Abstract
Learned associations between drugs and environment play an important role in addiction and are thought to be encoded within specific patterns of sparsely distributed neurons called neuronal ensembles. This hypothesis is supported by correlational data from in vivo electrophysiology and cellular imaging studies in relapse models in rodents. In particular, cellular imaging with the immediate early gene c-fos and its protein product Fos has been used to identify sparsely distributed neurons that were strongly activated during conditioned drug behaviors such as drug self-administration and context- and cue-induced reinstatement of drug seeking. Here we review how Fos and the c-fos promoter have been employed to demonstrate causal roles for Fos-expressing neuronal ensembles in prefrontal cortex and nucleus accumbens in conditioned drug behaviors. This work has allowed identification of unique molecular and electrophysiological alterations within Fos-expressing neuronal ensembles that may contribute to the development and expression of learned associations in addiction.
Keywords: conditioned cues, Daun02 inactivation, drug environment, self-administration, extinction, prefrontal cortex, nucleus accumbens
1. Neuronal ensembles in addiction
Learned associations play an important role in addiction. With repeated drug use, addicts learn to associate drug effects with stimuli or cues in the drug environment, such as drug paraphernalia, context, and people (Goldberg, 1976; O'Brien et al., 1986; Stewart et al., 1984; Wikler, 1973). Long after cessation of drug use, these cues can promote drug craving and relapse (O'Brien et al., 1986; Siegel, 1999; Wikler, 1973). These associations are complex combinations of many different stimuli or cues that are used to recognize people, places, and events with a high degree of resolution. Thus any neural mechanism capable of encoding these associations must have a comparably high degree of resolution. However, nearly all investigations of the molecular and cellular mechanisms of learning and behavior in addiction research manipulate neural activity or assess molecular and cellular alterations in all neurons, or all neurons of a given cell type, at the resolution of discrete brain sites (Bowers et al., 2010; Hyman et al., 2006; Kalivas et al., 2005; Kalivas, 2009; Koob, 2006; Lu et al., 2006; Mameli and Luscher, 2011; Nestler et al., 1993; Nestler, 2001; Pickens et al., 2011; Russo et al., 2010; White and Kalivas, 1998; Wolf and Ferrario, 2010b). While having important effects on drug-related behaviors, alterations at the level of brain sites do not have sufficient resolution to encode complex highly specific learned associations and cannot distinguish between the many distinct learned associations that are encoded within the same brain site.
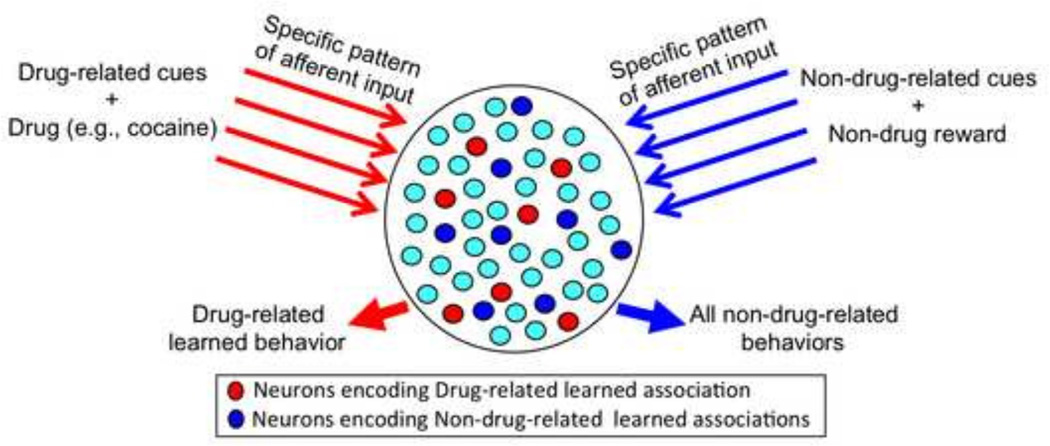
Instead, learned associations are hypothesized to be encoded within sparsely distributed patterns of neurons called neuronal ensembles (Buzsaki and Moser, 2013; Guzowski et al., 2004; Knierim and Zhang, 2012; Nicolelis et al., 1997; Pennartz et al., 1994; Pennartz et al., 2009; Penner and Mizumori, 2012; Schwindel and McNaughton, 2011), an idea based on the ‘cell assemblies’ hypothesis of Donald Hebb (1949). The particular ensemble activated during behavior is determined by the specific activity pattern of afferent inputs that convey information about interoceptive stimuli (from the body), exteroceptive stimuli (from the environment), and previous experience (from association cortex) (Figure 1) (Best et al., 2001; Deadwyler and Hampson, 1995; Deadwyler and Hampson, 1997; Doetsch, 2000; Guzowski et al., 2004; Johnson et al., 2009; Leutgeb et al., 2005; Mittler et al., 1994; Nicolelis et al., 1997). Those neurons that receive the most integrated stimulatory input will be part of the activated ensemble. Considering the small proportion of neurons estimated to compose a neuronal ensemble (approximately 1–5%) (Bossert et al., 2011; Chawla et al., 2005; Cruz et al., 2013; Fanous et al., 2012; Guzowski et al., 1999; Guzowski et al., 2004; Koya et al., 2009) and the thousands to millions of neurons available in a given brain area, then the number of possible ensemble patterns is enormous and capable of encoding learned associations with sufficient resolution. This number is increased further because neuronal ensembles encoding different learned associations can intermingle in the same brain area and individual neurons can participate in different neuronal ensembles (Schwindel and McNaughton, 2011).
Figure 1.
Schematic drawing to illustrate how different activity patterns of stimulatory afferent input to a brain area can select different patterns of neurons called ‘neuronal ensembles’ that mediate distinct learned associations. The longer red arrows symbolize different patterns of afferent input activated by Drug and Drug-related cues while the longer blue arrows symbolize different patterns of afferent input activated by Non-drug rewards and Non-drug-related cues. Those neurons that receive the highest and most persistent levels of integrated stimulatory input will be part of the neuronal ensemble. The red ovals symbolize neurons that receive the highest levels of excitatory drug-related afferent inputs and encode drug-related learned associations that mediate drug-related behaviors while the blue ovals symbolize neurons that receive the highest levels of excitatory non-drug-related afferent inputs and encode non-drug-related learned associations that mediate non-drug-related behaviors. In the real brain, each of these neuronal ensembles is comprised of thousands to millions of neurons in many different brain areas.
