SUMMARY
REP sequences are highly structured elements found downstream of ~500 genes in Escherichia coli that result in extensive stem-loop structures in their mRNAs. However, their physiological role has remained elusive. Here, we show that REP sequences can down-regulate translation, but only if they are within 15 nt of a termination codon; a spacing of 16 nt has no effect suggesting that the REP element acts to stall ribosome movement. Ribosome stalling leads to cleavage of the mRNA and induction of the trans-translation process. Using nrdAB as a model, we find that its regulation can be partially reversed by overexpression of RNA helicases, and can be fully overcome upon UV stress, emphasizing the importance of this regulatory process. Since 50% of REP-associated genes have these elements within the critical 15 nt, these findings identify a regulatory mechanism with the potential to affect translation from a large number of genes.
INTRODUCTION
RNase R is a 3′ to 5′ exoribonuclease (Zuo and Deutscher, 2001; Cheng and Deutscher, 2002) that plays an important role in multiple aspects of RNA metabolism including mRNA turnover, non-stop mRNA decay and quality control of stable RNAs (Cheng and Deutscher, 2003, 2005; Richards et al., 2006) in Escherichia coli. In recent studies from our laboratory, it was shown that RNase R is subject to a complex regulatory mechanism (Chen and Deutscher, 2005, 2010; Liang and Deutscher, 2010, 2012a, 2012b, 2013; Liang et al., 2011). Most RNase R is sequestered on ribosomes in exponential phase cells, where it is stable (Liang and Deutscher, 2013). In contrast, the free form of the enzyme is turned over rapidly, presumably because it is deleterious to cells due to its promiscuous action on essential RNA molecules (Liang and Deutscher, 2013). The stability of RNase R is determined by acetylation of a single lysine residue, Lys544 (Liang et al., 2011), which stimulates binding of the trans-translation factors, tmRNA and SmpB, to the C-terminal region of the enzyme (Liang and Deutscher, 2010). The binding of tmRNA-SmpB stabilizes association of HslUV and Lon proteases with the N-terminal region and enables them to initiate proteolysis (Liang and Deutscher, 2012a). During the process of trans-translation, RNase R is recruited to ribosomes in a tmRNA-SmpB dependent manner, to participate in the degradation of non-stop messages, and as a result, it is protected and is stable (Liang and Deutscher, 2013). Since stationary phase RNase R is not acetylated because of the absence of the acetylating enzyme, Pka, tmRNA-SmpB binds poorly, and thus RNase R is completely stable (Liang et al., 2011; Liang and Deutscher, 2012b).
RNase R is able to act on essentially all RNAs including those with extensive secondary structure, such as the repetitive extragenic palindromic (REP) sequences (Cheng and Deutscher, 2002, 2005). In fact, cells lacking both RNase R and polynucleotide phosphorylase (PNPase) are inviable, and REP-containing fragments accumulate in their absence (Cheng and Deutscher, 2005). REP sequences are highly conserved, 35–40 bp elements present at ~ 500 positions on the E. coli chromosome in intergenic regions downstream of stop codons (Rudd, 1998). REP sequences can occur singly, but more often are found as clusters, which lead to stable stem-loop structures in mRNA upon transcription (Higgins et al., 1998). REP sequences are known to increase mRNA stability as anti-decay hairpins and to cause transcription termination at Rho-dependent attenuators (Espéli et al., 2001; Khemici and Carpousis, 2004). Moreover, REP sequences can serve as the binding sites for DNA polymerase I and Integration Host Factor, and they provide necessary cleavage sites for DNA gyrase to unwind DNA (Gilson et al., 1990; Engelhorn et al., 1995; Espéli and Boccard, 1997). However, very little is actually known about the physiological role of REP sequences. Given the fact that ~80% of RNase R is bound to ribosomes in growing cells and that RNase R is involved in degradation of REP-containing messages (Cheng and Deutscher, 2005; Liang and Deutscher, 2013), it was of considerable interest to examine whether the degradation of REP-containing mRNAs utilizes a mechanism similar to that of non-stop mRNA decay.
We show here that, similar to non-stop mRNA, RNase R binding to ribosomes is stimulated by REP-containing messages. However, this stimulation is lost when the REP sequence is moved beyond a certain distance from the stop codon, and as a consequence, the mRNA is more stable and protein produced from the message is elevated >3-fold. Altering the distance between the termination codon and the REP sequence revealed a sharp cutoff between 15 nt and 16 nt for the three genes examined, similar to the number of nucleotides occupied by ribosomes in toe-printing assays (Hartz et al., 1989). Interestingly, as with non-stop mRNA, the decrease in mRNA and protein levels, dependent on a closely-spaced downstream REP sequence, is due to trans-translation, as shown by the effect of replacing wild type tmRNA with a mutant form of the RNA. We also find that for the nrdA gene this REP-based translational regulation is dependent on RNA helicases. Inactivation of six RNA helicases in combination decreases the level of this REP-containing mRNA and of NrdA protein produced from it, whereas over-expression of any one of the RNA helicases leads to increased mRNA and NrdA protein levels. Additionally, we find that under UV stress conditions, the REP-based translational repression of nrdA is diminished through elimination of trans-translation, confirming its physiological relevance. Inasmuch as ~50% of all REP sequences are within 15 nt of a stop codon, these findings identify a major cellular process that has the potential to limit protein expression from a large number of genes. A model is presented to explain how REP sequences regulate protein translation.
RESULTS
In recent work (Liang and Deutscher, 2013), we found that most RNase R is sequestered on ribosomes in growing cells which stabilizes it and prevents it from acting on sensitive cellular RNAs. Binding of RNase R to ribosomes is stimulated by non-stop mRNA, a known substrate of the enzyme during the process of trans-translation. Since mRNAs containing REP sequences are also known substrates for the RNase (Cheng and Deutscher, 2005), it was of interest to determine whether they also promoted ribosome binding, and to determine the mechanism of their turnover by RNase R.
