Abstract
Alcohol is a potent somnogen and one of the most commonly used “over the counter” sleep aids. In healthy non-alcoholics, acute alcohol decreases sleep latency, consolidates and increases the quality (delta power) and quantity of NREM sleep during the first half of the night. However, sleep is disrupted during the second half. Alcoholics, both during drinking periods and during abstinences, suffer from a multitude of sleep disruptions manifested by profound insomnia, excessive daytime sleepiness, and altered sleep architecture. Furthermore, subjective and objective indicators of sleep disturbances are predictors of relapse. Finally, within the USA, it is estimated that societal costs of alcohol-related sleep disorders exceeds $18 billion. Thus, although alcohol-associated sleep problems have significant economic and clinical consequences, very little is known about how and where alcohol acts to affect sleep.
In this review, we have described our attempts to understand how and where alcohol acts to affect sleep. We have conducted a series of experiments using two different species, rats and mice, as animal models, and a combination of multi-disciplinary experimental methodologies to examine and understand anatomical and cellular substrates mediating the effects of acute and chronic alcohol exposure on sleep-wakefulness.
The results of our studies suggest that the sleep-promoting effects of alcohol may be mediated via alcohol’s action on the mediators of sleep homeostasis: adenosine (AD) and the wake-promoting cholinergic neurons of the basal forebrain (BF). Alcohol, via its action on AD uptake, increases extracellular AD resulting in the inhibition of BF wake-promoting neurons. Lesions of the BF cholinergic neurons or blockade of AD A1 receptors results in attenuation of alcohol-induced sleep promotion, suggesting that AD and BF cholinergic neurons are critical for sleep-promoting effects of alcohol.
Since binge alcohol consumption is a highly prevalent pattern of alcohol consumption and disrupts sleep, we examined the effects of binge drinking on sleep-wakefulness. Our results suggest that disrupted sleep homeostasis may be the primary cause of sleep disruption observed following binge drinking. Finally, we have also shown that insomnia and associated sleep disruptions, observed during acute withdrawal, are caused due to impaired sleep homeostasis.
Based on our findings, we suggest that alcohol may disrupt sleep homeostasis to cause sleep disruptions.
Keywords: alcohol dependence, adenosine, basal forebrain, binge drinking, cholinergic, sleep deprivation, theta, delta, electroencephalogram
Introduction
Since the dawn of civilization, humankind has used alcohol for various reasons, including as a relaxant and for euphoric effects. Although the word “alcohol” in organic chemistry refers to any organic compound where the carbon atom of an alkyl group or a substituted alkyl group is bound to a hydroxyl group (−OH). However, innormal usage, the word alcohol is commonly used to describe ethanol or any beverage that contains alcohol. Alcohol is among the most highly abused drugs worldwide and a leading preventable cause of premature disability and death, accounting for an estimated 6 to 9% of all deaths (Rehm et al., 2009; Shield et al., 2013).
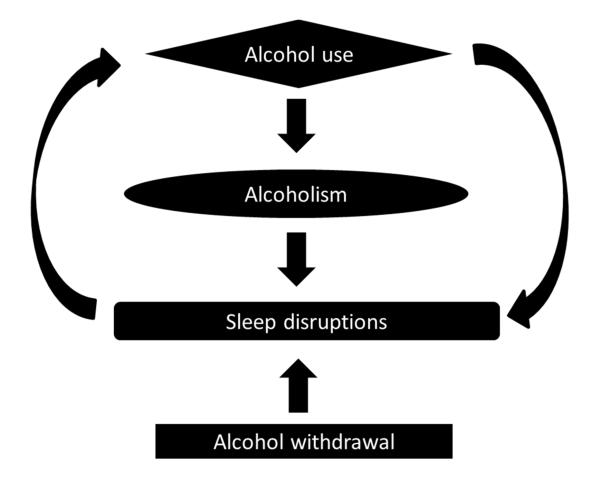
Alcohol is a potent somnogen and has a profound impact on sleep (Fig. 1). Acute alcohol intake, in non-alcoholic social drinkers, reduces the time to fall asleep (sleep onset latency), and consolidates and enhances the quality (delta power) and the quantity of NREM sleep. It is this sleep-promoting characteristic of alcohol that makes it one of the most commonly used “over the counter” sleep aids (Johnson, Roehrs, Roth, & Breslau, 1998; Roehrs & Roth, 2001, 2012). However, alcohol-induced sleep promotion is short-lived and sleep is severely disrupted during the second half of the night.
Figure 1.
Alcohol has complex interactions with sleep.
Alcoholics also suffer from severe and protracted sleep disruptions manifested by profound insomnia, excessive daytime sleepiness, and altered sleep architecture (Brower & Perron, 2010; Colrain, Turlington, & Baker, 2009). Furthermore, subjective and objective indicators of sleep disturbances are predictors of relapse (Brower & Perron, 2010). Finally, within the United States, it is estimated that the cost of alcohol-related problems exceeds $180 billion, out of which more than $18 billion is associated with alcohol-related sleep disorders. Thus, although alcohol-associated sleep problems have a significant socio-economic impact on the individual, the individual’s family and society, very little is known about how and where alcohol acts to affect sleep.
About 5 years ago, we started our research program to understand the mechanisms mediating the effects of alcohol on sleep-wakefulness. In this review, we have described what we have uncovered about how and where alcohol acts to affect sleep.
To understand the neuronal substrates responsible for mediating the effects of alcohol on sleep, it is essential to understand how sleep is regulated. Therefore, we will begin this review by describing the fundamentals of sleep regulation, followed by a description about the effect of alcohol on human and animal sleep. Finally, we will provide a summary of our published as well as unpublished/preliminary findings.
Fundamentals of sleep
Sleep is an immense topic. It is difficult to describe sleep in a few paragraphs. In this section, we have attempted to provide some fundamentals. An interested reader is recommended to some excellent reviews (Brown, Basheer, McKenna, Strecker, & McCarley, 2012; Datta & Maclean, 2007; McCarley, 2007; Rosenwasser, 2009).
Sleep has always fascinated humankind. There is a myriad of treatises and reviews, scientific and non-scientific, trying to explain the phenomenon of sleep, yet none have been comprehensive enough to gain general acceptance. It is now well established that sleep is neither a unitary nor a passive process. Rather, intricate neuronal systems via complex mechanisms are responsible for the initiation and maintenance of sleep.
Sleep is defined as a rapidly reversible state of immobility and greatly reduced sensory responsiveness (Campbell & Tobler, 1984). An additional important criterion that is included in the definition of sleep is that sleep is homeostatically regulated, i.e., lost sleep is made up with an increased ‘drive’ for sleep and a consequent ‘sleep rebound’ (Campbell & Tobler, 1984).
Sleep is not a homogenous state. Rather, it is a continuum of states. The different components of the sleep continuum in mammalian species could broadly be divided into two major states: non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. A combination of electrophysiological measurements including 1) electroencephalogram (EEG), which traces the electrical activity of the brain, 2) the electro-oculogram (EOG), which measures eye movements, and 3) the electromyogram (EMG), which measures the electrical activity of muscles, is used objectively to identify different stages of the sleep-W cycle (Datta & Maclean, 2007).
Physiology of sleep
The state of active wakefulness is characterized by the presence of low-voltage, high-frequency (> 15 Hz) waves in the EEG, REMs in the EOG, and high-amplitude activity in the EMG. In humans, NREM sleep is divided into three stages.
Stage I is characterized by relatively low-voltage, mixed-frequency activity (3– 7 Hz) and vertex sharp waves in the EEG.
Stage II is characterized by slow (< 1 Hz) oscillation with distinctive sleep spindles (waxing and waning of 12–14-Hz waves lasting between 0.5–1.0 sec) and K-complex waveforms (a negative sharp wave followed immediately by a slower positive component).
Stage III (also termed as slow-wave sleep) is characterized by the dominance of high-amplitude, low-frequency (< 4 Hz) waves in the EEG. This state is the deepest stage of NREM sleep.
In laboratory animals (cats, rats, and mice), NREM sleep is generally not subdivided into stages. It is identified by the presence of high-amplitude, low-frequency waves in the EEG.
REM sleep is characterized by an ensemble of concomitant events including: 1) low-amplitude and high-frequency waves in the EEG; 2) very low or complete absence of activity in the EMG (muscle atonia), and 3) singlets and clusters of rapid eye movements (REMs) in the EOG. Supplemental to these polysomnographic signs, other REM sleep-specific physiological signs in mammals are 1) phasic ponto-geniculo-occipital (PGO) activity, 2) tonic hippocampal theta activity, 3) myoclonic twitches, most apparent in the distal limb musculature; 4) pronounced fluctuations in cardio-respiratory rhythms and core body temperature, and 5) penile erection and clitoral tumescence.
In adult humans and non-human primates, circadian distribution of the sleep period is mainly monophasic. While NREM sleep occupies the majority of time during the first half of sleep time, REM sleep is predominant in the second half.
In laboratory animals (mouse, rat, and cat), circadian distribution of the sleep period is polyphasic and the NREM-REM sleep cycles are shorter and continue throughout sleep periods during day and night.
Cellular substrates of sleep-wakefulness
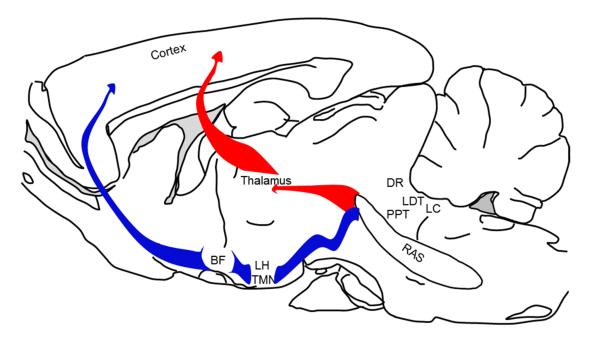
Multiple neuronal systems contribute to the promotion and maintenance of the awake state, which is characterized by cortical activation and behavioral arousal. Using predominantly glutamate as the neurotransmitter, neurons within the brainstem reticular activating system (RAS), via two major ascending relays, control cortical activation that occurs during wakefulness and REM sleep [Fig. 2; Brown et al., 2012; Jones, 2005].