In this review, we discuss neuronal ensembles in conditioned drug behaviors such as drug self-administration and context- and cue-induced reinstatement of drug seeking (Crombag et al., 2008b; Pickens et al., 2011). We focus on the prefrontal cortex and nucleus accumbens within the corticostriatal circuitry that play critical roles in conditioned drug behaviors. To support a role for neuronal ensembles in these behaviors, we begin with evidence accumulated from correlations between lever pressing and neuronal activity that was assessed using in vivo electrophysiology or cellular imaging with the immediate early gene (IEG) c-fos and its protein product Fos in activated neurons. The subsequent description of neurobiological mechanisms provides a more detailed understanding of the relationship between neural activity and c-fos gene activation and provides the basis for recently developed c-fos promoter-based techniques that (1) allow causal role evidence for Fos-expressing neuronal ensembles mediating these conditioned drug behaviors and (2) allow us to identify unique molecular and electrophysiological alterations within these ensembles. The overall goal of this review is to provide support for the hypothesis that sparsely distributed Fos-expressing neurons in the corticostriatal circuitry act together as a unit to form neuronal ensembles that encode and mediate conditioned drug behaviors. A more detailed review of the techniques themselves can be found in (Cruz et al., 2013).
2. Correlates of Fos-expressing neuronal ensembles in drug self-administration
In vivo electrophysiology in drug self-administration models
Since the early 1990’s, nearly all studies of neuronal ensembles in addiction examined correlations between single unit recordings of neurons and lever-pressing for intravenous cocaine, heroin, or drug-related cues in self-administration models in rats (Carelli et al., 1993; Carelli and Deadwyler, 1997; Chang et al., 1994; Chang et al., 1996; Deadwyler et al., 2004; Kiyatkin and Brown, 2007; Woodward et al., 2000). In the nucleus accumbens, cocaine or heroin self-administration has both tonic and phasic effects on single unit activity (Carelli and Deadwyler, 1996; Carelli et al., 1999; Carelli, 2002a; Chang et al., 1997b; Chang et al., 1998; Kiyatkin and Rebec, 1996; Kiyatkin and Rebec, 1999; Kiyatkin et al., 2000; Peoples et al., 1998a; Peoples and Cavanaugh, 2003; Peoples et al., 2004). Drug infusions throughout a self-administration session increase dopamine levels that tonically inhibit most neurons but carry low-resolution information (Haracz et al., 1998; Kiyatkin and Rebec, 1996; Kiyatkin, 2002; Peoples et al., 1998b; Peoples et al., 1999; Peoples and Cavanaugh, 2003; Peoples et al., 2004). At the same time, lever-pressing for drugs correlates with increases or decreases of glutamate-mediated phasic firing in response to either drug reward or to cues associated with drug reward (Carelli et al., 1993; Chang et al., 2000; Guillem et al., 2014; Haracz et al., 1998; Kiyatkin and Rebec, 1996; Kiyatkin and Rebec, 1999; Kiyatkin, 2002; Peoples et al., 1997; Wakabayashi and Kiyatkin, 2014). A key finding has been that different rewards such as cocaine, heroin, water, or sucrose induce phasic firing in largely different sets of neurons, which suggests that the stimulus properties of each reward are encoded in distinct ensembles (Cameron and Carelli, 2012; Carelli and Deadwyler, 1994; Carelli, 2002a; Carelli, 2002b; Carelli and Wondolowski, 2003; Chang et al., 1998; Deadwyler et al., 2004; Opris et al., 2009; Roop et al., 2002). Environmental cues associated with drug reward can also induce phasic firing either prior to drug reward presentation or during drug-free conditions (Carelli, 2002a; Chang et al., 1994; Chang et al., 1997b; Chang et al., 1998; Chang et al., 2000; Wheeler et al., 2011). Many of these neurons are activated only by cues that were previously associated with one reward (cocaine) but not by another reward (food) (Carelli and Ijames, 2001; Carelli, 2002b) which suggests that reward-associated cues or contexts are also encoded in distinct ensembles. Similar types of drug- and cue-induced phasic firing have been observed in different areas of the prefrontal cortex (Chang et al., 1997a; Chang et al., 1997b; Chang et al., 1998; Chang et al., 2000; Guillem et al., 2010; West et al., 2014). Overall, these studies support the hypothesis that distinct neuronal ensembles encode different learned associations underlying drug- and non-drug related behaviors.
c-fos and Fos expression in drug self-administration models
Cellular imaging of IEGs, such as c-fos, zif268, and arc, has also been used to provide correlative evidence for neuronal ensembles in addiction. These IEGs are rapidly induced within activated neurons (Cohen and Greenberg, 2008; Herdegen and Leah, 1998; Morgan and Curran, 1991) and their mRNA or protein products have long been used as markers of behaviorally activated neurons. C-fos mRNA and its protein product Fos have been the most commonly used IEG markers of neuronal activity in addiction research (Brenhouse and Stellar, 2006; Crombag et al., 2002; Graybiel et al., 1990; Hope et al., 1992; Konradi et al., 1994; Moratalla et al., 1993; Persico et al., 1993; Steiner and Gerfen, 1993; Young et al., 1991), with the first paper published in 1989 (Robertson et al., 1989). Immunohistochemical assays indicated that Fos expression is increased during cocaine or heroin self-administration throughout the corticostriatal circuitry, including medial prefrontal and orbitofrontal cortex, nucleus accumbens core and shell subregions, and the dorsal striatum (Ben-Shahar et al., 2004; Larson et al., 2010; Madsen et al., 2012; Martin-Garcia et al., 2014; Pich et al., 1997; Thiel et al., 2010; Zahm et al., 2010). Even when drug is not onboard, contextual cues and discrete cues previously paired with drug self-administration activate many of the same prefrontal cortex and striatal areas during reinstatement of lever pressing (Bastle et al., 2012; Bossert et al., 2011; Cruz et al., 2014; Fanous et al., 2012; Fanous et al., 2013; Hamlin et al., 2008; Kufahl et al., 2009; Mahler and Aston-Jones, 2012; Neisewander et al., 2000; Shalev et al., 2003; Zavala et al., 2007; Zhou et al., 2013) or exposure to the paired context alone without lever responding (Doherty et al., 2013). Perhaps due to higher variability, in situ hybridization and quantitative PCR assays for c-fos mRNA identify activation of only a subset of these same brain areas (Celentano et al., 2009; Daunais et al., 1993; Daunais et al., 1995; Hearing et al., 2008a; Hearing et al., 2008b; Koya et al., 2006; Kuntz et al., 2008; Kuzmin and Johansson, 1999). Overall, cellular imaging studies indicate that c-fos mRNA and Fos protein are expressed within sparsely distributed neurons in many of the same prefrontal cortex and nucleus accumbens regions shown with in vivo electrophysiology studies using similar drug and cue conditions in drug self-administration and relapse models.