REP-containing mRNA Promotes RNase R Binding to Ribosomes and Depends on Location of the REP Sequence
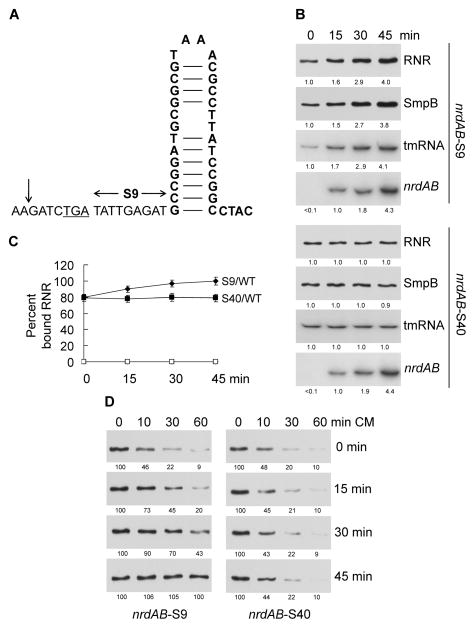
To ascertain whether a REP-containing mRNA could affect binding of RNase R to ribosomes, we made use of the nrdAB message which encodes the two subunits of ribonucleotide reductase. This mRNA contains multiple REP sequences in the intergenic region between the nrdA and nrdB genes and was previously found to accumulate in strains lacking RNase R and PNPase (Cheng and Deutscher, 2005). The sequence of the first REP element following nrdA is presented in Figure 1A. As shown in Figure 1B (top panel), overexpression of the nrdAB message leads to a 4-fold elevation of RNase R, and all of it is associated with ribosomes (Figure 1C). Ribosome binding does not occur in the absence of the trans-translation factor, SmpB (Figure 1C). RNase R is normally a very unstable protein with a half-life of 10 min (Chen and Deutscher, 2010; and Figure 1D, left panel). However, as the amount of the nrdAB mRNA increases, and more RNase R associates with ribosomes, the RNase is stabilized, and its turnover ceases (Figure 1D, left panel). These findings are identical to what was previously observed upon overexpression of a non-stop mRNA, including the concomitant elevation of the trans-translation factors, tmRNA and SmpB (Liang and Deutscher, 2013), and they suggest that decay of REP-containing mRNAs might also be dependent on the trans-translation process.
Figure 1.
Over-expression of nrdAB message increases the amount of RNase R and its binding to ribosomes depending on the distance between the stop codon and the downstream REP sequence. (A) Diagram of the sequence and structure of the first REP element following nrdA. Shown is the sequence beginning upstream of the stop codon (underlined), the normal 9 nt spacing beginning at the first nucleotide following the stop codon, and the site of the endonuclease cleavage indicated by a vertical arrow. (B) Total amount of RNase R (RNR), SmpB and tmRNA after over-expression of nrdAB message. Samples were taken at different times after induction of nrdAB and the amounts of the indicated components were determined by Western or Northern analysis. The spacing between nrdA stop codon and its downstream REP sequence was either the normal 9 nt (S9) or 40 nt (S40). Numbers under each lane are the relative amounts of the indicated components. (C) RNase R binding to ribosomes in WT and smpB mutant cells over-expressing nrdAB message. Samples were taken at different times after induction of nrdAB mRNAs with 9 nt (S9) or 40 nt (S40) between the nrdA stop codon and its downstream REP sequence. Binding of RNase R to ribosomes was determined as described in “Experimental Procedures”. The average of three independent experiments is shown. Error bars indicate the SEM. (D) Half-life of RNase R in cells over-expressing nrdAB message. Chloramphenicol was added to cultures at various times after induction of nrdAB mRNAs (shown on the right) to stop further protein synthesis and the amount of RNase R remaining after different times (shown at top) was determined by Western analysis as described in “Experimental Procedures”. A representative experiment carried out two times is presented. Numbers under each lane are the amount of RNase R remaining. Either 5 μg of total RNA or 5 μg of total protein was added to each lane.
However, the most interesting finding came when we increased the spacing between the nrdA termination codon and the REP sequence from its usual 9 nt (Figure 1A) to 40 nt. Upon overexpression of this message, there was no increase in the amount of RNase R or the trans-translation factors (Figure 1B, bottom panel), no stimulation of RNase R binding to ribosomes (Figure 1C), and no change in RNase R stability (Figure 1D, right panel). This unexpected outcome served as the impetus for all the remaining studies presented in this paper.
Lenthening the Spacing between the REP Sequence and Termination Codon Increases Translation
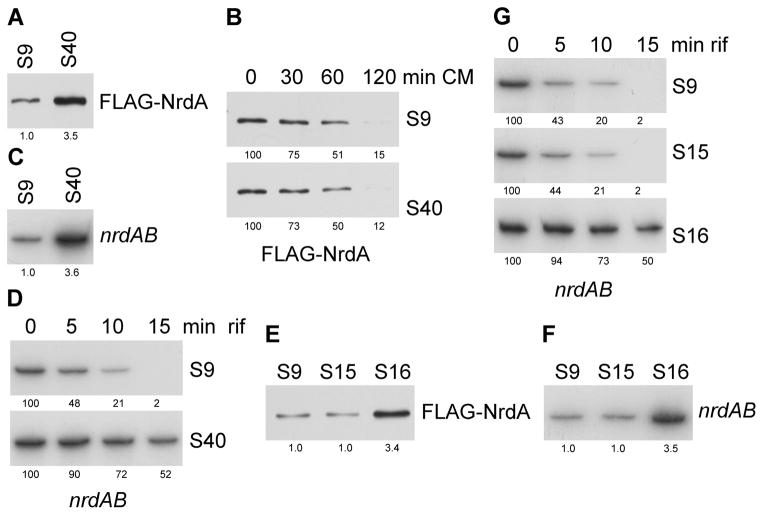
To examine in more detail the effects of increasing the spacing between the nrdA termination codon and the REP sequence, we measured the amount of NrdA protein and nrdAB message produced when the distance was the normal 9 nt and when it was increased to 40 nt. Western analysis of FLAG-tagged NrdA, encoded by the chromosome and expressed at normal levels, revealed that NrdA increased ~3.5-fold when the spacing increased from 9 nt to 40 nt (Figure 2A). This was not due to a change in the stability of NrdA as its half-life remained the same (Figure 2B). Rather, the elevation in NrdA protein reflected a similar elevation in the amount of nrdAB message (Figure 2C) which was a result of its stabilization. As shown in Figure 2D, the half-life of nrdAB mRNA increased from ~5 min to ~15 min as the spacing between the termination codon and the REP sequence was lengthened. These data lead to the interesting conclusion that at its normal location, 9 nt downstream of the nrdA termination codon, the REP sequence serves to down-regulate mRNA stability and translation of the nrdA gene.
Figure 2.