Figure 2.
A schematic sagittal view of a mammalian brain depicting neuronal systems mediating the ventral (blue) and dorsal (red) relays of the reticular activating system controlling cortical activation and behavioral arousal. Abbreviations: RAS = glutamatergic reticular activating system; LC = noradrenergic locus coeruleus; LDT and PPT = cholinergic laterodorsal and pedunculopontine tegmental nuclei; DR = serotonergic dorsal raphe; LH = orexinergic perifornical lateral hypothalamus; TMN = histaminergic tuberomammillary nucleus; BF = cholinergic basal forebrain.
The ventral relay from the RAS is the major pathway controlling cortical activation. This relay system consists of the ascending fibers from several brainstem nuclei, including noradrenergic locus coeruleus (LC), serotonergic raphe system (DRN) and via the medial forebrain bundle, pass through and/or synapsing with several wake-promoting centers including the histaminergic in the tuberomammillary (TMN) and the orexinergic in the perifornical hypothalamus, and project to the basal forebrain (BF). The BF, in turn, projects to and controls the activation of the cortex (Brown et al., 2012; Datta & Maclean, 2007; Jones, 2003; Thakkar, 2011).
The dorsal relay of the RAS includes the mesopontine cholinergic neurons localized in the pedunculopontine and laterodorsal tegmental nuclei (LDT/PPT), which send rich projections to several thalamic nuclei. The thalamus, in turn, sends widespread and overlapping projections to the cortex. Although the dorsal pathway has an important role in the generation of sleep spindles and delta activity, ablation of the thalamus has minimal effect on cortical activation or sleep cycle, suggesting that the dorsal relay may have a limited role in cortical activation and behavioral arousal (Brown et al., 2012; Buzsaki et al., 1988; Fuller, Sherman, Pedersen, Saper, & Lu, 2011; Kim et al., 2012; Villablanca & Salinas-Zeballos, 1972).
In addition, other neuromodulatory systems, such as the LC neurons, also give rise to highly diffuse projections throughout the brain and spinal cord, and simultaneously stimulate cortical activation and behavioral arousal. Although such neuromodulatory systems were thought to be redundant, the hypothalamic orexins/hypocretin system was found to be essential for the maintenance of muscle tone during wakefulness, since ablation of orexin neurons or knockdown of orexin receptors results in narcolepsy with cataplexy (McCarley, 2007; Thakkar et al., 1999).
Transitions from waking to NREM sleep involve coordinated inhibition of multiple arousal systems. A major source of inhibition involves homeostasis-mediated regulation of sleep and is discussed in a separate section below.
Another important source of sleep-related inhibition of arousal systems arises from the neurons located in the preoptic area and includes the ventrolateral preoptic nucleus and median preoptic nucleus (Brown et al., 2012). These neurons are activated during NREM sleep and exhibit state-dependent discharge patterns that are reciprocal to that observed in arousal systems (Sleep-on/Wake-Off discharge pattern). A majority of preoptic, sleep regulatory neurons synthesize GABA. Anatomical and functional evidence supports the hypothesis that the preoptic GABAergic neurons may exert inhibitory control over the monoaminergic, histaminergic, and hypocretin systems during sleep (Brown et al., 2012; Datta & Maclean, 2007).
Recently, neurons containing melanin concentrating-hormone (MCH) in the zonaincerto-hypothalamic region have also been implicated in sleep regulation (Konadhode et al., 2013; Monti, Torterolo, & Lagos, 2013). Optogenetic activation of MCH neurons suppresses wakefulness and promotes sleep (NREM and REM), suggesting that the MCH system may also have a prominent role in sleep control (Konadhode et al., 2013).
REM sleep is unique. Since its electrophysiological identification in the 1950s, multiple lines of evidence have shown that REM sleep is generated by oral pontine tegmentum neurons (McCarley, 2007). The perpetration of this paradoxical state that combines wakebrain activity with the most profound behavioral sleep occurs through interplay between REM-promoting (On) and REM-permitting (Off) neuronal systems within the pons. Mesopontine LDT/PPT cholinergic cells promote REM sleep by initiating processes of both forebrain activation and peripheral muscle inhibition (atonia) (Datta & Maclean, 2007; McCarley, 2007).
The dorsal relay, along with a major population of the LDT/PPT cholinergic neurons projecting to the cortex via the thalamus, is active during both wakefulness and REM sleep, and is responsible for promoting cortical activation. In addition, excitatory inputs from the brainstem glutamatergic, noradrenergic and serotonergic to the somatic and spinal cord motor neurons are responsible for the maintenance of muscle tone during wakefulness (Burgess & Peever, 2013). However, during wakefulness, a minor population of cholinergic LDT/PPT REM-On neurons that project to the brainstem is inhibited by the REM-Off LC and DRN neurons (Thakkar, Strecker, & McCarley, 1998).
During REM sleep, the LC and DRN neurons cease their activity, thereby lifting their inhibition on cholinergic REM-On neurons, allowing the expression of REM sleep (Datta & Maclean, 2007; McCarley, 2007). A combination of mechanisms, including disfacilitation (reduced glutamatergic, LC, and DRN tone) and active inhibition (increased brainstem cholinergic, glycinergic, and GABAergic tone), may be responsible for producing the muscle atonia observed during REM sleep (Thakkar, Sharma, Engemann, & Sahota, 2012).
Episodes of dreaming or nightmares are believed to occur only during REM sleep. It has been suggested that synchronized electrical field potentials in the pons, lateral geniculate nucleus, and occipital cortex (PGO waves) are the source of visual imagery observed during REM sleep (Brooks, 1968; Brown et al., 2012). The PGO waves are prominent observed in visual thalamocortical circuits and occur simultaneously with rapid eye movements associated with gaze direction in visual imagery (Brown et al., 2012).
Regulation of sleep
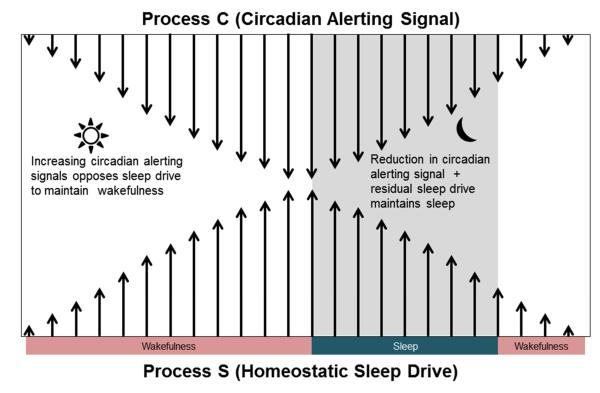
Current theory suggests that the physiological regulation of mammalian sleep is controlled by two components: 1) a homeostatic sleep process (Process S) which increases during wakefulness and declines during sleep and which interacts with 2) a circadian alerting process (Process C), that is independent of sleep and wakefulness (Borbély, 1982).
During normal, spontaneous wakefulness, rising homeostatic sleep pressure is compensated for by increasing circadian alerting signal, which helps in the maintenance of wakefulness. Conversely, during the night, diminution of the circadian alerting signal coupled with residual homeostatic sleep drive ensures the maintenance of sleep [Fig. 3; see Edgar, Dement, & Fuller, 1993]. The changes in sleep-wakefulness across 24 h, observed in humans and animals, are the result of combined influences of Process S and Process C. The assessment of the relative contributions of sleep homeostasis and circadian rhythmicity is complex.
Figure 3.
The interaction of the circadian alerting signal and the homeostatic sleep drive in the regulation of sleep-wakefulness is described. During the day, an increasing circadian alerting signal counteracts accumulating sleep pressure to maintain wakefulness. At night, the accumulating sleep pressure reaches its peak (threshold) whereby it overrides the circadian alerting drive and promotes sleep. During the night, sleep is maintained by the residual homeostatic sleep drive coupled with diminution of the circadian alerting signal (Adapted from Edgar et al., 1993).
Process C is regulated by the intrinsic body clock, the suprachiasmatic nucleus (SCN). Although neural mechanisms by which the circadian clock influences the sleep-wake system remain to be fully specified, the SCN is known to communicate with several sleep-wakefulness centers, including the preoptic area and the BF. Although complete ablation of the SCN eliminates sleep-wake rhythms, sleep loss results in a compensatory increase in the duration and intensity of sleep, suggesting that Process S is independent of Process C (Tobler, Borbély, & Groos, 1983).
Laboratory rodents are predominantly active during the dark period and sleep predominantly during the light period (Process C). They display a shorter sleep cycle interspersed with awake episodes, mainly due to a limited capacity for accumulation of sleep pressures. Despite this “polyphasic” pattern of sleeping, the homeostatic drive decreases during relatively consolidated periods of sleep, and accumulates during periods of wakefulness, suggesting that the same Process C and Process S are responsible for sleep regulation in nocturnal, polyphasic laboratory rodents (Franken, Tobler, & Borbély, 1991; Phillips, Robinson, Kedziora, & Abeysuriya, 2010; Tobler, Franken, Trachsel, & Borbély, 1992).
Sleep homeostasis
The core of sleep homeostasis is sleep propensity or sleep pressure. Sleep pressure starts to accumulate as soon as one is awake, and continues to accumulate until sleep is initiated. Once sleep is initiated, sleep pressure starts to dissipate. Sleep pressure is the manifestation of an accumulation of sleep homeostatic factor(s).
Although the search to identify the mediator of sleep pressure began more than 100 years ago, it was only in recent years that strict criteria were required to be fulfilled if a factor was to be considered as a homeostatic sleep factor. These required criteria are: 1) an increase in sleep propensity should result in the accumulation of homeostatic sleep factor, 2) the homeostatic sleep factor should act on brain regions and neurons regulating sleep-wakefulness, and 3) administration of the homeostatic sleep factor should promote sleep (Brown et al., 2012; Porkka-Heiskanen et al., 1997).