Neural activity and expression of c-fos and Fos
Although Fos expression and in vivo electrophysiology studies identify similar brain areas during lever responding for cocaine and heroin and related cues, the exact correspondence between Fos expression and electrophysiological activity at the cell level in intact brain of behaving animals is not known. Previous attempts to directly examine the relationship between Fos expression and electrophysiological activity in the striatum and hippocampal dentate gyrus of intact rats suggest that Fos expression correlates with synaptic activity levels and not with action potentials (Labiner et al., 1993; Sgambato et al., 1997). However, we do not know if the electrophysiological recordings in these studies were from Fos-positive or Fos-negative neurons. Indeed, the majority of recorded neurons were likely Fos-negative and thus would have had lower synaptic activity levels that were below the threshold for generating action potentials. Future in vivo electrophysiology experiments need to determine whether Fos expression or c-fos promoter activation is induced in the same neurons being recorded.
Regardless of whether action potentials are necessary for Fos expression or not, strong and persistent synaptic activity leads to both Fos expression and action potentials (Cohen and Greenberg, 2008; Curran and Morgan, 1986; Morgan and Curran, 1986; Morgan and Curran, 1988; Rajadhyaksha et al., 1999; Thomas and Huganir, 2004; Xing et al., 1996). The only difference may be that the synaptic activity threshold required for Fos expression is lower than that required for action potentials. Under these conditions, Fos expression still functions as a marker of the most electrophysiologically active neurons in a brain area, and this set of neurons likely contains most if not all of the neurons that generate action potentials responsible for conditioned behaviors.
3. Neurochemistry of c-fos and Fos expression in addiction research models
In addiction research, most c-fos and Fos expression studies have focused on the striatum, including the nucleus accumbens. The neurotransmitter systems that regulate drug-induced c-fos and Fos expression in striatal medium spiny neurons (MSNs) are similar to those that regulate in vivo electrophysiological activity. We focus here on glutamate and dopamine, the two most highly studied neurotransmitters in addiction research. The excitatory neurotransmitter glutamate mediates drug-induced c-fos induction in striatum of behaving animals; reviewed in (Cohen and Greenberg, 2008; Vanhoutte et al., 1999). NMDA glutamate receptor antagonists block drug-induced Fos expression (Berretta et al., 1992; Ghosh et al., 1994; Konradi et al., 1996; Ohno et al., 1994; Rajadhyaksha et al., 1999; Snyder-Keller, 1991; Torres and Rivier, 1993) while electrical or chemical stimulation of cortical glutamatergic inputs induces Fos expression in striatal neurons (Berretta et al., 1997; Berretta et al., 1999; Canales et al., 2002; Fu and Beckstead, 1992; Gerfen et al., 2002; Liste et al., 1995; Parthasarathy and Graybiel, 1997; Tschanz et al., 1991; Tschanz et al., 1994). Thus glutamate alone can induce c-fos and Fos expression.
Dopamine also plays a role in drug-induced c-fos induction in the striatum (Badiani et al., 1998; Labandeira-Garcia et al., 1996; Paul et al., 1995). Psychostimulant-induced Fos expression requires activation of both D1-type (Berretta et al., 1992; Young et al., 1991) and D2-type (LaHoste et al., 1993; Ruskin and Marshall, 1994) dopamine receptors. However, dopamine alone does not induce Fos expression. Anesthetics such as chloral hydrate or urethane have been used to attenuate glutamatergic input to the striatum during cocaine and amphetamine administration without altering drug-induced dopamine (Kreuter et al., 2004; Ryabinin et al., 2000; Torres and Rivier, 1993). Under these conditions, cocaine-induced dopamine could not induce Fos expression in the absence of glutamate.
The interaction between glutamate and dopamine on Fos expression can be explained, at least for D1-type MSNs, by the upstate-downstate hypothesis that proposes drug-induced dopamine enhances ongoing glutamate-mediated synaptic activity instead of dopamine inducing synaptic activity directly (Nicola et al., 2000; O'Donnell, 2003; Onn et al., 2000; Pennartz et al., 1994; Surmeier et al., 2007). When synapses are sufficiently activated by excitatory glutamatergic inputs on the dendritic spine head, the synapses transform from the downstate to the upstate. Concurrent drug-induced activation of D1-type dopamine receptors on the neck of the spine further enhances activity of the upstate synapses while attenuating activity in downstate synapses (Surmeier et al., 2007). This produces an effect at the systems level where drug-induced dopamine enhances activity of the set of neurons that receive the highest levels of ongoing glutamatergic excitatory input from cue-induced afferents, while attenuating activity in the majority of neurons that are receiving lower levels of excitatory afferent input; the net effect is that drug-induced dopamine enhances signal to noise during information processing in the striatum. Similar evidence for the upstate-downstate hypothesis has been found within the prefrontal cortex using in vivo electrophysiology (O'Donnell, 2003; Surmeier et al., 2007). It should be noted that this upstate-downstate explanation does not work for psychostimulant-induced Fos expression in D2-type MSNs in rats injected outside of their home cage; although dopamine enhancement of glutamatergic input in general still appears to be involved in Fos expression in these neurons (described below in the section entitled ‘cAMP/PKA pathway in c-fos promoter activation in D1-type MSNs’).
In summary, glutamate and dopamine act synergistically to mediate Fos expression. While glutamate alone can activate neural activity to induce Fos, drug-induced dopamine enhances ongoing glutamatergic excitatory input to induce Fos expression. We hypothesize that Fos is expressed in the small number of neurons that received the highest levels of cue-induced glutamatergic excitatory input during conditioned drug behavior.
4. Molecular and cellular mechanisms of drug-induced c-fos promoter activation
We know more about the molecular and cellular mechanisms of c-fos promoter activation in the brain than for almost any other mammalian promoter. Ever since the first demonstrations of c-fos induction in brain (Dragunow et al., 1987; Morgan et al., 1987), thousands of papers and many excellent reviews (Brami-Cherrier et al., 2009; Cohen and Greenberg, 2008; Farivar et al., 2004; Herdegen and Leah, 1998; Hughes et al., 1999; Lyons and West, 2011; Morgan and Curran, 1991; Nestler, 2001) have been written on the subject. Many of these studies used cell or slice cultures that were critical for determining candidate mechanisms, but not all these mechanisms are necessarily operating in the intact brains of behaving animals. Thus we attempt, whenever possible, to emphasize data confirmed using intact brains in behaving animals or slices from adult brains. In addition, we have not included data from knockout mice because these data are difficult to interpret due to the likely occurrence of many compensatory alterations induced within these mice that are unknown and may or may not be directly related to the deleted target gene. Lastly we refer to ‘c-fos promoter activation’ in this section because drug-induced c-fos and Fos expression levels are determined primarily by molecular and cellular activation of the c-fos promoter to induce transcription (Morgan and Curran, 1991).