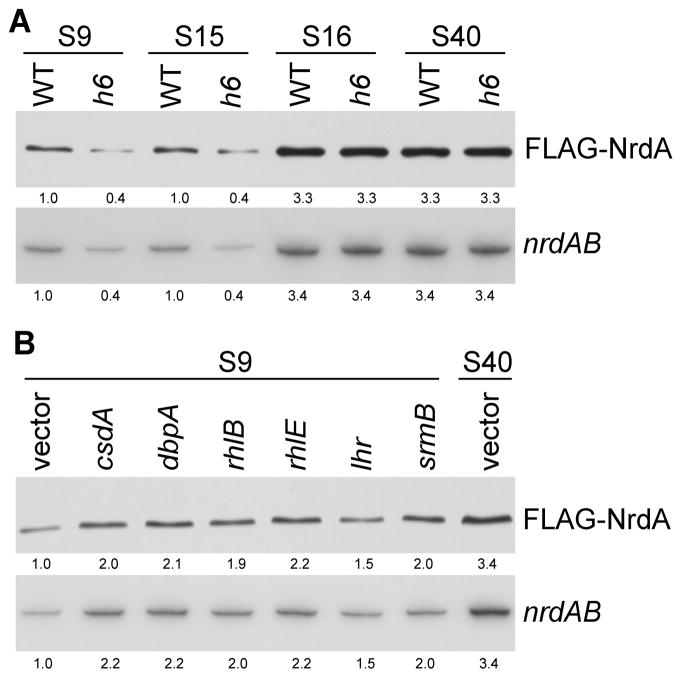
Amount and stability of NrdA protein and nrdAB mRNA in strains with different spacing between nrdA stop codon and its downstream REP sequence. (A) Amount of NrdA protein in strains with 9 nt (S9) or 40 nt (S40) between nrdA stop codon and its downstream REP sequence. (B) Half-life of NrdA protein in strains with 9 nt or 40 nt between nrdA stop codon and its downstream REP sequence. (C) Amount of nrdAB mRNA in strains with 9 nt or 40 nt between nrdA stop codon and its downstream REP sequence. (D) Half-life of nrdAB mRNA in strains with 9 nt or 40 nt between nrdA stop codon and its downstream REP sequence. (E) Amount of NrdA protein in strains with 9 nt, 15 nt (S15) or 16 nt (S16) between nrdA stop codon and its downstream REP sequence. (F) Amount of nrdAB mRNA in strains with 9 nt, 15 nt or 16 nt between nrdA stop codon and its downstream REP sequence. (G) Half-life of nrdAB mRNA in strains with 9 nt, 15 nt or 16 nt between nrdA stop codon and its downstream REP sequence. The amount of NrdA protein was determined by Western analysis using anti-FLAG antibody, and nrdAB message was determined by Northern blot with oligo N8 as described in “Experimental Procedures”. For panels B, D, and G, the time after chloramphenical (CM) or rifampicin (rif) addition is shown at the top. The numbers under each lane indicate the amount of NrdA or nrdAB mRNA present. Representative experiments carried out three times are presented. Samples were taken at an A550 of ~0.3 for these measurements. Either 40 μg of total RNA or 5 μg of total protein was added to each lane.
Regulation is Extremely Sensitive to REP Sequence Location
To define more exactly the spacing between the REP sequence and termination codon that leads to down-regulation of translation, we altered the distance between these two elements. The data presented in Figure 2E reveals that the regulation displays a sharp cutoff at a spacing beyond 15 nt. Thus, at a spacing of 15 nt, the amount of NrdA protein is the same as that at 9 nt, whereas extending the distance by 1 nt, to 16, relieves the down-regulation and leads to the same elevation of NrdA as is found at a spacing of 40 nt (Figure 2A). This extreme sensitivity to the position of the REP sequence extends to the amount of nrdAB message (Figure 2F) and to the effect on mRNA half-life (Figure 2G). These data define an all-or-none cutoff for the regulatory effect of a REP sequence on translation of NrdA. At a spacing of 15 nt or less, translation is fully down-regulated, whereas at 16 nt or more, this regulation is relieved. Interestingly, 15 nt are the number of residues protected by ribosomes in toe-printing experiments (Hartz et al., 1989).
Regulation of Translation Extends to Other REP Sequences
The foregoing results raised the question whether the regulation of translation by a REP sequence was a peculiarity of the nrdA gene or whether it was of more general significance. To answer this question, we selected two more genes for examination. One, avtA, encoding alanine-valine transaminase, contains a REP sequence only 5 nt from the termination codon. This spacing was lengthened to 16 nt, one more than the cutoff for regulation observed with nrdA. The data presented in Figure S1 show that for this gene as well, extending the spacing to 16 nt increases the amount of AvtA protein (Figure S1A) and avtA message (Figure S1B) about 3.5-fold. The second gene chosen, fsaA, which encodes fructose-6-phosphate aldolase, contains a REP sequence that is already greater than 15 nt away from the termination codon. In this case, we reduced the normal 28 nt spacing to 15 nt, one less than the cutoff for translational regulation with nrdA. As shown in Figure S2A and S2B, this reduction in spacing between the REP sequence and termination codon lowers the amount of FsaA protein and fsaA message greater than 3-fold. Thus, down-regulation of translation also occurs when a more distal REP sequence is brought to within the 15 nt cutoff. Based on these examples, we conclude that the presence of a REP sequence within 15 nt of a termination codon has the potential to exert a negative effect on the amount of protein produced from the adjacent upstream gene.
Distribution in Spacing between Termination Codon and E. coli REP Sequences
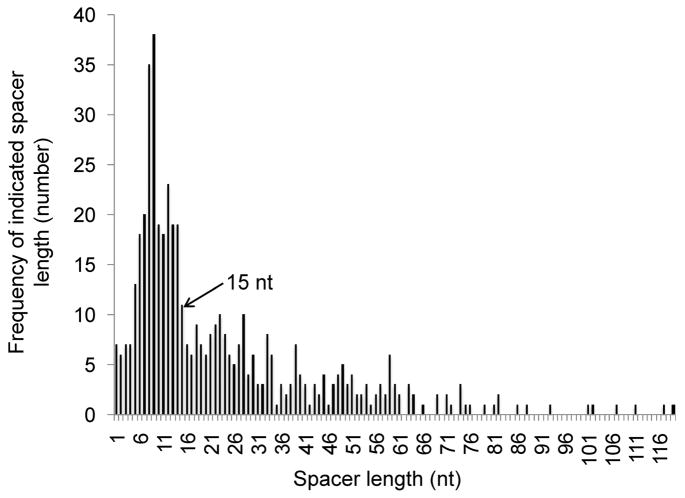
Given the aforementioned conclusions, it was of interest to determine the number of genes in E. coli that contained a REP sequence within 15 nt of an upstream termination codon. For this analysis, we made use of information present in EcoGene. org (Zhou and Rudd, 2013) employing the following criteria: 1) the REP is downstream of the stop codon and is proven or predicted to be part of the same message; 2) when part of a compound element containing multiple REP sequences only the spacing between the stop codon and the nearest REP sequence is considered; 3) possible alternative structures of compound elements are not examined. Based on these criteria, 496 REP sequences were identified, and a histogram of their distribution and frequency are presented in Figure 3. Of these, 260 REP sequences, or 52%, are within 15 nt of an upstream termination codon, and therefore, have the potential to be regulated by the mechanism described here.