Although several sleep factors have been identified and implicated to mediate the accumulation of sleep pressure during wakefulness, only adenosine (AD), nitric oxide (NO), prostaglandin D2 (PGD2), and cytokines appear to fulfill the established criteria to be considered as the homeostatic regulator(s) of sleep. Out of these four neuromodulators, only AD has gained the utmost attention because the remaining three (NO, PGD2, and the cytokine interleukin-1) mediate their sleep-promoting effects via AD. For example, NO and interleukin-1 increase AD release and inhibit neuronal activity in the -wake-promoting systems (Brown et al., 2012; Luk et al., 1999; Rosenberg, Li, Le, & Zhang, 2000). The somnogenic effects of PGD2 are mediated via prostaglandin receptor 1, localized predominantly in the meninges. Activation of prostaglandin receptor 1 results in an increase in AD (Hayaishi, Urade, Eguchi, & Huang, 2004; Mizoguchi et al., 2001).
AD links sleep with energy metabolism and neuronal activity. During wakefulness, energy (ATP) usage is high in wake-promoting systems, due to increased neuronal firing, synaptic activity, and synaptic potentiation. This increased energy usage during wakefulness is reflected in increased accumulation of extracellular AD, a breakdown product of ATP metabolism, which corresponds to increased accumulation of sleep pressure. The longer the period of wakefulness, the greater the accumulation of sleep pressure and/or AD and the longer it takes for sleep pressure to dissipate during sleep (Porkka-Heiskanen, 2013).
Experimentally, sleep homeostasis is examined by sleep deprivation followed by recovery sleep. During sleep deprivation, sleep pressure is measured by theta power in wake EEG and extracellular AD, most markedly in the wake-promoting BF region. Increased AD acts via A1 receptors (A1R) to inhibit wake-promoting neurons in the BF to promote sleep and enhance delta activity (Thakkar, Delgiacco, Strecker, & McCarley, 2003; Thakkar, Winston, & McCarley, 2003). Homeostatic response or dissipation of sleep pressure is measured by sleep latency along with the duration and intensity of recovery sleep that follows sleep loss (Borbély, 1982; Porkka-Heiskanen, 2013).
Now that we have described the fundamentals of sleep, let us describe what happens to sleep-wakefulness after acute and chronic alcohol consumption.
Alcohol and sleep: human studies
Since a detailed description of alcohol and sleep in human subjects in accompanying article(s), we have provided a brief overview here. Several laboratories have examined the effects of a single pre-bedtime dose (ranging from 0.25 to 1 g/kg) of alcohol in healthy non-alcoholic adult males and females [for reviews, see Ebrahim, Shapiro, Williams, & Fenwick, 2013; Roehrs & Roth, 2001]. The most consistent findings are that alcohol, irrespective of the dose, administered just before bedtime, reduces sleep onset latency, consolidates sleep, increases NREM (stage 3) sleep, and increases EEG power densities in the delta frequencies during NREM sleep, especially during the first half of the night.
Abstinence from alcohol (withdrawal) in alcoholics results in severe and protracted sleep disruptions. While acute withdrawal accompanies severe insomnia (difficulty in falling and staying asleep), insomnia, sleep fragmentation, and alteration in sleep architecture are observed for several years during sustained withdrawal from alcohol. Furthermore, subjective and objective indicators of insomnia including difficulty in falling asleep, decreased total sleep time, and decreased sleep efficiency are predictors of relapse [reviewed in Brower, 2001].
Alcohol and sleep: animal studies
Animal studies support human studies. Irrespective of the time of administration, acute alcohol treatment in rodents reduces sleep onset latency and increases NREM sleep (Hattan & Eacho, 1978; Hill & Reyes, 1978a; Kubota, De, Brown, Simasko, & Krueger, 2002; Mendelson & Hill, 1978). Local bilateral infusion of alcohol (0.047, 0.24, and 0.47 μmol) into the preoptic region dose-dependently increases NREM sleep (Ticho, Stojanovic, Lekovic, & Radulovacki, 1992). In contrast, intracerebroventricular (icv) application of alcohol (10 μL/5 nM) produces selective REM sleep changes (Prospero-García, Criado, & Henriksen, 1994).
Chronic alcohol exposure causes sleep disruptions in animals. While two studies have reported decreased REM during withdrawal after chronic alcohol exposure (Gitlow, Bentkover, Dziedzic, & Khazan, 1973; Rouhani et al., 1998), two other studies reported increased REM sleep (Kubota et al., 2002; Mendelson et al., 1978). Veatch (2006) found increased REM with a concomitant reduction in NREM sleep during the initial withdrawal period. While REM remained increased, non-REM sleep normalized within 4 days. Finally, 6 weeks of chronic exposure to 6% alcohol in a liquid diet increases wakefulness and reduces sleep (NREM and REM) during the light (sleep) period, but decreases wakefulness and increases sleep during the dark (active) period (Mukherjee, Kazerooni, & Simasko, 2008; Mukherjee & Simasko, 2009).
In summary, strong evidence from human and animal studies suggests that a single dose of alcohol reduces sleep onset latency and promotes NREM sleep. In humans, acute alcohol (especially a moderate to high dose) suppresses REM sleep during the first half of the sleep period.However, during the second half, there is an increase in REM sleep (REM rebound) along with an increase in wakefulness.. In contrast, both in humans and in animals, chronic alcohol exposure causes sleep disruptions, manifested by an increase in sleep onset latency and wakefulness along with a reduction in sleep during the normal sleep (light) period (insomnia), along with reduced wakefulness and increased sleep during the normal active (dark) period.
Alcohol and sleep: anatomical and cellular mediators
Initial studies implicated catecholamines, such as serotonin and norepinephrine, in mediating the sedative effects of alcohol (Hill & Reyes, 1978b). However, as described above, catecholamines have a limited modulatory role in mediating sleep promotion. For example, mice lacking norepinephrine do not display any sleep-wake abnormalities (Hunsley & Palmiter, 2003). A recent study confirmed that norepinephrine does not have a major role in the regulation of sleep-wakefulness. Rather; it is critical for maintenance of muscle tone during wakefulness and indirectly plays a role in muscle atonia that accompanies REM sleep (Burgess & Peever, 2013).
Alcohol-induced reduction in glutamate has been implicated in alcohol-induced suppression of REM sleep (Prospero-García et al., 1994). However, direct evidence supporting this hypothesis is lacking.
GABA was another potential candidate implicated in alcohol’s intoxicating effects (Breese et al., 2006; Wallner, Hanchar, & Olsen, 2006). In vivo and in vitro studies suggest that GABA and alcohol may act via GABAA receptors, similar to barbiturates or benzodiazepines, which are known hypnotics that induce sleep [reviewed in Wallner et al., 2006]. Therefore, alcohol-induced activation of GABAA receptors may result in sleep promotion. However, the effects of alcohol on GABAA receptors are complex and not clearly understood [reviewed in Aguayo, Peoples, Yeh, & Yevenes, 2002; Siggins, Roberto, & Nie, 2005; Wallner et al., 2006]. In addition, recent reports suggest that the intoxicating effects of alcohol may be due to activation of extrasynaptic δ subunit-containing GABAA receptors (Breese et al., 2006; Wallner et al., 2006). Although activation of extrasynaptic GABA receptors in the orexin zone of the lateral hypothalamus promotes sleep, GABAA receptors may have a minimal role in sleep promotion (Winsky-Sommerer, Vyazovskiy, Homanics, & Tobler, 2007). In addition, the role of GABA in sleep is complex. For example, extracellular GABA levels in the pons are highest during wakefulness and lowest during sleep (Thakkar et al., 2004; Vanini, Wathen, Lydic, & Baghdoyan, 2011). While activation of GABAA receptors in the pons promotes wakefulness, blockade of GABAA receptors promotes REM sleep (Boissard et al., 2002; Pollock & Mistlberger, 2003; Xi, Morales, & Chase, 2001). In addition, cortical-projecting GABA neurons in the BF activate the cortex and promote EEG desynchronization (Jones, 2005).
In an attempt to understand the cellular substrates mediating the effects of alcohol on sleep-wakefulness, we began by focusing on AD. AD appeared to be an ideal candidate mediating the effects of alcohol on sleep because AD is a sleep factor implicated in the homeostatic regulation of sleep (Radulovacki, Virus, Djuricic-Nedelson, & Green, 1984; Thakkar, Winston, et al., 2003). In addition, AD is a key mediator of neuronal responses to alcohol (Dunwiddie & Masino, 2001; Hack & Christie, 2003; Newton & Messing, 2006).
Strong and convincing evidence exists demonstrating the importance of AD and its A1R in mediating the effects of alcohol on ataxia (Dar, 1993, 1997, 2001; Phan, Gray, & Nyce, 1997; Saeed Dar, 2006). In addition, AD is also implicated in mediating the behavioral aspects of alcohol withdrawal, such as anxiety, tremors, and seizures (Batista, Prediger, Morato, & Takahashi, 2005; Concas et al., 1996; Kaplan, Bharmal, Leite-Morris, & Adams, 1999; Prediger, da Silva, Batista, Bittencourt, & Takahashi, 2006). Furthermore, acute alcohol exposure inhibits AD reuptake via an NBTI-sensitive equilibrative nucleoside transporter-1 (ENT1), whereas chronic exposure to alcohol down-regulates ENT1 expression (Krauss, Ghirnikar, Diamond, & Gordon, 1993; Nagy, Diamond, Casso, Franklin, & Gordon, 1990). The ENT1-null mice show reduced AD tone and reduced hypnotic (loss of righting reflex) and ataxic responses to alcohol, along with increased alcohol consumption. Treatment with an A1R agonist decreases alcohol consumption in ENT1-null mice; however, an A2AR agonist is ineffective (Choi et al., 2004).