The c-fos promoter
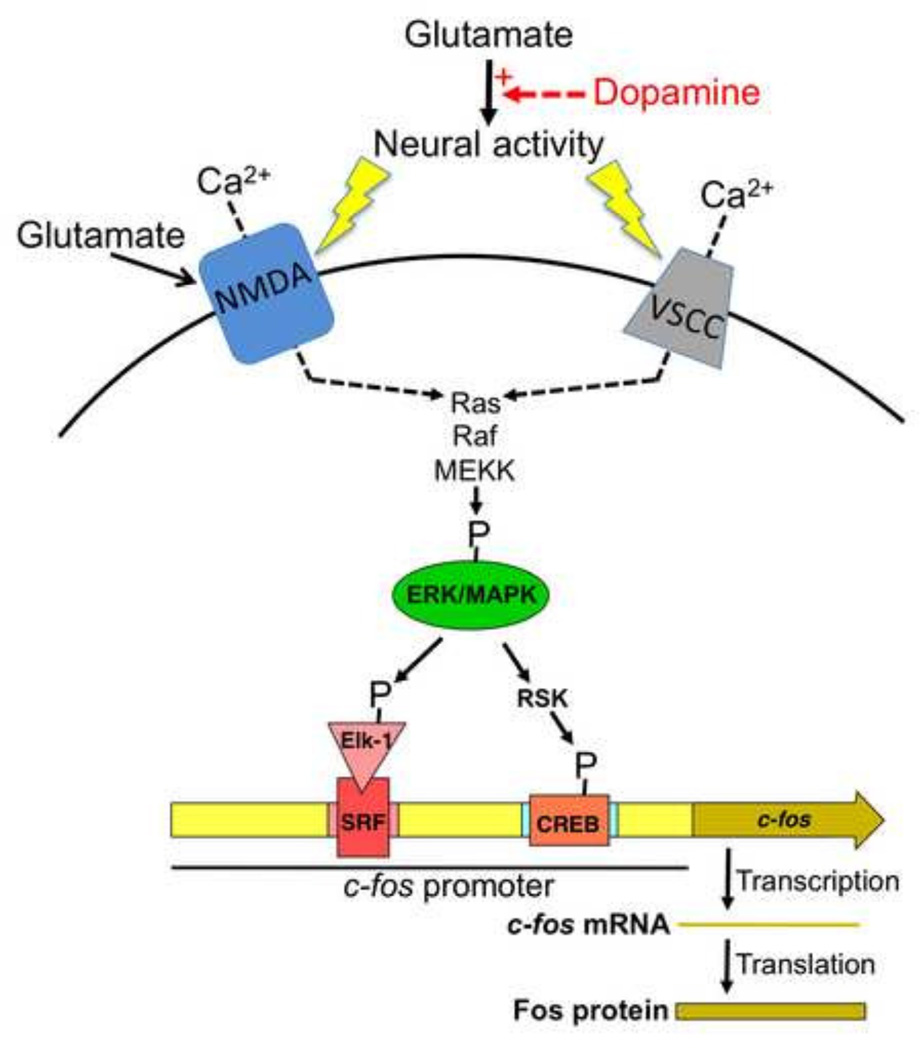
The two most critical components of the c-fos promoter for neural activity-induced expression are the serum response element (SRE) and the calcium response element (CaRE) (Figure 2); reviewed in (Chen et al., 1992; Cohen and Greenberg, 2008; Herdegen and Leah, 1998; Morgan and Curran, 1991). The transcription factor proteins serum response factor (SRF) and Elk-1 bind constitutively as a complex to the SRE while CREB binds constitutively to the CaRE. Ultimately both Elk-1 and CREB need to be phosphorylated to activate the c-fos promoter. The CaRE site is sometimes called the cyclic AMP response element (CRE), but since the cAMP/PKA pathway appears to play only an indirect role in activating CREB in the intact brain in addiction research models (see cAMP/PKA section below), we refer to this site only as CaRE.
Figure 2.
Schematic drawing to illustrate the neurochemistry and molecular mechanisms of c-fos and Fos expression in strongly activated neurons in prefrontal cortex and nucleus accumbens of awake behaving animals. Glutamate is the main excitatory neurotransmitter that increases neural activity. Dopamine enhances (red arrow and + sign) glutamate-mediated neural activation of the small proportion of neurons that have the highest levels of neural activity while inhibiting neural activation of the majority of neurons that have lower levels of neural activity. The detailed electrophysiological mechanisms underlying this glutamate-dopamine interaction are described in (Surmeier et al., 2007). Strong persistent neural activity induces calcium (Ca2+) influx through NMDA-type glutamate receptors and voltage-sensitive calcium channels (VSCCs) to levels that are sufficient for phosphorylating and activating ERK/MAPK via the Ras-Raf-MEKK pathway. ERK/MAPK activation leads to phosphorylation of Elk-1 that is associated with serum response factor (SRF) as well as phosphorylation of CREB via ribosomal S6 kinase (RSK). Elk-1/SRF and CREB are transcription factors that, when phosphorylated, can induce transcription of the coding sequence for c-fos. Transcribed c-fos mRNA and the translated protein product Fos can be used as markers of strongly activated neurons.
A critical role for calcium-dependent activation of the ERK/MAPK pathway
Drug-induced c-fos promoter activation in the brains of awake behaving rats is mediated primarily by calcium-dependent activation of the ERK/MAPK pathway (Bading et al., 1997; Bertran-Gonzalez et al., 2008; Brami-Cherrier et al., 2005; Chen et al., 1992; Cohen and Greenberg, 2008; Ferguson and Robinson, 2004; Mattson et al., 2005; Miller and Marshall, 2005; Radwanska et al., 2005; Sgambato et al., 1998a; Sgambato et al., 1998b; Valjent et al., 2000). Recent excellent reviews of the role of this pathway in corticostriatal circuitry can be found in (Brami-Cherrier et al., 2009; Cahill et al., 2014). In brief, neural activity initiates calcium influx through NMDA receptors and L-type voltage-sensitive calcium channels (VSCC) to activate a variety of calcium-dependent molecules, including Ras-GRP, that mediate calcium-dependent activation of the Ras/Raf kinase pathway that phosphorylates and activates ERK (Agell et al., 2002; Cahill et al., 2014) (Figure 2). Phosphorylated ERK then translocates to the nucleus where it can phosphorylate the transcription factors Elk-1 and CREB (via RSK: ribosomal S6 kinase) on the c-fos promoter (Besnard et al., 2011; Cahill et al., 2014; Chen et al., 1992; Cohen and Greenberg, 2008; Morgan and Curran, 1991).