Figure 3.
Distribution of spacer lengths from stop codon to REP sequence. Utilizing the EcoInterGene data from EcoGene. org, the distance between the last nucleotide of the stop codon and the first nucleotide of the downstream REP sequence was tallied for all E. coli genes with intergenic REP sequences. If multiple REP sequences were present, only the distance to the nearest one was considered. The arrow indicates the position of the 15-nt cutoff.
REP-Dependent Regulation Requires Trans-translation
The fact that REP sequences only affect translation when they are within a specific distance of a termination codon strongly suggests that they act by blocking ribosome movement and cause ribosomes to stall just prior to accessing the codon. If correct, it raises the possibility that trans-translation comes into play which is known to be stimulated by ribosome stalling. This idea was strengthened by the data in Figure 1 which showed elevation of trans-translation factors (panel B) and a requirement for SmpB (panel C) for increased RNase R binding upon overexpression of nrdAB with a spacing of 9 nt, but not 40 nt.
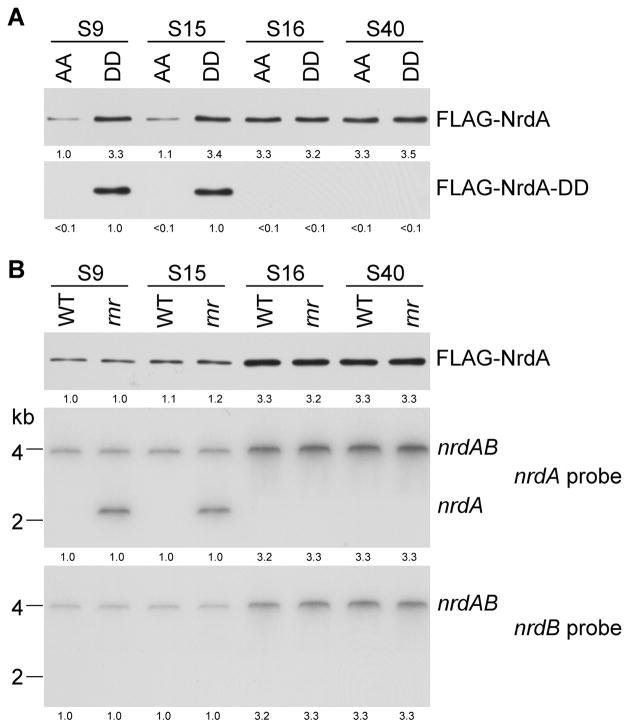
In order to determine directly whether trans-translation plays a role in REP-dependent regulation, we generated a strain containing a mutant tmRNA in which the last two alanine (AA) residues of the tagging peptide were changed to aspartic acids (DD in Figure 4A). As a result of this mutation, the truncated NrdA protein generated during trans-translation would not be degraded (Keiler et al., 1996; Gottesman et al., 1998). The data in Figure 4A show, as before, that the amount of NrdA is ~3.5 elevated with wild type tmRNA when the spacing is 16 or 40 nt compared to 9 or 15 nt. Most importantly, the amount of NrdA protein also increased ~3.5-fold in the DD strain compared to the WT strain (AA) in which the peptide tag normally leads to protein degradation, but only when the spacing is 9 nt or 15 nt; there is no effect of the DD tmRNA when the spacing is 16 nt or 40 nt, conditions in which the REP sequence has no effect, and there is no trans-translation. Moreover, NrdA can only be detected with antibody directed against the DD peptide tag when the spacing is 9 or 15 nt (Figure 4A). These data show directly that NrdA pulled down with FLAG-tag antibody is tagged with the DD-containing peptide encoded by the mutant tmRNA, and therefore, is not degraded. As a consequence, NrdA levels are not decreased when the REP sequence is spaced 9 or 15 nt from the stop codon. These data support the conclusion that trans-translation is responsible for the down-regulation of NrdA levels dependent on REP sequences. Identical results were found with AvtA (Figure S1C) and FsaA (Figure S2C) indicating that trans-translation affects these proteins as well when a REP sequence is within 15 nt of a stop codon.
Figure 4.
REP-based translational regulation is dependent on trans-translation. (A) The amount of total NrdA and tmRNA (DD) tagged NrdA protein present in wild type (AA) and tmRNA mutant (DD) strains. Multiple strains with different spacing between the nrdA stop codon and the downstream REP sequence were analyzed. The total amount of NrdA was determined with FLAG antibody and the amount of DD-tagged NrdA was determined by tmRNA (DD) antibody as described in “Experimental Procedures”. (B) Western and Northern blot analyses of NrdA protein and nrdAB message in strains containing or lacking RNase R and with different spacing between the nrdA stop codon and its downstream REP sequence. nrdA and nrdB specific probes were used to detect the individual nrdA and nrdB mRNAs, respectively. Numbers under each lane are the relative amount of the indicated component. Either 40 μg of total RNA or 5 μg of total protein were added to each lane.
Further support for the involvement of the trans-translation comes from examination of mRNA levels. As shown in Figure 1, S1 and S2, mRNA levels are reduced when the REP sequence is within 15 nt of the stop codon. It is known that during trans-translation the pre-existing mRNA present on the ribosomes is removed, and that this is due to the action of the exoribonuclease, RNase R (Ge et al., 2010). The data presented in Figure 4B show that nrdA mRNA is not degraded in an rnr mutant strain, confirming the role of RNase R in the process, and that only the nrdA portion of the nrdAB message accumulates; the corresponding nrdB portion is undetectable (Figure 4B). Based on these data, it appears that during trans-translation the nrdAB message is cleaved and the upstream portion containing nrdA is degraded by RNase R. Since decay of the downstream RNA containing nrdB continues even in the absence of RNase R, another RNase is implicated in its removal.
The identity of the endoribonuclease responsible for cleavage of the nrdAB message is not yet known, but mutant analysis indicated it is not due to the toxin, RelE (data not shown). Sequencing of the 3′ end of the nrdA mRNA that accumulates in the S9 strain lacking RNase R using 3′ RACE indicated that the cleavage occurs 4 nt upstream of the nrdA UGA stop codon; when the same experiment is carried out in the S15 strain, the cut site shifts 6 nt downstream into the stop codon, suggesting that mRNA cleavage occurs at a fixed position on the ribosome. Thus, stalling of a ribosome due to the presence of a REP sequence leads to cleavage of its associated message. This, in effect, generates a non-stop mRNA, explaining why trans-translation comes into play.
