Abstract
Oncolytic viruses and active immunotherapeutics have complementary mechanisms of action (MOA) that are both self amplifying in tumors, yet the impact of dose on subject outcome is unclear. JX-594 (Pexa-Vec) is an oncolytic and immunotherapeutic vaccinia virus. To determine the optimal JX-594 dose in subjects with advanced hepatocellular carcinoma (HCC), we conducted a randomized phase 2 dose-finding trial (n 5 30). Radiologists infused low-or high-dose JX-594 into liver tumors (days 1, 15 and 29); infusions resulted in acute detectable intravascular JX-594 genomes. Objective intrahepatic Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (15%) and Choi (62%) response rates and intrahepatic disease control (50%) were equivalent in injected and distant noninjected tumors at both doses. JX-594 replication and granulocyte-macrophage colony-stimulating factor (GM-CSF) expression preceded the induction of anticancer immunity. In contrast to tumor response rate and immune endpoints, subject survival duration was significantly related to dose (median survival of 14.1 months compared to 6.7 months on the high and low dose, respectively; hazard ratio 0.39; P = 0.020). JX-594 demonstrated oncolytic and immunotherapy MOA, tumor responses and dose-related survival in individuals with HCC.
Comment
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death in men and claims more than 700,000 lives per year worldwide.1,2 The age-adjusted incidence of HCC in the U.S. more than doubled within the past 35 years, and it is projected to continue to be the fastest-growing cancer in the U.S. for at least another 15 years. However, the prognosis remains dismal, with a 5-year survival rate slightly above 10%.2
The risk factors for HCC can be divided into two major groups: (1) viral hepatitis, including chronic hepatitis B and chronic hepatitis C infection; and (2) chronic nonviral hepatic inflammation, such as alcoholic and nonalcoholic fatty liver disease, autoimmune hepatitis, and primary biliary cirrhosis. Approximately 85% of HCCs arise in livers with chronic hepatitis or cirrhosis. In clinical practice, cirrhosis is recognized as a high-risk preneoplastic condition and HCC surveillance with abdominal ultrasound every 6 months is recommended for all individuals with cirrhosis by both the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL).3,4 The relationship between cirrhosis and increased incidence of HCC still remains poorly understood, but observational studies indicate that it is usually a chronic process with decades of preceding chronic inflammation and progressive fibrosis. Thus, one can speculate that the underlying inflammatory and fibrotic processes that lead to the distortion of normal liver architecture affect immune-surveillance and cellular homeostasis within the liver, possibly creating a “permissive” milieu for somatic mutations and uncontrolled clonal expansion.
In addition to the rising incidence of HCC and its poorly understood pathogenesis, HCC is resistant to conventional chemotherapy, and there is currently only one U.S. Food and Drug Administration (FDA)-approved drug for systemic use in unresectable HCC, sorafenib, a multi-tyrosine kinase inhibitor that provides a modest (2-month) benefit in extending patient survival.5 Recently, Heo et al. reported in Nature Medicine the first randomized clinical trial using an oncolytic viral therapy that showed improved overall survival in patients with advanced HCC.6 The concept that viral infections and vaccinations can lead to tumor remission dates back to 1904, with the first published case report of viral infection-induced cancer regression in a patient with chronic myeloid leukemia. Over the last century, several other examples of “natural” viral infections with oncolytic response have been reported, predominantly in patients with hematological malignancies.7 In addition, a few case reports relating West Nile virus, adenovirus, vaccinia, and mumps infection to solid tumor regression have been documented.7 These observations led to the first steps in oncolytic virotherapeutics in the 1990s.