As described in the previous neurochemistry section, glutamate and dopamine can act synergistically to depolarize neurons and induce calcium influx and ERK activation. Similarly, any neurochemical system that regulates electrophysiological activity and intracellular calcium levels, such as inhibitory receptors (e.g., GABAergic), excitatory receptors (e.g., acetylcholinergic), or modulatory receptors (e.g., presynaptic mGluR2/3 receptors), can affect ERK-mediated c-fos promoter activation (Herdegen and Leah, 1998; Lyons and West, 2011).
One of the key aspects of neuronal activation of the ERK pathway is that it requires consistent (not sporadic) high levels of calcium influx (Blank et al., 2004; Morgan and Curran, 1988; Xing et al., 1996). Thus, ERK-dependent activation of c-fos reflects a summation of activity-dependent calcium influx over seconds to minutes. Only strong consistent activity over this timeframe increases calcium levels past the threshold required to activate the c-fos promoter (Deisseroth and Tsien, 2002; Deisseroth et al., 2003; Ghosh et al., 1994; Konradi et al., 1996; Mermelstein et al., 2000; Morgan and Curran, 1989; Murphy et al., 2002; Wu et al., 2001). This mechanism agrees with the hypothesis that the c-fos promoter is induced in only the most consistently and strongly activated neurons observed with in vivo electrophysiology studies.
Possible alternative calcium-dependent signaling pathways
Other calcium-dependent intracellular signaling pathways (distinct from the ERK/MAPK pathway) may regulate c-fos promoter activation. Numerous cell and slice culture studies indicate that calcium-dependent CaM kinase IV can rapidly phosphorylate CREB within the first few minutes of neural activity before ERK activation takes over the dominant role; reviewed in (Deisseroth and Tsien, 2002). Although group I mGluR-mediated release of calcium from intracellular stores appears not to play a significant role in ERK activation or c-fos promoter activation (Choe et al., 2002; Wang et al., 2007), group I mGluR receptors are able to activate the ERK pathway using a novel calcium-independent Homer protein-dependent pathway (Mao et al., 2005; Yang et al., 2004). Another calcium-dependent molecule called DREAM can bind to and constitutively inhibit the c-fos promoter (Carrion et al., 1999). Increased levels of calcium during neural activity can bind to DREAM and attenuate DREAM-mediated inhibition of the c-fos promoter. While these alternative calcium-dependent pathways may play a role in c-fos promoter activation, they await confirmation in the intact brains of behaving (non-knockout) animals.
cAMP/PKA pathway in c-fos promoter activation in D1-type MSNs
Psychostimulant induction of c-fos and Fos in D1-type MSNs (Berretta et al., 1993; Bertran-Gonzalez et al., 2008) suggests a role for D1 dopamine receptor stimulation of the cAMP/PKA pathway in c-fos promoter activation. The D1/cAMP/PKA pathway can act synergistically with the glutamate/calcium/ERK pathway by a combination of electrophysiological and biochemical mechanisms. The electrophysiological mechanism is based on the upstate-downstate hypothesis described above. Dopamine-induced cAMP/PKA pathway enhances the electrophysiological effects of ongoing glutamatergic input onto MSN dendritic spines by modulating ion channels within synapses (Surmeier et al., 2007) that increases calcium-dependent ERK activation leading to c-fos promoter activation. One biochemical mechanism involves D1/cAMP/PKA phosphorylation of NMDA receptors in the synapse to enhance activation of the NMDA/calcium/ERK pathway (David et al., 2014; Ron, 2004). Another biochemical mechanism proposes that D1/cAMP/PKA activates a DARPP32-mediated mechanism that indirectly enhances NMDA/calcium activation of the ERK pathway (Valjent et al., 2005). However this mechanism is dependent largely on experiments with knockout mice that need to be validated in wild-type mice.
Enhanced glutamate-induced ERK in c-fos promoter activation in D2-type MSNs
When psychostimulants are administered to rats (but not mice) outside their home cage, as done in most behavioral assays, then c-fos and Fos are induced equally in D1-and D2-type MSNs (Badiani et al., 1999; Jaber et al., 1995; Mattson et al., 2005; Mattson et al., 2007; Uslaner et al., 2001); reviewed in (Badiani and Robinson, 2004). Since D2 receptor activation reduces cAMP levels (Stoof and Kebabian, 1981), it is unlikely that cAMP is necessary for c-fos promoter activation in these neurons. Badiani and Robinson have shown that excitatory glutamatergic inputs from the cortex to the striatum are much more active in rats outside their home cages than in rats kept in their highly habituated home cage environment (Badiani et al., 1998; Ferguson and Robinson, 2004). Since activation of glutamatergic inputs from the cortex to the striatum preferentially induce Fos in D2-type MSNs (Berretta et al., 1992; Berretta et al., 1997; Parthasarathy and Graybiel, 1997), psychostimulant-induced Fos expression in D2-type MSNs in rats injected outside their home cage is likely due to increased excitatory glutamatergic input and activation of calcium-dependent ERK in these neurons (Ferguson and Robinson, 2004).
Signaling from the synapse to the nucleus
One of the more important questions is how information about synaptic activity in dendritic spines is conducted to the nucleus for c-fos promoter activation (reviewed in (Bengtson and Bading, 2012; Bito et al., 1997; Matamales, 2012; Wiegert and Bading, 2011). Locally within dendritic spines, glutamatergic input can depolarize the synapse and activate NMDA and voltage-gated calcium channels that increase calcium influx and activate ERK and CaM kinase signaling to produce significant local effects within the activated spine (Bowers et al., 2010; Citri and Malenka, 2008; Luscher et al., 2000). Distinct from local regulation of synaptic function, some of the activated molecules (e.g., phospho-ERK) may be transported from the dendritic spines to the nucleus to activate the c-fos promoter (Deisseroth and Tsien, 2002; Hardingham et al., 2001; Zhai et al., 2013). However, the relatively long distances from these dendritic spines to the nucleus and the rapid activation of the c-fos promoter within minutes suggests that other mechanisms may be involved (Matamales, 2012). One possibility involves calcium-induced calcium release from endoplasmic reticulum (calcium waves) (Matamales, 2012). A simpler electrophysiological explanation is that synaptic depolarization events in dendritic spines are conducted rapidly by well-established cable properties of dendrites to the cell soma that integrates these depolarization events (Carter et al., 2007; Hille, 1992; Matamales, 2012). Once the depolarization threshold for activation of voltage-gated calcium channels is reached in the cell soma, calcium influx into the soma can activate the ERK (and CaM kinase IV) pathway and activate the c-fos promoter in the nucleus (Bengtson and Bading, 2012; Bito et al., 1997; Cahill et al., 2014).