RNA Helicases Affect Regulation by REP Sequences
Based on the data presented, we propose that those REP sequences within 15 nt of a stop codon stall ribosomes at a position that prevents completion of translation termination. Rather, the stalling leads to induction of the trans-translation process resulting in turnover of the associated mRNA and proteolysis of the almost completed protein. However, the block by the REP sequence is not complete. For each of the three genes examined, 25–30% of the encoded protein is made suggesting that in some instances the ribosome is able to move the additional nucleotides needed to allow completion of normal translation. One possibility to explain this is that the REP sequence is partially opened a portion of the time and this allows a ribosome to access the termination codon.
One group of factors that might participate in opening the REP sequence are the RNA helicases. To test for their possible involvement, we made use of a strain lacking six RNA helicases (h6), kindly provided by Dr. Chaitanya Jain. As shown in Figure 5A, the amount of NrdA protein and nrdAB message was reduced even further when six RNA helicases were absent, but only when the spacing between the termination codon and the REP sequence was 9 nt or 15 nt. Removal of any one of the six RNA helicases individually had no effect (data not shown) suggesting that more than one helicase can stimulate when translation is reduced by a REP sequence. Moreover, overexpression of any one of the RNA helicases in the S9 strain stimulated translation ~2-fold over the basal level (Figure 5B) indicating that each helicase has some stimulatory potential. However, in no case could a single RNA helicase increase translation of NrdA to the level obtained when the spacing is >15 nt (Figure 5B). Nonetheless, these data show that RNA helicases do contribute to the basal level of protein produced from a gene with a REP sequence within 15 nt of its stop codon.
Figure 5.
Effect of RNA helicases on REP-based translational regulation. (A) Amount of NrdA protein and nrdAB mRNA in wild type and in a mutant strain lacking six RNA helicases and with different spacing between the nrdA stop codon and its downstream REP sequence. The amount of NrdA was determined with FLAG antibody and the amount of nrdAB message by Northern analysis using oligo N8. (B) Amount of NrdA protein and nrdAB mRNA in wild type and in strains each over-expressing one RNA helicase. All strains had 9 nt spacing between the nrdA stop codon and its downstream REP sequence. The relative amount of NrdA or nrdAB mRNA is indicated below each lane. Either 40 μg of total RNA or 5 μg of total protein were added to each lane.
UV Stress Reverses REP-Dependent Regulation of NrdA
Inasmuch as a large number of genes may normally be down-regulated by a closely-spaced REP sequence, the question arises whether any physiological condition reverses this process and allows full production of the gene product. In an initial attempt to identify such a condition, we first examined UV stress as DNA damage was known to elevate nrdAB (Monje-Casas et al., 2001). As shown in Figure 6, UV treatment completely reversed the down-regulation of the nrdAB message and NrdA protein in the S9 strain elevating them to the same level found when the REP sequence is 40 nt downstream of the stop codon (Figure 6A). Moreover, the UV treatment completely eliminated trans-translation of NrdA (Figure 6B) indicating that ribosomes no longer were stalled by a REP sequence 9 nt downstream of the termination codon. Interestingly, UV treatment did not stimulate either avtA or fsaA indicating that the UV effect is specific for nrdAB. Although the mechanism of UV-induced stimulation of nrdAB is not fully understood, these data clearly show that REP-dependent translational regulation does have physiological relevance, and they raise the possibility that specific signals will lead to stimulation of expression from different REP-regulated genes.
Figure 6.
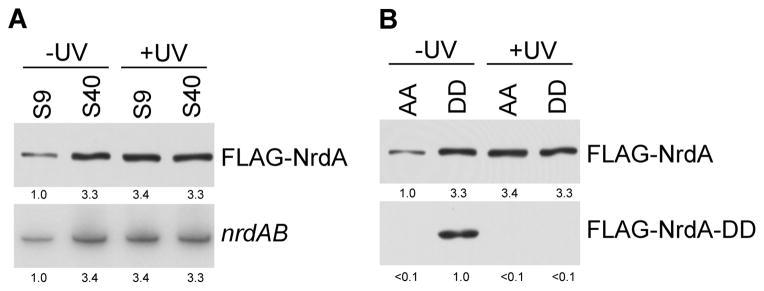
UV stress attenuates REP-based translational repression of NrdA by elimination of trans-translation. (A) The amount of NrdA protein and nrdAB mRNA in strains with either 9 nt (S9) or 40 nt spacing (S40) between the nrdA stop codon and its downstream REP sequence. Cells were untreated or exposed to UV treatment as described in “Experimental Procedures”. NrdA was detected with FLAG antibody, nrdAB with oligo N8. (B) Amount of total NrdA and tmRNA (DD) tagged NrdA protein in wild type (AA) and tmRNA mutant (DD) strains containing 9 nt between the nrdA stop codon and its downstream REP sequence with or without UV treatment. NrdA (DD) was detected with tmRNA (DD) antibody as described in “Experimental Procedures”. Cells were treated with UV as described in “Experimental Procedures” and the amount of NrdA protein and nrdAB message was determined by Western or Northern analysis as in panel A. Either 40 μg of total RNA or 5 μg of total protein were added to each lane.
DISCUSSION
The studies presented here identify a mechanism of translational regulation dependent on REP sequences. Our findings indicate that the presence of a REP sequence within 15 nt of a termination codon down-regulates the amount of protein produced from the affected gene ~3- to 4-fold compared to the same gene with the REP sequence spaced 16 or more nt away. Based on the three genes examined, this down-regulation occurs both when the REP sequence is within 15 nt and is moved further away and when it is initially at a greater distance and is moved closer. Our studies also reveal that the decrease in protein synthesis is a consequence of the process of trans-translation which leads to a reduction of the mRNA, tagging of the nascent polypeptide chain directed by tmRNA, and ultimately, degradation of the protein product. We conclude, based on these observations, that the presence of a REP sequence spaced within 15 nt of a stop codon causes ribosomes to pause prior to completion of translation resulting in induction of the trans-translation process, a known consequence of ribosome pausing (Gillet and Felden, 2001; Withey and Friedman, 2002).
In support of this conclusion, we found that a single nucleotide change in spacing between the stop codon and the REP sequence, from 16 to 15 nt, is sufficient to induce the trans-translation process which leads to a dramatic decrease in the steady-state amount of the nrdAB message and NrdA. Identical results were obtained with two other genes, avtA and fsaA. These findings are completely consistent with the REP sequence acting as a barrier to ribosome movement (Figure 7). The fact that the critical spacing is at 15/16 nt strongly suggests that only when the REP sequence is at least 16 nt downstream can the A site of the ribosome sit directly opposite the stop codon to allow the termination mechanism to proceed. At 15 nt spacing, the ribosome is unable to translocate to a position that allows termination, and as a consequence, the ribosome pauses. These data suggest that the leading edge of the ribosome extends 15 to 16 nt from the A site, in close agreement with information derived from toeprinting experiments (Hartz et al., 1989).