The focus of oncolytic virotherapy is to engineer viruses that will exploit tumor-restricted signaling pathways for viral replication and tumor cell lysis. In 2011, Breitbach et al.8 reported a phase 1 clinical trial using a genetically engineered oncolytic poxvirus, JX-594. In that study, the authors demonstrated JX-594 dose-related replication and transgene expression in patients with various advanced-stage, treatment-refractory, metastatic solid tumors. JX-594 is a smallpox-vaccine derivative of Wyeth-strain vaccinia virus. The Wyeth strain has been used safely in millions of people as part of a worldwide vaccination program to eradicate smallpox. The JX-594 virus carries the following modifications: (1) an inactivated thymidine kinase gene to increase tumor specificity, as this enzyme is commonly activated in malignant but not in healthy cells by way of activation of the EGFR-RAS pathway; (2) insertion of two transgenes: one encoding human granulocyte-macrophage colony-stimulating factor (hGM-CSF) to stimulate antitumor immunity, by promoting mobilization and maturation of myeloid and dendritic cells; and the other encoding β-galactosidase (β-gal), a surrogate marker to assess viral replication (Fig. 1A). Of note, this virus is safely administered intravenously, resistant to complement and antibody-mediated neutralization, and capable of carrying multiple therapeutic transgenes.
Fig. 1.
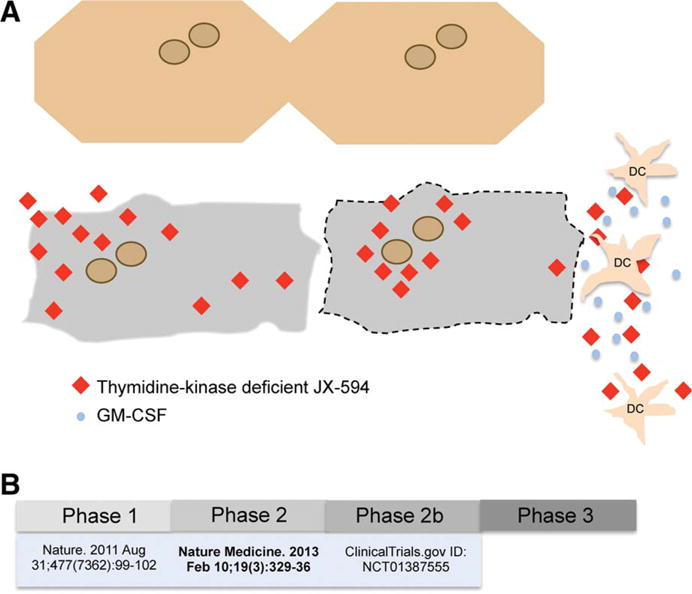
(A) Schematic representation of JX-594 replication (red diamonds) exclusively in malignant liver cells (in gray), and not within healthy hepatocytes (cream color), leading to hGM-CSF transgene expression (blue dots) and dendritic cell (DCs) activation, and culminating in targeted cancer cell death. (B) Overview of clinical trials using JX-594 virus in patients with advanced HCC.
This recent follow-up study by Heo et al. reports a multicenter, multinational (U.S.A., Canadian, and Korean), randomized, phase 2, stratified (viral and non-viral), parallel-group dose-finding clinical trial in patients with advanced HCC. The goal of this study was to determine the optimal JX-594 dose in this population. The authors compared safety, intrahepatic tumor response, induction of immunity, and overall survival in patients with advanced HCC who received low-dose (108 plaque-forming units [PFU]) versus high-dose (109 PFU) JX-594. The oncovirus was injected directly into the tumor and when possible distributed among up to five intrahepatic tumors. The intratumor injections were repeated at the three timepoints: days +1, +15, and +29, resulting in acute JX-594 “viremia” in both dose groups. Demographic and baseline characteristics of the 29 subjects enrolled in this trial were well balanced between the two randomized groups. Over 95% of subjects had Barcelona Clinic Liver Cancer (BCLC) stage C (advanced disease), but most (>85%) had well compensated cirrhosis (Child-Pugh class A). Extrahepatic spread was unknown in over 80% of the enrolled subjects; and 4 of 16 patients (25%) who received high-dose JX-594 had experienced disease progression while previously on sorafenib. Patients were excluded if they had tumors impinging on the biliary tract due to the risk of therapy-induced edema leading to biliary obstruction, known central nervous system malignancy, symppitomatic ascites, severe and/or unstable cardiac disease, exfoliative skin conditions, or any underlying immunodeficiency condition. Patients were also excluded if they received any anticancer therapy within the first 4 weeks of JX-597 injection, or if they were pregnant, nursing, or using antiviral agents with antivaccinia activity (such as ribavirin, adefovir, and pegylated interferon).