Several clinical studies also support the role of AD in alcohol-induced behaviors. For example, AD via its action on A1R promotes NREM sleep, enhances EEG power in delta frequency, and suppresses REM sleep, which is remarkably similar to the effects of acute alcohol intake on sleep (Basheer, Strecker, Thakkar, & McCarley, 2004; Benington, Kodali, & Heller, 1995; Landolt & Gillin, 2001). Caffeine, a nonspecific AD receptor antagonist, offsets the intoxicating effects of alcohol and improves alertness (Franks, Hagedorn, Hensley, Hensley, & Starmer, 1975; Hasenfratz, Bunge, Dal Prá, & Bättig, 1993; Kerr, Sherwood, & Hindmarch, 1991; Liguori & Robinson, 2001). Combined administration of caffeine and alcohol can increase the development of alcohol tolerance in humans (Fillmore, 2003). Alcoholics display an impaired response to sleep deprivation, suggesting an impaired AD system, especially since AD has a critical role in sleep homeostasis (Irwin et al., 2002).
Interestingly, the sedative effects are observed during the descending phase of the BAC curve (Erblich, Earleywine, Erblich, & Bovbjerg, 2003; Martin, Earleywine, Musty, Perrine, & Swift, 1993; Schweizer & Vogel-Sprott, 2008), suggesting the sleep-promoting effects of alcohol are relatively delayed. If it were the case that alcohol promotes sleep via its direct action on (sleep-promoting) GABA neurons, then the sedative effects would be more likely to be observed, much earlier, in the ascending phase of the BAC curve. This relatively delayed sleep-promoting response fits well with alcohol’s effect on AD uptake, accumulation of extracellular AD, and inhibition of wake-promoting systems, resulting in sleep promotion. Thus, based on the evidence described above, we began our quest to understand the role of AD in the sleep-promoting effects of alcohol.
Sleep homeostasis in acute alcohol-induced sleep promotion
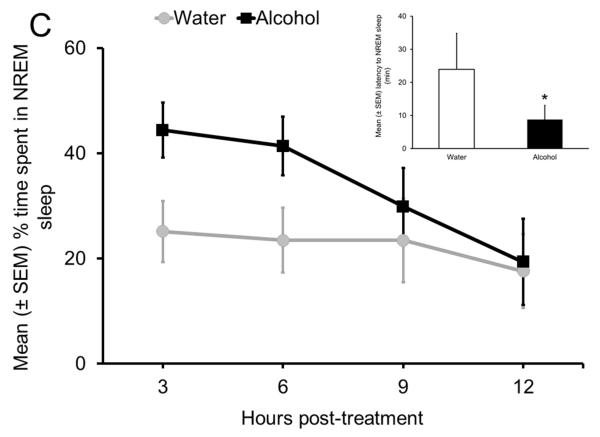
In the first set of experiments, we examined the effect of alcohol on sleep promotion in freely behaving Sprague Dawley (SD) rats. The experimental design was simple. Rats were instrumented for electrographic recording of the EEG and EMG to examine and quantify sleep-wake states. In order to examine the potency of alcohol in sleep promotion, we administered alcohol (3g/kg; intragastric) at the onset of the circadian active period when rats are maximally active and all wake-promoting systems are very active. Our results suggest that alcohol administration at dark onset significantly reduced sleep onset latency (inset in Fig. 4C) and increased NREM sleep (Fig. 4C). While wakefulness was reduced, REM sleep remained unaffected. In addition, the maximal effect of alcohol was observed during the first 6 h after alcohol administration. These results suggest that alcohol is able to counteract a strong wake-promoting drive initiated by combined activation of all wake-promoting centers to promote NREM sleep.
Figure 4.
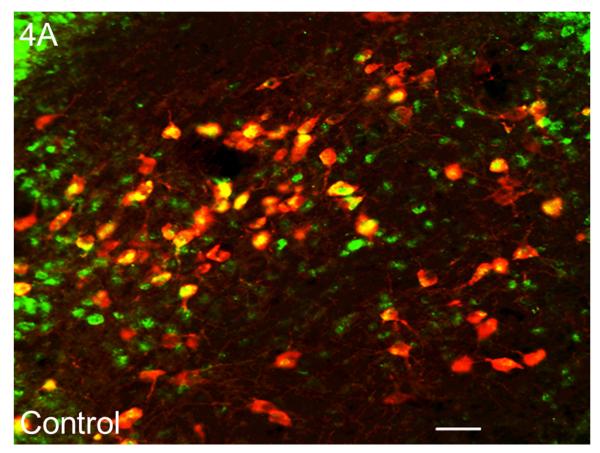
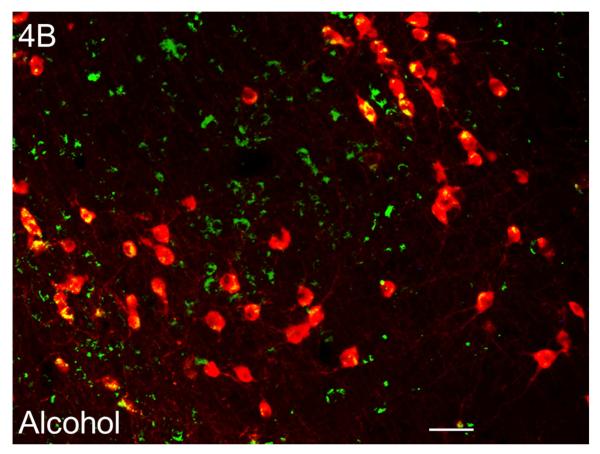
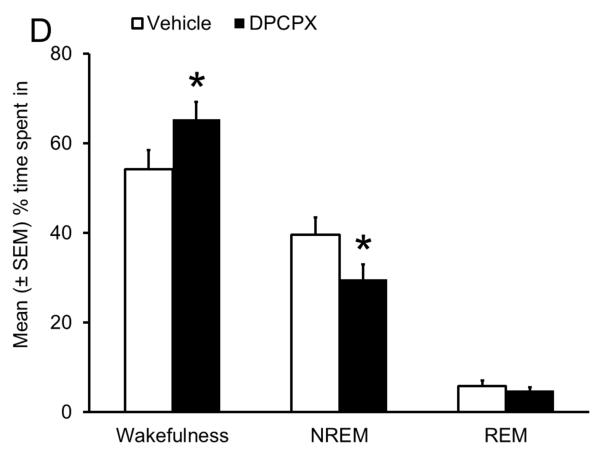
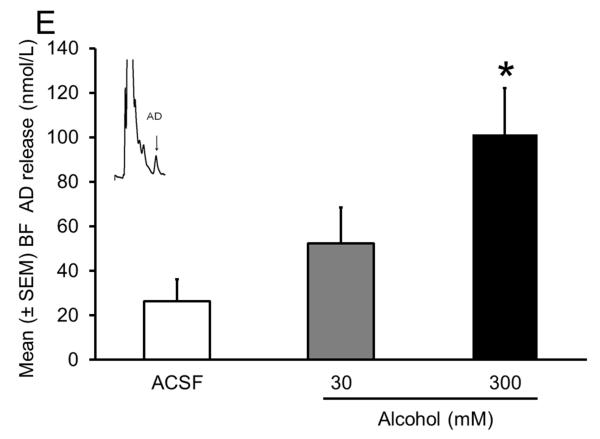
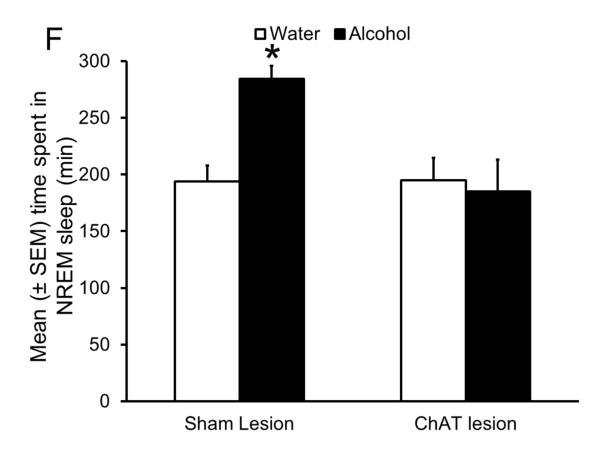
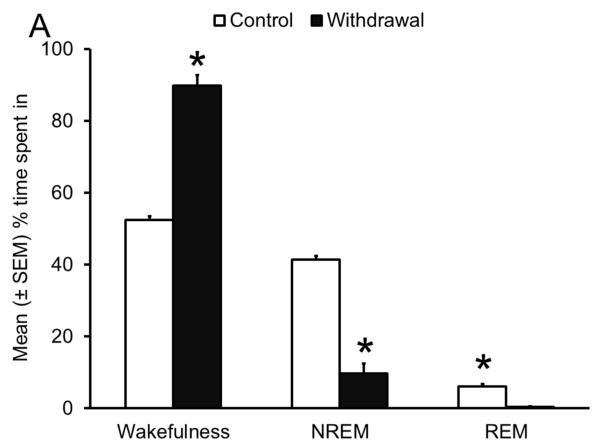
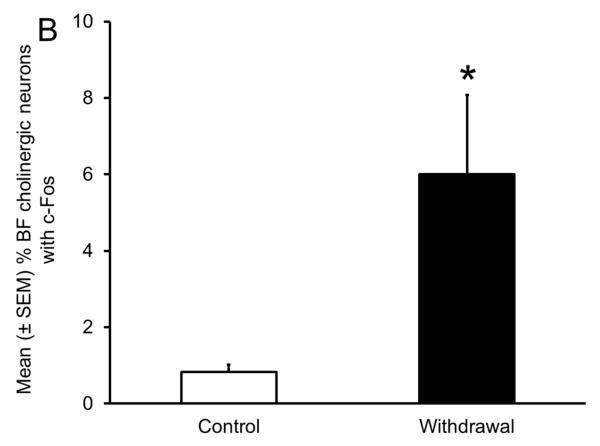
Sleep homeostasis in sleep-promoting effects of alcohol. A single dose (3 g/kg; intragastric) of alcohol, administered at the onset of the circadian active period, inhibits wake-promoting cholinergic neurons of the BF (Panel A & B) to promote NREM sleep as shown by a significant reduction in sleep onset latency (inset in Panel C) coupled with a significant increase in the amount of time spent in NREM sleep (Panel C). However, blockade of AD A1R in the BF resulted in an attenuation of alcohol- induced sleep promotion (Panel D), whereas local, reverse microdialysis perfusion of pharmacological relevant doses of alcohol into the BF significantly increased extracellular AD in the BF (Panel E; inset describes AD chromatogram) implicating adenosinergic mechanisms in alcohol-induced sleep promotion [adapted from Sharma et al., 2010b and Thakkar et al., 2010]. Lastly, in contrast to sham controls, animals with a selective lesion of the BF cholinergic neurons displayed attenuated alcohol-induced sleep [Panel F; unpublished data]; *= p < 0.05.