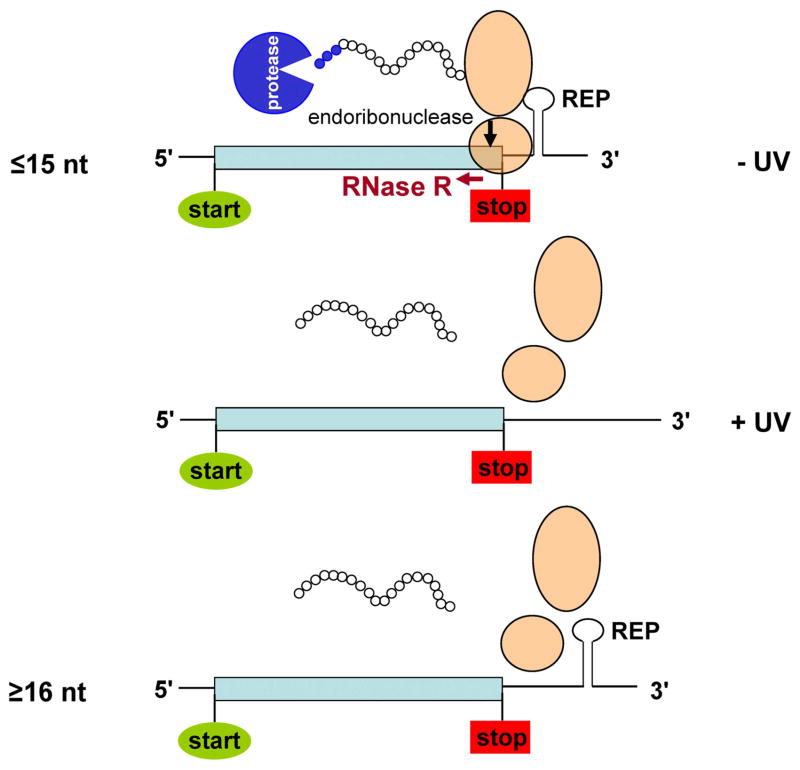
Figure 7.
A model to explain how REP sequences down-regulate translation. When the distance between a stop codon and the downstream REP sequence is 15 nt or less, ribosomes stall just before the stop codon because the REP sequence prevents further movement. As a consequence, an endonuclease cleaves the mRNA generating a non-stop message that initiates the process of trans-translation. RNase R degrades the upstream portion of the cleaved message, and the polypeptide with a tmRNA-directed tag is removed by proteases allowing ribosomes to recycle. Upon UV stress, translation is able to be completed and trans-translation does not occur. Therefore, the mRNA and its protein products are not degraded and the down-regulation is reversed. When the spacing is greater than 15 nt, normal translation occur
Once the ribosome pauses, an endoribonuclease, as yet unidentified, cleaves the mRNA (Figure 7), in effect, generating a non-stop mRNA. In the WT nrdAB message (9 nt spacing) this cleavage occurs 4 nt upstream of the stop codon. When the spacing is increased to 15 nt, the cleavage site moves 6 nt downstream to within the stop codon. These findings argue that once a ribosome pauses because further movement is blocked by a REP sequence, an as yet unknown endoribonuclease cleaves the mRNA at a fixed location on the ribosome that is within the A site and 16 nt upstream of the REP sequence. The cleavage site is independent of the mRNA sequence itself. These observations are consistent with a model in which an endoribonuclease binds to a paused ribosome at a specific position such that its catalytic site cleaves the phosphodiester bond between the 2nd and 3rd nt of the codon at the A site. An alternative possibility is that the cleavage activity is an intrinsic property of the ribosome itself, but since some toxins are already known that cleave an mRNA within the A site (Christensen and Gerdes, 2003; Pedersen et al., 2003), this latter explanation appears less likely. Moreover, these data are in complete agreement with the earlier findings of Hayes and Sauer (2003) that the codon within the A site is cleaved during ribosome pausing.
Based on our data, many REP sequences are located at positions in which they would down-regulate the full protein production capability of the upstream gene. This raises the interesting question of what physiological role is served by this regulatory mechanism. At first glance, it might seem wasteful to destroy an almost completed protein as a means to down-regulate protein production. On the other hand, it could provide a means to rapidly provide a supply of an urgently needed protein as a stalled ribosome would likely have a queue of additional ribosomes carrying almost finished proteins right behind it. Once the block was lifted, these proteins would be completed and could very quickly become available. Inasmuch as the elevation in protein amount is only ~3.5-fold, the queue would only need to be 2 to 3 ribosomes, likely too small to be observed in ribosome profiling experiments (Li et al., 2014). Moreover, such queues would be transient, disappearing as ribosomes became unstalled due to trans-translation and re-appearing as others stalled due to interaction with the REP.
As shown in the case of NrdA, a protein needed for DNA synthesis, UV stress is a physiological condition that induces this up-regulation, and this is mediated through the action of RNA helicases. In this situation, opening of a few based pairs in the REP sequence is sufficient to allow ribosomes to complete translation, and this would be true for other genes containing closely spaced REP sequences as well. On the other hand, the physiological trigger that induces an RNA helicase to act on other genes is likely to be different, as UV treatment does not have the same effect on avtA or fsaA.
As yet, it is unclear whether there are any special physiological roles associated with the 260 genes that contain REP sequences within the 15 nt that would induce stalling. Analysis of these genes has not revealed any obvious commonalities in their metabolic roles or other properties that might explain why they are subjected to down-regulation by REP sequences. Nevertheless, these studies have expanded our understanding of translational regulation and identified an important function for REP sequences.
EXPERIMENTAL PROCEDURES
Materials
Antibody against RNase R was prepared and purified as described previously (Cheng and Deutscher, 2002; Liang and Deutscher, 2010). Anti-FLAG M2 mAb was from Sigma. Anti-rabbit and anti-mouse IgG HRP conjugate were obtained from Santa Cruz Biotechnology. [γ-32P]ATP was purchased from PerkinElmer Life Sciences. RNeasy mini kit was from Qiagen. Protease inhibitor cocktail was purchased from Roche. M-MLV reverse transcriptase, RNasin, T4 polynucleotide kinase, T4 RNA ligase and pGEM-T vector were from Promega. The ASKA constructs (Kitagawa et al., 2005) for overexpression of RNA helicases CsdA, DbpA, RhlB, RhlE, Lhr and SrmB were kindly provided by Dr. Chaitanya Jain, University of Miami. The tmRNA (DD) antibody was a gift from Dr. Christopher Hayes, University of California Los Angeles. All other materials were reagent grade.