Treatment with JX-594 was considered safe and well tolerated by all subjects; no treatment-related deaths were reported. As expected based on data from the phase I clinical trial, flu-like symptoms occurred in all individuals over the first 12–24 hours after injection. Side effects were comparable among both groups, except for anorexia, which was present in 31% of the patients who received high-dose, but in none who received low-dose JX-594.
Intrahepatic tumor response was assessed by dynamic magnetic resonance imaging (MRI) at week 8 posttreatment in comparison with baseline MRI performed between days −14 and 0. Based on previous studies demonstrating JX-594-induced tumor hypovascularity and necrosis,9 the authors chose two methods, modified Response Evaluation Criteria in Solid Tumors (mRE-CIST) and modified Choi (mChoi) criteria to assess response to therapy. Developed by AASLD-Journal of the National Cancer Institute (JNCI), mRECIST criteria factor in maximal dimension of the liver lesion and area of parenchymal contrast enhancement visible during the hepatic arterial phase of imaging (arterial enhancement) as markers of growth and tumor viability.10 The mChoi criteria were adapted from the “original” Choi criteria developed initially for computed tomography (CT) scans. These criteria include tumor enhancement characteristics to assess the effect of treatment on perfusion and development of tumor necrosis. Decreases in tumor size from baseline >10% in longest diameter or decreases in tumor density >15% define significant tumor response to therapy.11 Both criteria account for cases where tumors have decreased arterial enhancement but increased swelling and edema and, therefore, an “artificial” increased diameter. Overall, mRECIST and mChoi response rates at week 8 were 46% and 62%, respectively, with no significant difference between the two dose groups.
Induction of humoral and cellular anticancer immunity was detected and equivalent in injected and non-injected tumors at both doses, similar to intrahepatic tumor response rates. Specifically, antibody-mediated complement-dependent cytotoxicity induction against at least one HCC cell line was similar in both high-and low-dose groups. Interferon gamma (IFNγ) producing T cells in response to stimulation with β-gal peptides were detected by enzyme-linked immunosorbent spot (ELISPOT) analysis at days 29 and 57 after JX-594 treatment in both groups but with distinct kinetics. In the low-dose group, IFNγ-producing T cells peaked at day 157, whereas in the high-dose group IFNγ-producing T cells peaked at day 129 after treatment. These results illustrate the systemic effect of JX-594; in one high-dose subject, cytotoxic T-cell activity was detected up to 1.5 years after treatment, suggesting a durable effect of therapy.
The median overall survival in patients who received high-dose JX-594 was more than double that of patients who received low-dose therapy (14.1 months versus 6.7 months, respectively, P = 0.02). More important, within the group who received high-dose of JX-594, the overall survival of the six patients who had previously failed systemic therapy (four of whom had disease progression while on sorafenib treatment) was 13.6 months; two patients were still alive 25 months posttreatment. This study highlights a potential new strategy to selectively potentiate the immune system to recognize and eliminate malignant cells while healthy cells are spared. Oncolytic viruses will likely increase immune responses as a consequence of increased inflammation due to release of intracellular contents by viral-induced lysis.
Enthusiasm should be tempered by the fact that this is a small study, with safety as a primary endpoint. Further investigation is essential to gain insight into the potential mechanisms that led to increased survival in patients who received high-dose JX-594 compared to those who received low-dose, despite the similar intrahepatic tumor response rates and levels of humoral and cellular anticancer immunity measured in both groups. A more detailed clinical characterization in future studies may elucidate which patients with advanced HCC are most likely to benefit from virotherapy. A phase 2b multinational clinical trial (Fig. 1B) using JX-594 in patients with advanced HCC who have failed sorafenib (NCT01387555)12 is under way. We look forward to new insights that will come from studying this therapy in a larger population.
Footnotes
Potential conflict of interest: Dr. Taddei consults for Onyx and received grantsfrom Bayer
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Sherman JBaM. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EOfRaToC. EASL — EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 8.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 9.Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 12.Biotherapeutics J. http://wwwclinicaltrialsgov/ct2/show/NCT01387555.