Since the wake-promoting BF is the final node in the ventral arm of the RAS (see Fig. 2) and is involved in the regulation of sleep homeostasis, we hypothesized that the strong sleep-promoting effect of alcohol may be mediated via inhibition of the wake-promoting neurons of the BF. To test our hypothesis, we examined the effects of alcohol on the activation of BF cholinergic wake-promoting neurons in SD rats. We used c-Fos as a marker of neuronal activation and double-label (choline acetyltransferase and c-Fos) immunohistochemistry (IH) to examine the expression of c-Fos in the BF cholinergic neurons. We found that alcohol (3 g/kg; intragastric) administration at dark onset significantly reduced the activation of wake-promoting neurons in the BF (Fig. 4A & 4B). Thus, alcohol inhibits the BF wake-promoting neurons and suppresses cortical desynchronization to promote sleep.
The next logical question was: How does alcohol inhibit BF wake-promoting neurons? AD acts via A1R to inhibit BF wake-promoting neurons and promote sleep (Thakkar, Delgiacco, et al., 2003; Thakkar, Winston, et al., 2003). There are no A2A receptors in the BF (Basheer et al., 2004). Thus, we examined the effects of bilateral BF infusion of an A1R receptor antagonist, 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX), on alcohol-induced sleep at dark onset. We chose DPCPX because it promotes wakefulness (Van Dort, Baghdoyan, & Lydic, 2009). Since our experiment was performed at dark onset when animals are maximally awake and spent > 80% time in wakefulness, infusion of DPCPX will have minimal effects on spontaneous wake-promotion due to “ceiling” effects. However, since alcohol promotes sleep at dark onset, then DPCPX will suppress alcohol-induced sleep and promote wakefulness. Indeed, pretreatment with DPCPX resulted in an attenuation of alcohol-induced sleep promotion (Fig. 4D), implicating AD and its A1R, critical components of sleep homeostasis, in alcohol-induced sleep promotion (Thakkar, Engemann, Sharma, & Sahota, 2010).
To further demonstrate the role of AD in the BF in alcohol-induced sleep, we examined the effect of alcohol on extracellular release of AD in the BF. We used microdialysis to measure AD release. To provide specificity of alcohol’s action on the BF, we used reverse microdialysis and locally delivered pharmacologically relevant doses of alcohol through the same microdialysis probe. We found that local microdialysis perfusion of alcohol produced a dose-dependent increase in extracellular AD in the BF with the maximal increase observed with a 300 mM dose of alcohol perfused (Fig. 4E). Reverse microdialysis perfusion of a 300 mM dose of alcohol provides a concentration of 33 mM directly outside the probe. This concentration of alcohol is in the range of alcohol concentration observed in the brain after a systemic administration of a sedative dose (~3 g/kg) of alcohol (Sharma, Engemann, Sahota, & Thakkar, 2010b). This study provided the first evidence demonstrating that alcohol acts directly in the brain to increase AD.
Finally, if the wake-promoting cholinergic neurons of BF are responsible for acute alcohol-induced sleep promotion, then lesions of the cholinergic neurons may attenuate the sleep-promoting effects of alcohol. In this unpublished study, we used 192-IgG-saporin (SAP) to selectively lesion BF cholinergic neurons. Our results suggest that as compared to controls (infused with ACSF), bilateral infusion of SAP in the BF resulted in a significant reduction (> 70%) in the number of cholinergic neurons in the BF. In addition, alcohol administration in lesioned animals resulted in an attenuated alcohol-induced sleep promotion. While control animals showed a significant increase in the NREM sleep following alcohol (3 g/kg) administration, as compared to systemic water administration, a day earlier, lesioned animals did not show such an increase in NREM sleep after alcohol (3 g/kg) administration (Fig. 4F). These results strongly implicate the wake-promoting BF cholinergic neurons as a critical anatomical substrate mediating the sleep-promoting effects of alcohol.
In summary, based on the results of our studies described above, we suggest that alcohol may act on the wake-promoting cholinergic neurons in the BF to increase extracellular AD. Increased AD promotes sleep by inhibiting BF wake-promoting neurons via A1R.
Sleep homeostasis in withdrawal-induced sleep disruptions
As described above, alcohol may promote sleep by its action on AD in the BF. The next question we asked was: Are sleep disruptions observed during alcohol withdrawal due to an impaired AD system in the BF?
Thus, in our next series of experiments, we examined the role of the AD system in the BF on sleep disruptions observed during alcohol withdrawal. We used the Majchrowicz’s chronic binge alcohol protocol to induce alcohol withdrawal in SD rats (Majchrowicz, 1975). The Majchrowicz’s protocol has been extensively used to induce alcohol dependence and understand the effects of alcohol dependency and alcohol withdrawal in experimental animals [> 300 papers have cited the original article in the last 20 years (Faingold, 2008)]. This relatively easy-to-use method offers other advantages including 1) mimicking high blood alcohol levels and heavy alcohol consumption in alcoholics, 2) rapid induction of alcohol dependency and relatively high and sustained blood alcohol concentration achieved within a short duration, and 3) high incidence of overt signs of withdrawal. A brief description of the Majchrowicz protocol used is as follows: 2 h before the light onset, experimental animals were primed with an intragastric administration of a 5 g/kg dose of alcohol mixture [100 mL of 35% alcohol (v/v in water) containing 8.5 g infant formula (PBM Nutritionals, Georgia, VT)]. Subsequent doses were adjusted depending on the level of intoxication, assessed by observing the behavior of the animal [see Table 1 in Sharma, Bradshaw, Sahota, & Thakkar, 2010a] and was administered every 8 h for 4 days (treatment days). The controls received the control mixture (8.5 g of infant formula in 100 mL of water; 10 mL/kg) at the same time of day for 4 days.
Table 1. Effects of binge alcohol exposure on indices of sleep homeostasis in mice.
Controls were exposed to water, whereas mice in the Alcohol group were exposed to voluntary binge alcohol (20% v/v) drinking for 4 h in the DID paradigm (see text for details).
| Indices of sleep homeostasis | Control (n = 4) | Alcohol (n = 4) | |||
|---|---|---|---|---|---|
| Hour 2 | Hour 6 | Hour 2 | Hour 6 | ||
|
Sleep
deprivation (6 hours) |
EEG Theta (4-9 Hz) power (%) | 100 ± 8.3 | 130.4 ± 8.3* | 100 ± 8.3 | 89.6 ± 8.3 |
| BF AD release (%) | 100 ± 13.8 | 283.2 ± 18.3* | 100 ± 8.3 | 92.9 ± 15.6 | |
|
Recovery
sleep (3 hours) |
EEG NREM Delta (1-4 Hz) power (%) | 100 ± 14.2 | 34.6 ± 4.7 | ||
| #Sleep onset latency (min) | 10 ± 3.5 | 112 ± 28.6* | |||
| NREM sleep (%) | 30.9 ± 3.0 | 19.3 ± 5.4* | |||
Sleep onset latency is defined as the amount of minutes between end of sleep deprivation and first non-interrupted 60 sec bout of NREM sleep;
denotes p < 0.05.
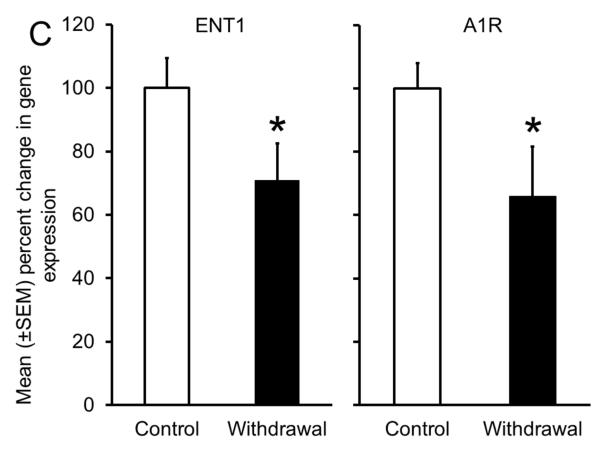
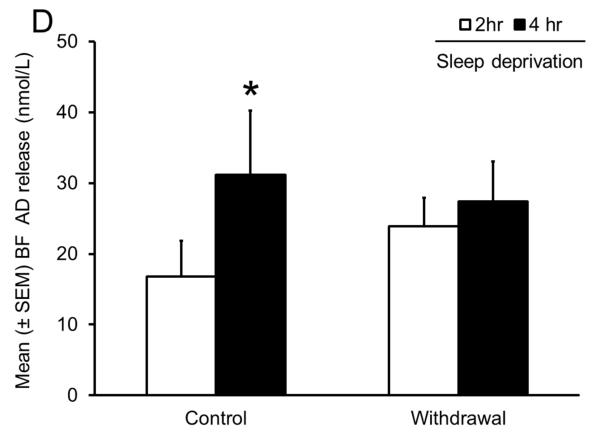
As shown in Fig. 5A, during acute alcohol withdrawal, rats displayed significant increases in wakefulness, both during their sleep (light) and active (dark) period with a concomitant reduction in sleep (NREM and REM), suggesting insomnia-like symptoms which have been observed during acute alcohol withdrawal in humans. In addition, alcohol-dependent rats displayed a significant increase in the number of cholinergic neurons with c-Fos expression in the BF (Fig. 5B), suggesting an activation of the wake-promoting system. Furthermore, rats undergoing alcohol withdrawal did not display an increase in extracellular AD during sleep deprivation, suggesting an impaired sleep homeostatic response (Fig. 5D). To further investigate the cause of impaired sleep homeostasis, we examined the expression of ENT1 and A1R (critical for regulation of AD tone), in the BF of rats undergoing alcohol withdrawal and found a reduction in the expression of ENT1 and A1R genes (Fig. 5C). These results provided strong support for the role of AD in alcohol’s effects on sleep (Sharma, Engemann, Sahota, & Thakkar, 2010a) and were the first to demonstrate that sleep disruptions observed during alcohol withdrawal may be due to disrupted sleep homeostasis caused by alcohol-induced down-regulation of the AD system. Indeed, recent studies have confirmed that human alcoholics display a blunted sleep homeostatic response (Armitage, Hoffman, Conroy, Arnedt, & Brower, 2012; Brower, Hoffman, Conroy, Arnedt, & Armitage, 2011).