Bacterial Strains and Growth Conditions
All strains used were derivatives of E. coli K12 strain MG1655(Seq)rph+. DNA encoding 3x FLAG sequence was fused to the N-termini of the coding sequences of chromosomal nrdA, avtA and fsaA, respectively, following a previously published recombineering protocol (Liang and Deutscher, 2010), using gene specific primers (Table S1). This procedure was also used to change the spacing between the stop codon and the downstream REP sequence of these three genes (Table S1). The last two alanines of the tagging peptide encoded by tmRNA were both changed to aspartic acid with primer T1 (Table S1). The resulting gene mutations were confirmed by DNA sequencing.
Cells were grown at 37 °C in liquid culture in YT medium. Antibiotics, when present, were at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml. Exponential phase cells were collected at an A550 of ~0.3, and cells grown overnight were used as stationary phase samples. For UV treatment, 5 ml exponential phase cells were placed in a petri dish (60 mm) and irradiated with 100 J/m2 of UV-C (254 nm) in a UV crosslinker (CL-1000, UVP). The treated cells were then grown at 37 °C for 10 min.
Induction of nrdAB Message
The coding sequences for the nrdA and nrdB genes and the intergenic region between them were amplified by PCR with primers N6 and N7 (Table S1). The PCR product was purified and digested with NcoI and HindIII, and then cloned into the corresponding sites on pBAD24. Cells harboring the construct were grown in YT medium with 0.2% glucose (to inhibit leaky expression of nrdAB) to an A550 of ~0.3, and were collected by centrifugation. The cell pellet was washed once in YT medium and then grown in the same medium containing 0.02% arabinose for the indicated times to induce expression of nrdAB mRNA. Cells grow slowly after induction.
Ribosome Isolation
Cells were disrupted in lysis buffer (50 mM Tris-HCl, pH 7.5, 300 mM NH4Cl, 20 mM MgCl2, 2 mM β-ME and 1mg/ml DNase I) containing protease inhibitor cocktail as described (Liang et al., 2011). The suspension was centrifuged at 30,000g for 10 min to remove cell debris. The supernatant fraction was further centrifuged at 100,000g for 90 min and the resulting supernatant and pellet fractions were used to detect the amount of unbound and ribosome-bound RNase R (Liang and Deutscher, 2013).
Measurement of RNase R and NrdA Protein Half-life
Cells were grown under normal conditions to an A550 of ~0.3. A portion of the cells was collected for the zero time point and chloramphenicol was added to the remaining culture at 200 μg/ml. Cells were collected at the indicated times, lysed by sonication, and assayed by immunoblotting to determine the amount of RNase R and FLAG-NrdA remaining.
Measurement of nrdAB mRNA Decay
Cells were grown to an A550 of ~0.3, and rifampicin (0.45 mg/ml) was then added to inhibit further transcription (Ge et al., 2010). Samples were taken at the indicated time points and total RNA was extracted. The nrdAB mRNA was probed with 32P-labelled oligo N8 (Table S1).
Overexpression of RNA Helicases
Cells producing His6-tagged RNA helicases were grown at 37°C in YT medium supplemented with 34 μg/ml chloramphenicol to an A550 of ~0.3, and 0.1 mM IPTG was added to induce the expression of proteins for 30 min. Samples were collected for Western blot and Northern blot analyses.
Pull-down Assay
Cells were ruptured by sonication in binding buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM DTT, 0.5% NP-40, 1 mM PMSF) containing protease inhibitor cocktail. Anti-FLAG M2-agarose suspension (100 μl per 200 ml of bacterial culture) was then added and incubated overnight with the crude extract at 4°C. The beads were washed 3 times with 500 μl of binding buffer containing 0.5 M NaCl to remove non-specific contaminants, and the bound proteins were eluted with FLAG peptide (1 mg/ml in binding buffer, 50 μl per 200 ml bacterial culture). FLAG tagged and tmRNA (DD) tagged proteins in the eluant were detected by Western blot analysis.
Western Blot Analysis
Proteins were resolved on either 8% or 12% gels and subjected to immunoblotting. RNase R, FLAG tagged NrdA, AvtA, FsaA and SmpB, and tmRNA (DD) tagged NrdA, AvtA and FsaA were detected by purified RNase R antibody (1:10,000 dilution), anti-FLAG M2 mAbs (1:1000 dilution), and tmRNA (DD) antibody (1:5,000 dilution), respectively. Underexposed films were used for quantification by Quantity One (Bio-Rad).
Northern Blot Analysis
RNA was extracted from cells with the RNeasy mini kit according to the manufacturer’s protocol and separated by electrophoresis on a 6% polyacrylamide/7.5 M urea gel or a 1.2% agarose gel. Prehybridization and hybridization with 32P-labelled DNA oligonucleotides (Table S1) complementary to nrdA, nrdB, avtA, fsaA and tmRNA was performed as described previously (Liang et al., 2009).
3′ RACE Analysis
Eighty pmols of oligo L1 (Table S1) were phosphorylated using T4 polynucleotide kinase and ligated to 10 μg of total RNA using T4 RNA ligase as previously described (Liu and Gorovsky, 1993). Free oligonucleotides were then removed by centrifugal ultrafiltration. For RT-PCR, 2 μg of oligo L1- treated total RNA was used for first-strand cDNA synthesis by M-MLV in the presence of oligo L2 (Table S1). L2 and nrdA gene-specific primer N10 (Table S1) were used for 3′ end amplification. PCR conditions were as follows: 3 min at 94°C, followed by 30 cycles of 30 s at 94°C; 30 s at 60°C; and 1 min at 72°C. PCR products were ligated to pGEM-T vector and then sequenced. Six colonies were randomly selected for sequencing and each gave the same result.
Supplementary Material
Highlights.