Summary for c-fos promoter activation in addiction research models
The described neurochemical and molecular biological mechanisms all support the idea that c-fos promoter activation is an indicator of strong and persistent calcium influx into synapses of MSNs that received the most excitatory glutamatergic input. Drug-induced dopamine can synergistically enhance c-fos promoter activation in these strongly activated MSNs. It should be noted that a lack of Fos expression in a neuron does not imply a complete lack of neural activity, only that it is not depolarized strongly or persistently enough to produce enough intracellular calcium to activate the ERK signalling pathway. The earlier section describing in vivo electrophysiology and cellular imaging studies indicate that the neurons receiving the most excitatory glutamatergic input are determined by the context and cues present during drug administration. Altogether these data support the hypothesis that Fos-expressing neurons in corticostriatal circuitry can act together as a unit to form neuronal ensembles that encode and mediate conditioned drug behaviors. It is important to note that we treat c-fos and Fos only as markers of activated neurons in this hypothesis and do not imply that these molecules are directly involved in neuronal ensemble function. To test our hypothesis, we developed several techniques that exploit c-fos promoter activation in behaviorally activated neurons.
4. Causal roles for neuronal ensembles in behavior
In one sense, we have been able to identify sparsely distributed neuronal ensembles using c-fos mRNA and Fos protein expression for a long time. However, we have not been able to test the hypothesis until very recently. To examine causal roles for neuronal ensembles in learned behavior, one requires a method for manipulating one set of sparsely distributed neurons that were activated specifically by one set of stimuli without affecting surrounding neurons activated by distinct sets of stimuli. Existing inactivation methods, such as lesions, reversible inactivation with GABAergic agonists, receptor antagonists, or optogenetic and DREADD technologies (Rogan and Roth, 2011) either inactivate most neurons or selected neurons based on cell type, regardless of their activation state during behavior or stimuli presentation. To selectively manipulate behaviorally activated neuronal ensembles, we exploited the selective activation of the c-fos promoter in neurons strongly activated during behavior.
Daun02 inactivation was the first procedure to demonstrate a causal role for endogenously produced neuronal ensembles in a learned behavior (Bossert et al., 2011; Cruz et al., 2014; Fanous et al., 2012; Koya et al., 2009). This procedure, reviewed in (Cruz et al., 2013), inactivates only neurons that were previously activated during a specific behavior or during reactivation of a specific memory (Koya et al., 2009). We used c-fos-lacZ transgenic rats with a transgene that contains a c-fos promoter that induces transcription of the lacZ coding sequence, leading to translation of the protein product β-galactosidase (βgal) in strongly activated neurons, but not in surrounding non-activated neurons (Kasof et al., 1995a; Kasof et al., 1995b; Kasof et al., 1996). Once β-gal is the induced in strongly activated neurons, the inactive prodrug Daun02 is injected into the brain area of interest where it is catalyzed by β-gal to the active product daunorubicin that appears to induce apoptotic cell death of the previously activated neurons (Bakina and Farquhar, 1999; Farquhar et al., 2002; Ghosh et al., 2000). In Daun02 inactivation experiments, c-fos-lacZ rats are exposed on ‘Induction day’ to the previously drug-associated training- or extinction-related context and cues, or a novel environment as a control condition, for 15–30 min to induce β-gal protein in the activated neurons. Daun02 is injected into the brain 90 min later when β-gal levels are maximal (Koya et al., 2009). On ‘Test day’, three days later, the rats are exposed to training- or extinction-related context and cues to assess whether inactivation of the previously activated βgal-expressing neurons altered the ability of the cues and contexts to reactivate the same neurons and induce context-specific behavior.
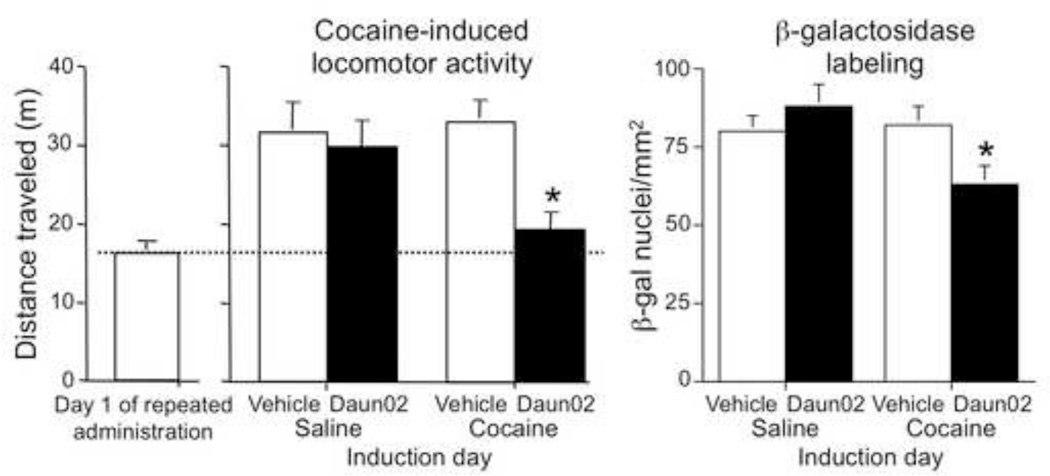
The first application of the Daun02 inactivation procedure was context-specific sensitization of cocaine-induced locomotor activity where the sensitized response is expressed in only the drug-paired environment (A) and not in a non-drug-paired environment (B) (Anagnostaras and Robinson, 1996; Badiani and Robinson, 2004; Crombag et al., 2002; Hope et al., 2006; Koya et al., 2009; Marin et al., 2009; Mattson et al., 2008; Robinson et al., 1998; Stewart et al., 1984; Stewart and Vezina, 1991; Uslaner et al., 2003; Vezina and Stewart, 1984; Vezina et al., 1989). We first found that context-specific cocaine-induced locomotion induced Fos in ~3% of nucleus accumbens neurons. In the next experiment, intra-accumbens Daun02 injections 90 min after cocaine-induced reactivation of the drug-paired neuronal ensemble in the drug-paired environment (A) on Induction day attenuated subsequent cocaine-induced reactivation of the drug-paired ensemble and the sensitized locomotor response in the drug-paired environment (A) on Test day (Figure 3). In control animals, however, intra-accumbens Daun02 injections 90 min after cocaine-induced activation of a non-drug-paired neuronal ensemble in a non-drug-paired environment (B) on Induction day did not attenuate subsequent cocaine-induced reactivation of the drug-paired ensemble or the sensitized locomotor response in the drug-paired context (A) on Test day. This indicates that activation of a context-specific pattern of accumbens neurons is necessary for context-specific sensitization of cocaine-induced locomotion in rats.