Figure 5.
Impaired sleep homeostasis is the cause of sleep disruptions observed during alcohol withdrawal. A significant increase in wakefulness coupled with a concomitant reduction in sleep (NREM and REM) (Panel A), along with increased activation of wake- promoting BF cholinergic neurons (Panel B) was observed during acute alcohol withdrawal. In addition, there was a significant reduction in the expression of ENT1 and A1R in the BF (Panel C) during alcohol withdrawal, resulting in an absence of a sleep deprivation-induced increase in AD in the BF [Panel D; adapted from Sharma et al., 2010]; *= p < 0.05.
Sleep homeostasis in binge alcohol-induced sleep disruptions
Human alcohol dependency is associated with voluntary oral self-ingestion of alcohol. Thus, one limitation of our above-described studies may be that we used forced alcohol administration, and not voluntary alcohol self-administration. In addition, since binge drinking is highly prevalent in our society and sleep disruptions are common in binge drinkers (Popovici & French, 2013), the next set of experiments was designed to examine the effects of voluntary binge drinking on sleep-wakefulness. We used C57BL/6J mice and exposed them to voluntary binge drinking under a modified version of the “drinking in the dark” (DID) paradigm. The basic paradigm was to offer each experimental mouse a single tube containing 20% alcohol (v/v; in tap water), instead of its usual water bottle, for a period of 4 h, beginning 3 h from the onset of the circadian dark period, for one day, and monitor their sleep (Crabbe, Harris, & Koob, 2011; Mulligan et al., 2011; Rhodes, Best, Belknap, Finn, & Crabbe, 2005).
We chose to use C57BL/6J mice because they are known to self-administer high amounts of alcohol in a relatively short period and consume alcohol for post-ingestive intoxicating effects, and not simply for taste or caloric fulfillment (Belknap, Crabbe, & Young, 1993; Rhodes et al., 2007; Sprow & Thiele, 2012). We chose the DID method because it is simple, easy to use, reliable, and extensively used with high face validity in mimicking human binge drinking (Mulligan et al., 2011; Sharma, Sahota, & Thakkar, 2014a, 2014b; Sprow & Thiele, 2012).
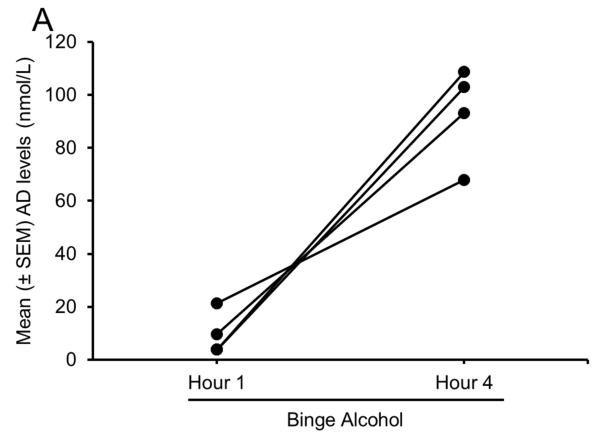
Our first experiment examined AD release in the BF during binge drinking. Unpublished preliminary results from our ongoing study suggest that each of the 4 alcohol-naïve mice exposed to a single episode of binge drinking displayed a profound increase in extracellular AD in the BF during the last hour of alcohol consumption as compared to during the first hour (Fig. 6A).
Figure 6.
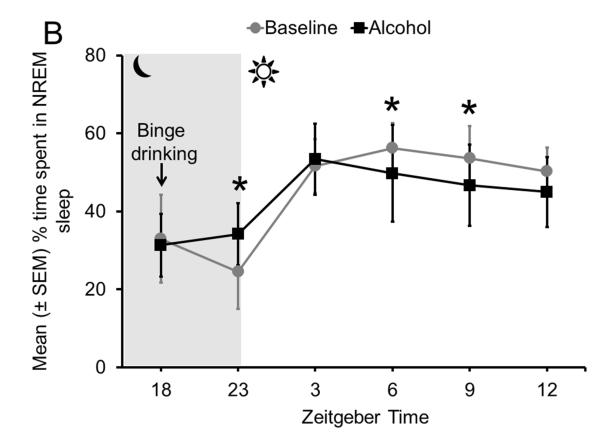
Sleep homeostasis in binge alcohol-induced sleep disruptions. All 4 animals displayed an increase in AD in the BF during 4 h of binge alcohol consumption (Panel A) coupled with a significant increase in NREM sleep during the active period, immediately after the completion of 4 h of binge drinking. However, during the subsequent 12 h of the light (sleep) period, NREM sleep was significantly reduced, especially during the last 9 h of the light period. No change was observed during the first 3 h of the light period, suggesting that Process C, regulating the timing of sleep, remained unaffected (Panel B).
Does increased AD in the BF result in sleep promotion? Our next experiment examined sleep-wakefulness following a single episode of binge drinking. Adult male C57BL/6J mice implanted with sleep recording electrodes were exposed to a single 4-h session of alcohol self-administration (under DID protocol as described above). Sleep was continuously recorded for 21 h, beginning with the onset of alcohol drinking (3 h after dark onset) and ending at the onset of the next dark period, 21 h later. Baseline recording was performed on the previous day by exposing the animals to water and mimicking alcohol-day conditions. The unpublished/preliminary results of our study suggest that alcohol consumption resulted in a robust sleep promotion during the dark period, immediately after alcohol consumption was complete, suggesting that alcohol-induced AD release in the BF may be the cause of sleep promotion (Fig. 6B). These results provide support to our hypothesis that alcohol increases AD and promotes sleep.
In contrast to the sleep promotion observed immediately after completion of binge alcohol consumption, there was a significant increase in wakefulness with a concomitant reduction in sleep during the subsequent light (sleep) period. Fine grain analysis revealed that the amount of time spent in sleep-wakefulness during the first 3 h of the light period was comparable to baseline, suggesting that Process C, which regulates sleep timing, remained unaffected. However, sleep was profoundly reduced during the subsequent 9 h of the light period (Fig. 6B). These results suggest that alcohol intake disrupts Process S or sleep homeostasis, but may have a minimal effect on Process C, or the circadian controller of sleep. The next group of experiments was designed to confirm the effects of alcohol intake on sleep homeostasis.
Sleep deprivation followed by recovery sleep is an essential paradigm to examine the sleep homeostasis process (Porkka-Heiskanen, 2013). Therefore, we examined the effects of alcohol consumption on the indicators of sleep homeostasis during sleep deprivation and recovery sleep. Alcohol-naïve mice (instrumented with sleep-recording electrodes and bilateral guide cannulas in the BF) were exposed to a single episode (4 h) of alcohol self-administration. Controls were exposed to water. On completion, mice were left undisturbed until the onset of sleep deprivation, 11 h after the completion of alcohol/water consumption. Sleep deprivation was performed during the last 6 h of the light period and carried out by gentle handling. The gentle handling protocol of sleep deprivation minimizes stress and involves keeping the animals awake by (gentle) tactile and auditory stimuli (Sharma et al., 2010a; Thakkar, Winston, et al., 2003). After 6 h of sleep deprivation, animals were allowed to recover sleep for 3 h. Electrographic recordings of sleep-wakefulness and spectral analysis of the EEG were performed to examine theta power during sleep deprivation, the amount of time spent in recovery sleep, and delta power during recovery sleep. In addition, microdialysis sampling was performed to measure the extracellular levels of AD. Our unpublished/preliminary results are described in Table 1. Mice exposed to voluntary alcohol consumption displayed a blunted homeostatic response, as shown by absence of sleep deprivation-induced increase in 1) theta power; 2) extracellular AD levels in the BF; 3) NREM delta power during recovery sleep, and 4) recovery sleep. These results support our previous results and our hypothesis that alcohol consumption disrupts sleep homeostasis and causes sleep disruptions. We believe that this is the first step toward understanding the mechanism of sleep disruptions induced by binge drinking. Still, many questions remained unanswered such as a) how long do the effects of acute binge drinking on sleep homeostasis last, b) if sleep homeostasis is affected, does it play any role in reducing sleep-promoting effects of subsequent alcohol consumption which we have observed in our recent study (Sharma et al., 2014b), and c) what is the molecular mechanism responsible for altered sleep homeostasis?