REP sequences down-regulate translation, but only when within 15 nt of a stop codon
REP-dependent translational regulation requires trans-translation
Overexpression of RNA helicases or UV stress reverse REP-dependent regulation
This work identifies a regulatory process that can alter translation of many genes
Acknowledgments
This work was supported by Grants GM16317 to M.P.D. and GM58560 to K.E.R. from the National Institutes of Health and to W.L. from the Taishan Scholar Construction Foundation of Shandong Province. We thank Dr. Christopher Hayes for tmRNA (DD) antibody and Dr. Chaitanya Jain for RNA helicase constructs. We also thank members of the laboratory for critical comments on the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
W.L. and M.P.D. designed research; W.L. performed research; K.E.R. contributed new reagents or analytic tools; W.L., K.E.R., and M.P.D. analyzed data; W.L., K.E.R., and M.P.D. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen C, Deutscher MP. Elevation of RNase R in response to multiple stress conditions. J Biol Chem. 2005;280:34393–34396. doi: 10.1074/jbc.C500333200. [DOI] [PubMed] [Google Scholar]
- Chen C, Deutscher MP. RNase R is a highly unstable protein regulated by growth phase and stress. RNA. 2010;16:667–672. doi: 10.1261/rna.1981010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZF, Deutscher MP. Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J Biol Chem. 2002;277:21624–21629. doi: 10.1074/jbc.M202942200. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Deutscher MP. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc Natl Acad Sci USA. 2003;100:6388–6393. doi: 10.1073/pnas.1231041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZF, Deutscher MP. An important role for RNase R in mRNA decay. Mol Cell. 2005;17:313–318. doi: 10.1016/j.molcel.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Christensen SK, Gerdes K. RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol Microbiol. 2003;48:1389–1400. doi: 10.1046/j.1365-2958.2003.03512.x. [DOI] [PubMed] [Google Scholar]
- Engelhorn M, Boccard F, Prentki P, Geiselmann J. In vivo interaction of the Escherichia coli integration host factor with its specific binding sites. Nucleic Acids Res. 1995;23:2959–2965. [PubMed] [Google Scholar]
- Espéli O, Boccard F. In vivo cleavage of Escherichia coli BIME-2 repeats by DNA gyrase: genetic characterization of the target and identification of the cut site. Mol Microbiol. 1997;26:767–777. doi: 10.1046/j.1365-2958.1997.6121983.x. [DOI] [PubMed] [Google Scholar]
- Espéli O, Moulin L, Boccard F. Transcription attenuation associated with bacterial repetitive extragenic BIME elements. J Mol Biol. 2001;314:375–86. doi: 10.1006/jmbi.2001.5150. [DOI] [PubMed] [Google Scholar]
- Ge Z, Mehta P, Richards J, Karzai AW. Non-Stop mRNA decay initiates at the ribosome. Mol Microbiol. 2010;78:1159–1170. doi: 10.1111/j.1365-2958.2010.07396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet R, Felden B. Emerging views on tmRNA-mediated protein tagging and ribosome rescue. Mol Microbiol. 2001;42:879–885. doi: 10.1046/j.1365-2958.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- Gilson E, Perrin D, Hofnung M. DNA polymerase I and a protein complex bind specifically to E. coli palindromic unit highly repetitive DNA: implications for bacterial chromosome organization. Nucleic Acids Res. 1990;18:3941–52. doi: 10.1093/nar/18.13.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Gold L. Selection of the initiator tRNA by Escherichia coli initiation factors. Genes Dev. 1989;3:1899–1912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- Hayes CS, Sauer RT. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol Cell. 2003;12:903–911. doi: 10.1016/s1097-2765(03)00385-x. [DOI] [PubMed] [Google Scholar]
- Higgins CF, McLaren RS, Newbury SF. Repetitive extragenic palindromic sequences, mRNA stability and gene expression: evolution by gene conversion? Gene. 1988;72:3–14. doi: 10.1016/0378-1119(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Keiler KC, Waller PR, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- Khemici V, Carpousis AJ. The RNA degradosome and poly(A) polymerase of Escherichia coli are required in vivo for the degradation of small mRNA decay intermediates containing REP stabilizers. Mol Microbiol. 2004;51:777–790. doi: 10.1046/j.1365-2958.2003.03862.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Deutscher MP. A novel mechanism for ribonuclease regulation: transfer-messenger RNA (tmRNA) and its associated protein SmpB regulate the stability of RNase R. J Biol Chem. 2010;17:29054–29058. doi: 10.1074/jbc.C110.168641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Deutscher MP. Transfer-messenger RNA-SmpB protein regulates ribonuclease R turnover by promoting binding of HslUV and Lon proteases. J Biol Chem. 2012a;287:33472–33479. doi: 10.1074/jbc.M112.375287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Deutscher MP. Post-translational modification of RNase R is regulated by stress-dependent reduction in the acetylating enzyme Pka (YfiQ) RNA. 2012b;28:37–41. doi: 10.1261/rna.030213.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Deutscher MP. Ribosomes regulate the stability and action of the exoribonuclease RNase R. J Biol Chem. 2013;288:34791–34798. doi: 10.1074/jbc.M113.519553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Li C, Liu F, Jiang H, Li S, Sun J, Wu X, Li C. The Arabidopsis homologs of CCR4-associated factor 1 show mRNA deadenylation activity and play a role in plant defence responses. Cell Res. 2009;19:307–316. doi: 10.1038/cr.2008.317. [DOI] [PubMed] [Google Scholar]
- Liang W, Malhotra A, Deutscher MP. Acetylation regulates the stability of a bacterial protein: growth stage-dependent modification of RNase R. Mol Cell. 2011;44:160–166. doi: 10.1016/j.molcel.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gorovsky MA. Mapping the 5′ and 3′ ends of Tetrahymena thermophila mRNAs using RNA ligase mediated amplification of cDNA ends (RLM-RACE) Nucleic Acids Res. 1993;21:4954–4960. doi: 10.1093/nar/21.21.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C. Expression analysis of the nrdHIEF operon from Escherichia coli.Conditions that trigger the transcript level in vivo. J Biol Chem. 2001;276:18031–18037. doi: 10.1074/jbc.M011728200. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Zavialov AV, Pavlov MY, Elf J, Gerdes K, Ehrenberg M. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell. 2003;112:131–140. doi: 10.1016/s0092-8674(02)01248-5. [DOI] [PubMed] [Google Scholar]
- Richards J, Mehta P, Karzai AW. RNase R degrades non-stop mRNAs selectively in an SmpB-tmRNA-dependent manner. Mol Microbiol. 2006;62:1700–1712. doi: 10.1111/j.1365-2958.2006.05472.x. [DOI] [PubMed] [Google Scholar]
- Rudd KE. Linkage map of Escherichia coli K12, edition 10: the physical map. Microbiol Mol Biol Rev. 1998;62:985–1019. doi: 10.1128/mmbr.62.3.985-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withey JH, Friedman DI. The biological roles of trans-translation. Curr Opin Microbiol. 2002;5:154–159. doi: 10.1016/s1369-5274(02)00299-0. [DOI] [PubMed] [Google Scholar]
- Zhou J, Rudd KE. EcoGene 3.0. Nucleic Acids Res. 2013;41:D613–D624. doi: 10.1093/nar/gks1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Deutscher MP. Exoribonuclease superfamilies: Structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001;29:1017–1026. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.