D. L. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986 Nov 20;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring L. C., Buckley A., Daynes R. A. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J Clin Invest. 1985 Oct;76(4):1585–1591. doi: 10.1172/JCI112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldminz D., Kupper T. S., McGuire J. Keratinocyte membrane-associated epidermal cell-derived thymocyte-activating factor (ETAF). J Invest Dermatol. 1987 Jan;88(1):97–100. doi: 10.1111/1523-1747.ep12465135. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Mochizuki D., Kronheim S., Price V., Cosman D., Urdal D., Gillis S., Conlon P. Regulation of antibody production in vitro by granulocyte-macrophage colony stimulating factor. J Mol Cell Immunol. 1986;2(4):199–207. [PubMed] [Google Scholar]
- Hauser C., Saurat J. H., Schmitt A., Jaunin F., Dayer J. M. Interleukin 1 is present in normal human epidermis. J Immunol. 1986 May 1;136(9):3317–3323. [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Chua A. O., Flood P., McGuire J., Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987 Aug;80(2):430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. Interleukin 1 and other human keratinocyte cytokines: molecular and functional characterization. Adv Dermatol. 1988;3:293–307. [PubMed] [Google Scholar]
- Kupper T. S., Lee F., Coleman D., Chodakewitz J., Flood P., Horowitz M. Keratinocyte derived T-cell growth factor (KTGF) is identical to granulocyte macrophage colony stimulating factor (GM-CSF). J Invest Dermatol. 1988 Aug;91(2):185–188. doi: 10.1111/1523-1747.ep12464470. [DOI] [PubMed] [Google Scholar]
- Kupper T., Flood P., Coleman D., Horowitz M. Growth of an interleukin 2/interleukin 4-dependent T cell line induced by granulocyte-macrophage colony-stimulating factor (GM-CSF). J Immunol. 1987 Jun 15;138(12):4288–4292. [PubMed] [Google Scholar]
- Le P. T., Tuck D. T., Dinarello C. A., Haynes B. F., Singer K. H. Human thymic epithelial cells produce interleukin 1. J Immunol. 1987 Apr 15;138(8):2520–2526. [PubMed] [Google Scholar]
- Lowenthal J. W., MacDonald H. R. Binding and internalization of interleukin 1 by T cells. Direct evidence for high- and low-affinity classes of interleukin 1 receptor. J Exp Med. 1986 Oct 1;164(4):1060–1074. doi: 10.1084/jem.164.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Larsen A. D., Tushinski R. J. Interleukin 1 regulates hematopoietic activity, a role previously ascribed to hemopoietin 1. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5267–5271. doi: 10.1073/pnas.84.15.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey P. J., Bressler L., Park L. S., Alpert A., Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987 Aug 15;139(4):1113–1119. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Ristow H. J. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be interleukin 1 or a related protein. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1940–1944. doi: 10.1073/pnas.84.7.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A., Tsai S., Faller D. V. Interleukin 1 induces cultured human endothelial cell production of granulocyte-macrophage colony-stimulating factor. J Clin Invest. 1987 Jan;79(1):48–51. doi: 10.1172/JCI112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtieri M., Santoli D., Caracciolo D., Kreider B. L., Altmann S. W., Tweardy D. J., Gemperlein I., Mavilio F., Lange B., Rovera G. Establishment and characterization of an undifferentiated human T leukemia cell line which requires granulocyte-macrophage colony stimulatory factor for growth. J Immunol. 1987 Jun 1;138(11):4042–4050. [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]