Summary and conclusion
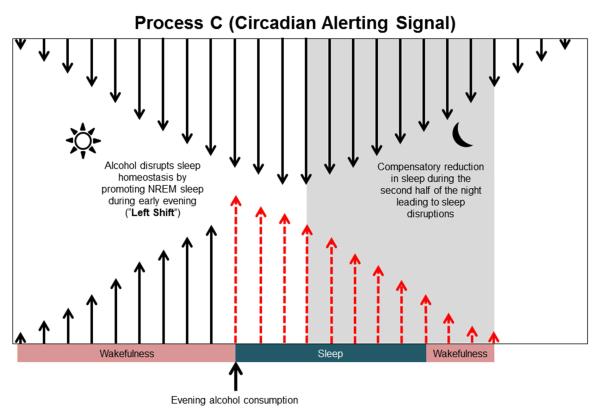
As described above, we have gathered strong and convincing evidence suggesting that alcohol has disruptive effects on sleep homeostasis. We have shown that AD and the wake-promoting BF may be the major cellular substrates responsible for alcohol’s effect on sleep. Additionally, because both AD and the wake-promoting BF are the mediators of sleep homeostasis, it is not surprising that alcohol may mediate its sleep-promoting effects by shifting (“left shift”) the homeostatic drive (Fig. 7). Thus, alcohol consumed during the evening (Dawson, 1996) has a profound NREM sleep-promoting effect and is followed by sleep disruptions, especially during the latter half of the sleep time. We suggest that this may be due to disruption of sleep homeostasis.
Figure 7.
A putative mechanism describing the disruption of sleep homeostasis after alcohol consumption. Since alcohol is a potent somnogen, consumption of alcohol during the evening hours (after 6:00 PM) will promote NREM sleep and disrupt sleep homeostasis (“left shift”), resulting in sleep disruptions manifested by a compensatory reduction in NREM sleep during the second half of the night (see Fig. 3 to compare).
Interestingly, human studies suggest that acute alcohol consumption, (especially moderate to high doses) in non-alcoholics results in strong REM sleep suppression, followed by REM rebound (Ebrahim et al., 2013; Roehrs & Roth, 2001). However, we did not observe a REM sleep-suppressing effect in our rodent studies, most likely because rodents spend less than 4% of their total active (dark) period in REM sleep.
We have also demonstrated that impaired sleep homeostasis is the cause of increased wakefulness and reduced sleep observed in alcohol-dependent rats. Indeed, insomnia, as shown by the presence of increased sleep latency and decreased total sleep, is the most prominent sleep disturbance observed in alcoholics during early recovery (Brower, Aldrich, Robinson, Zucker, & Greden, 2001; Drummond, Gillin, Smith, & DeModena, 1998; Kolla, Mansukhani, & Schneekloth, 2011). There are no reports suggesting that alcoholics, especially during early withdrawal, suffer from sleep disorders of circadian rhythms, such as delayed or advanced sleep phase syndrome or irregular sleep-wake rhythms. In addition, as compared to healthy controls, alcoholics do not show any major differences in dim light melatonin onset, which is a reliable phase marker of circadian rhythm (Conroy et al., 2012).
We want to emphasize that we do not suggest that alcohol has no effect on circadian rhythms. In fact, there is strong and convincing evidence suggesting that alcohol has a profound interaction with circadian rhythms (Agapito, Barreira, Logan, & Sarkar, 2013; Brager, Ruby, Prosser, & Glass, 2011; Chen, Kuhn, Advis, & Sarkar, 2006; Frank et al., 2013; Lindsay, Glass, Amicarelli, & Prosser, 2014; Logan, Williams, & McClung, 2014; Partonen, 2012; Perreau-Lenz & Spanagel, 2008; Prosser, Mangrum, & Glass, 2008; Rosenwasser, 2010; Ruby, Prosser, & Glass, 2006; Seggio, Logan, & Rosenwasser, 2007; Spanagel, Rosenwasser, Schumann, & Sarkar, 2005). We suggest that alcohol may have minimal impact on the circadian regulation of sleep and a more predominant effect on the homeostatic regulation of sleep.
Does alcohol mediate its sleep promoting effects only via AD and BF? Again, we do not want to claim that AD and the BF are the only mediators of alcohol-induced sleep promotion. However, since this area is immensely under-researched, there is very little information available about how and where alcohol acts to promote sleep.
According to the two-process model of sleep, sleep is controlled by two processes: Process C and Process S. Thus, for alcohol to promote sleep, it must act on either Process S or on Process C, or on both Process S and Process C. Based on our findings, we suggest that alcohol disrupts Process S to promote sleep. Since BF and AD are key substrates involved in sleep homeostasis, we suggest that AD and BF may be critical in mediating alcohol-induced sleep. It is entirely possible that, along with AD and BF, alcohol may concurrently (directly or indirectly) inhibit multiple wake-promoting systems [for an example, see Sharma, Sahota, & Thakkar, 2014c] while activating sleep-promoting systems. More research is necessary to understand how and where alcohol acts to promote sleep and cause sleep disruptions.
In conclusion, based on our results, we suggest that alcohol disrupts sleep homeostasis to affect sleep and cause sleep disruptions.
Acknowledgments
This work is supported by resources, including the use of facilities, from Research Services, Harry S. Truman Memorial Veterans Hospital, and funded by research grants (AA020334 and AA0174720) from the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapito MA, Barreira JC, Logan RW, Sarkar DK. Evidence for possible period 2 gene mediation of the effects of alcohol exposure during the postnatal period on genes associated with maintaining metabolic signaling in the mouse hypothalamus. Alcoholism: Clinical and Experimental Research. 2013;37:263–269. doi: 10.1111/j.1530-0277.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABA(A) receptors as molecular sites of ethanol action. Direct or indirect actions? Current Topics in Medicinal Chemistry. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann R, Conroy DA, Arnedt JT, Brower KJ. Effects of a 3-hour sleep delay on sleep homeostasis in alcohol dependent adults. Sleep. 2012;35:273–278. doi: 10.5665/sleep.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Progress in Neurobiology. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Batista LC, Prediger RD, Morato GS, Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology (Berl) 2005;181:714–721. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Research. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. The European Journal of Neuroscience. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcoholism: Clinical and Experimental Research. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, et al. Basis of the gabamimetic profile of ethanol. Alcoholism: Clinical andExperimental Research. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC. Waves associated with eye movement in the awake and sleeping cat. Electroencephalography and Clinical Neurophysiology. 1968;24:532–541. doi: 10.1016/0013-4694(68)90042-4. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. The American Journal of Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. European Archives of Psychiatry and Clinical Neuroscience. 2011;261:559–566. doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Medical Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiological Reviews. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Peever JH. A noradrenergic mechanism functions to couple motor behavior with arousal state. Current Biology. 2013;23:1719–1725. doi: 10.1016/j.cub.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. The Journal of Neuroscience. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neuroscience and Biobehavioral Reviews. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. Journal of Neurochemistry. 2006;97:1026–1033. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nature Neuroscience. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, Baker FC. Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep. 2009;32:1341–1352. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mascia MP, Cuccheddu T, Floris S, Mostallino MC, Perra C, et al. Chronic ethanol intoxication enhances [3H]CCPA binding and does not reduce A1 adenosine receptor function in rat cerebellum. Pharmacology, Biochemistry, & Behavior. 1996;53:249–255. doi: 10.1016/0091-3057(95)00208-1. [DOI] [PubMed] [Google Scholar]
- Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiology International. 2012;29:35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS. Brain adenosinergic modulation of acute ethanol-induced motor impairment. Alcohol and Alcoholism Suppl. 1993;2:425–429. [PubMed] [Google Scholar]
- Dar MS. Mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination: possible involvement of cAMP. Brain Research. 1997;749:263–274. doi: 10.1016/s0006-8993(96)01263-2. [DOI] [PubMed] [Google Scholar]
- Dar MS. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Research Bulletin. 2001;55:513–520. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neuroscience and Biobehavioral Reviews. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Temporal drinking patterns and variation in social consequences. Addiction. 1996;91:1623–1635. doi: 10.1046/j.1360-0443.1996.911116234.x. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcoholism: Clinical and Experimental Research. 2013;37:539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. The Journal of Neuroscience. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M, Erblich B, Bovbjerg DH. Biphasic stimulant and sedative effects of ethanol: are children of alcoholics really different? Addictive Behaviors. 2003;28:1129–1139. doi: 10.1016/s0306-4603(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Current Protocols in Neuroscience. 2008 doi: 10.1002/0471142301.ns0928s44. Chapter 9, Unit 9.28. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Alcohol tolerance in humans is enhanced by prior caffeine antagonism of alcohol-induced impairment. Experimental and Clinical Psychopharmacology. 2003;11:9–17. doi: 10.1037//1064-1297.11.1.9. [DOI] [PubMed] [Google Scholar]
- Frank E, Sidor MM, Gamble KL, Cirelli C, Sharkey KM, Hoyle N, et al. Circadian clocks, brain function, and development. Annals of the New York Academy of Sciences. 2013;1306:43–67. doi: 10.1111/nyas.12335. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neuroscience Letters. 1991;130:141–144. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- Franks HM, Hagedorn H, Hensley VR, Hensley WJ, Starmer GA. The effect of caffeine on human performance, alone and in combination with ethanol. Psychopharmacologia. 1975;45:177–181. doi: 10.1007/BF00429058. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. The Journal of Comparative Neurology. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlow SE, Bentkover SH, Dziedzic SW, Khazan N. Persistence of abnormal REM sleep response to ethanol as a result of previous ethanol ingestion. Psychopharmacologia. 1973;33:135–140. doi: 10.1007/BF00429083. [DOI] [PubMed] [Google Scholar]
- Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Critical Reviews in Neurobiology. 2003;15:235–274. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- Hasenfratz M, Bunge A, Dal Prá G, Bättig K. Antagonistic effects of caffeine and alcohol on mental performance parameters. Pharmacology, Biochemistry, & Behavior. 1993;46:463–465. doi: 10.1016/0091-3057(93)90380-c. [DOI] [PubMed] [Google Scholar]
- Hattan DG, Eacho PI. Relationship of ethanol blood level to REM and non-REM sleep time and distribution in the rat. Life Sciences. 1978;22:839–846. doi: 10.1016/0024-3205(78)90607-0. [DOI] [PubMed] [Google Scholar]
- Hayaishi O, Urade Y, Eguchi N, Huang ZL. Genes for prostaglandin dsynthase and receptor as well as adenosine A2A receptor are involved in the homeostatic regulation of nrem sleep. Archives Italiennes de Biologie. 2004;142:533–539. [PubMed] [Google Scholar]
- Hill SY, Reyes RB. Effects of chronic and acute ethanol administration on sleep in laboratory rats. Journal of Studies on Alcohol. 1978a;39:47–55. doi: 10.15288/jsa.1978.39.47. [DOI] [PubMed] [Google Scholar]