Figure 3.
Daun02 inactivation of Fos-expressing neuronal ensembles in rat nucleus accumbens attenuated sensitized cocaine-induced locomotion and β-galactosidase-labeled neural activity (shown on the y-axes) on Test day only when Daun02 was injected into the nucleus accumbens following cocaine injections to rats in the Cocaine-paired locomotor activity chamber on Induction day. The x-axes indicate conditions used on Induction day three days prior to Test day. On induction day, Vehicle or Daun02 was injected into the nucleus accumbens 90 min following systemic injections of Saline or Cocaine to rats. Daun02 did not attenuate cocaine-induced locomotion or neural activity when non-drug-associated neurons were activated in the Saline-injected rats. The column on the left indicates cocaine-induced locomotion on the first day of repeated injections during sensitization training, and is provided only to show that Daun02 attenuated cocaine-induced locomotion on test day in sensitized rats to approximately the same level as that seen in non-sensitized rats. * indicates a significant difference (p<0.05) between Vehicle and Daun02 groups following cocaine injections three days earlier on induction day. Modified figure from (Koya et al., 2009).
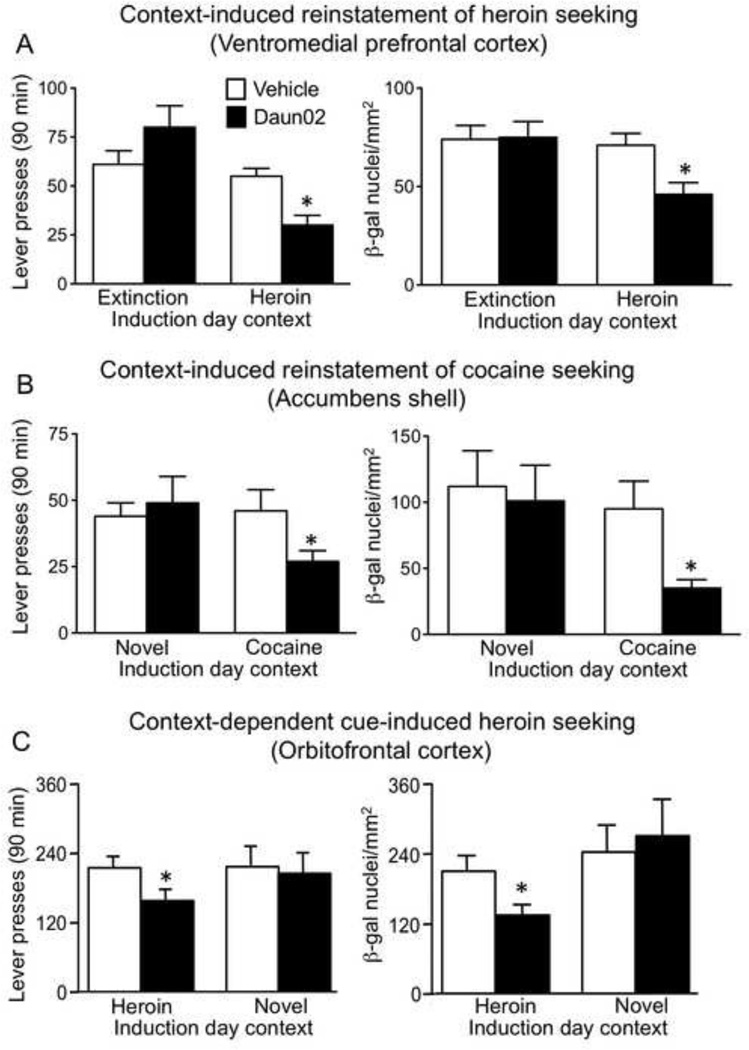
We then used the Daun02 inactivation procedure in two studies that assessed functional roles for neuronal ensembles in a rat model of drug relapse, called context-induced reinstatement of drug seeking (Crombag et al., 2008a), which is based on the ABA renewal model (Bouton and Bolles, 1979). Rats were first trained to self-administer drugs in one context (A); each drug infusion was paired with discrete cues (light or light+tone). Then drug-reinforced lever responding in the presence of the discrete drug cue was extinguished in a non-drug context (B). Subsequently, context-induced reinstatement of drug seeking was assessed by re-exposing rats to the discrete cues in the drug-associated (A) context (Crombag and Shaham, 2002). We specifically assessed roles for ventral mPFC ensembles in context-induced reinstatement of heroin seeking (Bossert et al., 2011) and for accumbens shell ensembles in context-induced reinstatement of cocaine seeking (Cruz et al., 2014). We found that context-induced reinstatement of heroin seeking correlated with Fos induction in ~6% of ventral mPFC neurons (Bossert et al., 2011) while context-induced reinstatement of cocaine seeking correlated with Fos induction in ~3.4% of accumbens shell neurons (Cruz et al., 2014). In the next set of experiments, we found that prior Daun02 injections into ventral mPFC or accumbens shell after exposure to the drug-associated context (A) decreased subsequent context-induced β-gal expression and reinstatement of drug seeking in context A (Figure 4A and 4B). Daun02 injections following exposure to the control extinction-associated context (B) or to a novel context (C) did not alter β-gal expression or reinstatement of drug seeking in context A on test day. These data indicate that the ability of specific cues and contexts to induce reinstatement of drug seeking is mediated by specific patterns of Fos-expressing neuronal ensembles that are selected by these cues and contexts.
Figure 4.
Daun02 inactivation of Fos-expressing neuronal ensembles in different drug relapse models in rats. Lever-pressing behavior on test day is indicated on the y-axes of graphs in the left-hand column. Neural activity, indicated by number of β-galactosidase (β-gal)-labeled neurons, on test day is indicated on the y-axes of graphs in the right-hand column. The x-axes indicate conditions used on Induction day three days prior to Test day. Panel A: Daun02 (but not Vehicle) injections into ventromedial prefrontal cortex (vmPFC) following exposure to the Heroin context (but not the Extinction context) on Induction day attenuated context-induced reinstatement of heroin seeking (lever pressing) and neural activity three days later on Test day. Modified figure from (Bossert et al., 2011). Panel B: Daun02 (but not Vehicle) injections into nucleus accumbens shell following exposure to the Cocaine context (but not to a Novel context) on Induction day attenuated context-induced reinstatement of cocaine seeking (lever pressing) and neural activity three days later on Test day. Modified figure from (Cruz et al., 2014). Panel C: Daun02 (but not Vehicle) injections into orbitofrontal cortex (OFC) following exposure to the Heroin context (but not to a Novel context) on Induction day attenuated cue-induced heroin seeking (lever pressing) and neural activity three days later on Test day. Modified figure from (Fanous et al., 2012). * indicates a significant difference (p<0.05) between Vehicle and Daun02 groups following exposure to the drug context three days earlier on induction day.