- Hill SY, Reyes RB. Effects of L-tryptophan and ethanol on sleep parameters in the rat. Psychopharmacology (Berl) 1978b;58:229–233. doi: 10.1007/BF00427384. [DOI] [PubMed] [Google Scholar]
- Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–526. [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biological Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–186. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Frontiers in Bioscience. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends in Pharmacological Sciences. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–162. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Sherwood N, Hindmarch I. Separate and combined effects of the social drugs on psychomotor performance. Psychopharmacology (Berl) 1991;104:113–119. doi: 10.1007/BF02244564. [DOI] [PubMed] [Google Scholar]
- Kim A, Latchoumane C, Lee S, Kim GB, Cheong E, Augustine GJ, et al. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20673–20678. doi: 10.1073/pnas.1217897109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BP, Mansukhani MP, Schneekloth T. Pharmacological treatment of insomnia in alcohol recovery: a systematic review. Alcohol and Alcoholism. 2011;46:578–585. doi: 10.1093/alcalc/agr073. [DOI] [PubMed] [Google Scholar]
- Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, et al. Optogenetic stimulation of MCH neurons increases sleep. The Journal of Neuroscience. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss SW, Ghirnikar RB, Diamond I, Gordon AS. Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Molecular Pharmacology. 1993;44:1021–1026. [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcoholism: Clinical and Experimental Research. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Liguori A, Robinson JH. Caffeine antagonism of alcohol-induced driving impairment. Drug and Alcohol Dependence. 2001;63:123–129. doi: 10.1016/s0376-8716(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Lindsay JH, Glass JD, Amicarelli M, Prosser RA. The mammalian circadian clock in the suprachiasmatic nucleus exhibits rapid tolerance to ethanol in vivo and in vitro. Alcoholism: Clinical and Experimental Research. 2014;38:760–769. doi: 10.1111/acer.12303. [DOI] [PubMed] [Google Scholar]
- Logan RW, Williams WP, 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behavioral Neuroscience. 2014;128:387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk WP, Zhang Y, White TD, Lue FA, Wu C, Jiang CG, et al. Adenosine: a mediator of interleukin-1beta-induced hippocampal synaptic inhibition. The Journal of Neuroscience. 1999;19:4238–4244. doi: 10.1523/JNEUROSCI.19-11-04238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Medicine. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Hill SY. Effects of the acute administration of ethanol on the sleep of the rat: a dose-response study. Pharmacology, Biochemistry, and Behavior. 1978;8:723–726. doi: 10.1016/0091-3057(78)90272-1. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Majchrowicz E, Mirmirani N, Dawson S, Gillin JC, Wyatt RJ. Sleep during chronic ethanol administration and withdrawal in rats. Journal of Studies on Alcohol. 1978;39:1213–1223. doi: 10.15288/jsa.1978.39.1213. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Torterolo P, Lagos P. Melanin-concentrating hormone control of sleep-wake behavior. Sleep Medicine Reviews. 2013;17:293–298. doi: 10.1016/j.smrv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Kazerooni M, Simasko SM. Dose-response study of chronic alcohol induced changes in sleep patterns in rats. Brain Research. 2008;1208:120–127. doi: 10.1016/j.brainres.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Simasko SM. Chronic alcohol treatment in rats alters sleep by fragmenting periods of vigilance cycling in the light period with extended wakenings. Behavioural Brain Research. 2009;198:113–124. doi: 10.1016/j.bbr.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Harris RA, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. The Journal of Biological Chemistry. 1990;265:1946–1951. [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacology & Therapeutics. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Partonen T. Clock gene variants in mood and anxiety disorders. Journal of Neural Transmission. 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Spanagel R. The effects of drugs of abuse on clock genes. Drug News & Perspectives. 2008;21:211–217. doi: 10.1358/dnp.2008.21.4.1213350. [DOI] [PubMed] [Google Scholar]
- Phan TA, Gray AM, Nyce JW. Intrastriatal adenosine A1 receptor antisense oligodeoxynucleotide blocks ethanol-induced motor incoordination. European Journal of Pharmacology. 1997;323:R5–R7. doi: 10.1016/s0014-2999(97)00147-7. [DOI] [PubMed] [Google Scholar]
- Phillips AJ, Robinson PA, Kedziora DJ, Abeysuriya RG. Mammalian sleep dynamics: how diverse features arise from a common physiological framework. PLoS Computational Biology. 2010;6:e1000826. doi: 10.1371/journal.pcbi.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Research. 2003;962:68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- Popovici I, French MT. Binge drinking and sleep problems among young adults. Drug and Alcohol Dependence. 2013;132:207–215. doi: 10.1016/j.drugalcdep.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T. Sleep homeostasis. Current Opinion in Neurobiology. 2013;23:799–805. doi: 10.1016/j.conb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- Prospero-García O, Criado JR, Henriksen SJ. Pharmacology of ethanol and glutamate antagonists on rodent sleep: a comparative study. Pharmacology, Biochemistry, and Behavior. 1994;49:413–416. doi: 10.1016/0091-3057(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. The Journal of Pharmacology and Experimental Therapeutics. 1984;228:268–274. [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr., et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain, and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Research & Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9:728–738. doi: 10.1007/s13311-012-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Li Y, Le M, Zhang Y. Nitric oxide-stimulated increase in extracellular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity. The Journal of Neuroscience. 2000;20:6294–6301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Research Reviews. 2009;61:281–306. doi: 10.1016/j.brainresrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neuroscience and Biobehavioral Reviews. 2010;34:1249–1255. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Dall’Ava-Santucci J, Bajenaru O, Emmanouilidis E, Tran G, Manicom R, et al. Effects of muscimol or homotaurine on sleep-wake states in alcohol-dependent rats during withdrawal. Pharmacology, Biochemistry, and Behavior. 1998;59:955–960. doi: 10.1016/s0091-3057(97)00521-2. [DOI] [PubMed] [Google Scholar]
- Ruby C, Prosser RA, Glass JD. Ethanol attenuates photic phase-resetting in Syrian hamsters in vivo. Society of Neuroscience Abstracts. 2006;156:5. [Google Scholar]
- Saeed Dar M. Co-modulation of acute ethanol-induced motor impairment by mouse cerebellar adenosinergic A1 and GABA(A) receptor systems. Brain Research Bulletin. 2006;71:287–295. doi: 10.1016/j.brainresbull.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacology, Biochemistry, and Behavior. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Bradshaw K, Sahota P, Thakkar MM. Acute binge alcohol administration reverses sleep-wake cycle in Sprague Dawley rats. Alcoholism: Clinical and Experimental Research. 2014;38:1941–1946. doi: 10.1111/acer.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. Journal of Neurochemistry. 2010a;115:782–794. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann SC, Sahota P, Thakkar MM. Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcoholism: Clinical and Experimental Research. 2010b;34:813–818. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Nicotine administration in the cholinergic basal forebrain increases alcohol consumption in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2014a;38:1315–1320. doi: 10.1111/acer.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Rapid tolerance development to the NREM sleep promoting effect of alcohol. Sleep. 2014b;37:821–824. doi: 10.5665/sleep.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep. 2014c;37:525–533. doi: 10.5665/sleep.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield KD, Gmel G, Kehoe-Chan T, Dawson DA, Grant BF, Rehm J. Mortality and potential years of life lost attributable to alcohol consumption by race and sex in the United States in 2005. PLoS One. 2013;8:e51923. doi: 10.1371/journal.pone.0051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacology & Therapeutics. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcoholism: Clinical and Experimental Research. 2005;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiology & Behavior. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM. Histamine in the regulation of wakefulness. Sleep Medicine Reviews. 2011;15:65–74. doi: 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;122:1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcoholism: Clinical and Experimental Research. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Research Online. 1999;2:112–120. [PubMed] [Google Scholar]
- Thakkar MM, Sharma R, Engemann SC, Sahota PK. Adenosine and Glycine in REM sleep regulation. In: Mallick BN, Pandi-Perumal SR, McCarley RW, Morrison A, editors. Rapid Eye Movement Sleep: Regulation and Function. Cambridge University Press; Cambridge: 2012. pp. 256–265. [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. The Journal of Neuroscience. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Tao R, Yunren B, Winston S, Chen L, McCarley RW. GABA release in the pontine reticular formation is lowest during REM sleep. Society for Neuroscience Abstracts. 2004;895:5. [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. The Journal of Neuroscience. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticho SR, Stojanovic M, Lekovic G, Radulovacki M. Effects of ethanol injection to the preoptic area on sleep and temperature in rats. Alcohol. 1992;9:275–278. doi: 10.1016/0741-8329(92)90065-i. [DOI] [PubMed] [Google Scholar]
- Tobler I, Borbély AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neuroscience Letters. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- Tobler I, Franken P, Trachsel L, Borbély AA. Models of sleep regulation in mammals. Journal of Sleep Research. 1992;1:125–127. doi: 10.1111/j.1365-2869.1992.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. The Journal of Neuroscience. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Wathen BL, Lydic R, Baghdoyan HA. Endogenous GABA levels in the pontine reticular formation are greater during wakefulness than during rapid eye movement sleep. The Journal of Neuroscience. 2011;31:2649–2656. doi: 10.1523/JNEUROSCI.5674-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol: Clinical and Experimental Research. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Villablanca J, Salinas-Zeballos ME. Sleep-wakefulness, EEG and behavioral studies of chronic cats without the thalamus: the ‘athalamic’ cat. Archives Italiennes de Biologie. 1972;110:383–411. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacology & Therapeutics. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunit-containing receptors. The European Journal of Neuroscience. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Induction of wakefulness and inhibition of active (REM) sleep by GABAergic processes in the nucleus pontis oralis. Archives Italiennes de Biologie. 2001;139:125–145. [PubMed] [Google Scholar]