We also used Daun02 to demonstrate a causal role for orbitofrontal cortex (OFC) neuronal ensembles in cue-induced heroin seeking (Fanous et al., 2012). Rats were first trained to lever press for heroin paired with discrete cue (light+tone) presentation and then kept in their home cage for 14 d. On test day, re-exposure to the discrete cues in the training context under extinction conditions increased lever responding and induced Fos expression in ~12% of OFC neurons. In the next experiment, we used the Daun02 inactivation procedure to assess a causal role for context-dependent Fos-expressing neuronal ensembles in the lateral OFC in cue-induced heroin seeking. We found that prior injections of Daun02 into OFC after exposure to the heroin-associated environment attenuated subsequent cue-induced β-gal expression and heroin seeking on test day (Figure 4C). In contrast, prior Daun02 injections after exposure to a novel control environment (without levers or the discrete cues to activate a different pattern of OFC neurons) had no effect on subsequent cue-induced β-gal expression or heroin seeking on test day.
In all four studies, the drug-associated contexts and cues increased Fos expression in prefrontal cortex and nucleus accumbens. Selective inactivation of these Fos-expressing neurons in the test groups attenuated subsequent reactivation of these neurons and drug seeking behavior when exposed again to the drug-associated contexts and cues on test day. Inactivation of Fos-expressing neurons that were activated by alternate contexts or cues in the control groups did not attenuate subsequent reactivation of the drug-associated neurons or behaviors on test day. These studies provide evidence that drug-associated contexts and cues activate specific patterns of Fos-expressing neuronal ensembles that mediate the conditioned drug behaviors. They also provide the rationale for identifying unique molecular, structural, and electrophysiological alterations within Fos-expressing neurons.
5. FACS sorting of selectively activated neuronal ensembles
Based on the above studies, molecular neuroadaptations within context- and cue-selected Fos-expressing neurons are likely to play unique and important roles in conditioned drug behaviors. We recently developed an antibody-based fluorescence-activated cell sorting (FACS) method to purify activated Fos-expressing neurons and assess gene expression (Guez-Barber et al., 2011; Guez-Barber et al., 2012), similar to the previously described GFP expression-based FACS method (Lobo et al., 2006; Lobo, 2009). In our method, reviewed in (Cruz et al., 2013), cells from adult brain tissue are enzymatically dissociated and then neurons are labeled with fluorescent NeuN antibodies while strongly activated neurons are labeled with fluorescent Fos or β-gal antibodies. The labeled cells are forced to pass single file through a narrow flow cell in a flow cytometer and sorted according to their light-scattering and immunofluorescent characteristics. Gene expression can then be assessed using qPCR and microarrays.
Until recently, nearly all molecular neuroadaptations had been found in brain homogenates that did not distinguish between neurons activated or not activated during behavior (Kalivas, 2009; Koob et al., 2004; Nestler et al., 1993; Nestler, 2001; Nestler, 2005; Olive et al., 2012). It had long been speculated that gene expression in the small proportion of neurons activated during behavior may be diluted or masked using these brain homogenates. To test this idea, acute methamphetamine was injected rats and IEG expression in brain homogenates was compared with IEG expression in FACS-separated Fos-positive and Fos-negative neurons in the dorsal striatum (Liu et al., 2014). Methamphetamine induced 3–20-fold increases of the IEGs arc, homer-2, c-fos, fosB and its isoforms (ΔfosB and a novel isoform ΔfosB-2) in Fos-positive, but not Fos-negative neurons, while IEG mRNA induction was 10-fold lower or absent when assessed in unsorted samples from single dorsal striatum homogenates. Thus FACS is useful for identifying unique alterations in behaviorally activated neurons.
FACS was used to assess unique gene alterations in β-gal and Fos-expressing striatal neurons in context-specific sensitization of cocaine-induced locomotion (Guez-Barber et al., 2011). Fos-expressing neuronal ensembles in nucleus accumbens in ventral striatum had already been shown to mediate context-specific sensitization of cocaine-induced locomotion (Koya et al., 2009). Cocaine-induced gene expression in activated striatal neurons following FACS purification of β-gal neurons from c-fos-lacZ transgenic rats 90 min after test injections of cocaine to naïve rats or cocaine-sensitized rats and compared this to gene expression in control rats that received test injections of saline (Guez-Barber et al., 2011). Microarray and qPCR analyses indicate that expression of the IEG activity markers arc, fosB, and nr4a3 were increased in activated neurons from cocaine-injected rats but not in non-activated neurons from the same cocaine-injected rats or in all neurons from saline-injected rats. Notably, gene expression was not significantly different between the two control groups: non-activated neurons from cocaine-injected rats and all neurons from saline-injected rats.
FACS was also used to assess gene expression in Fos-expressing neurons from the prefrontal cortex, including the OFC, following cue-induced heroin seeking in rats (Fanous et al., 2013). OFC neuronal ensembles were previously shown to play a causal role in mediating cue-induced heroin seeking after prolonged withdrawal (Fanous et al., 2012). Rats were first trained to self-administer heroin and then remained in their home cages for 14–30 days. On test day, half of the rats were re-exposed to the training context for 90 min under extinction conditions, while control rats were kept in their home cage. After FACS separation of Fos-positive and negative neurons, qPCR analyses indicated that cue-induced heroin seeking increased expression of the IEG activity markers arc, fosB, egr1, and egr2 in Fos-positive neurons but not in Fos-negative neurons from the same ‘test’ rats or inall neurons from the ‘no test’ rats that were left in the home cage after drug self-administration training. Again, gene expression was not significantly different between the two control groups: non-activated neurons from rats exposed to the heroin cue and all neurons from rats kept in their home cages.
In both studies, IEGs were induced only within the behaviorally activated Fos-expressing neurons and not at all in the Fos-negative neurons. This all-or-nothing activation of IEGs in Fos-expressing neurons does not fit with the more graduated range of neuronal activity levels observed from in vivo electrophysiology experiments. As suggested above in our section on neurobiology of c-fos promoter activation, it is likely that only a small proportion of neurons experience enough strong and consistent activation so that calcium influx crosses the threshold for induction of c-fos and other IEGs. It is notable that the IEGs induced selectively in Fos-expressing neurons are either transcription factors (e.g., fosB) capable of inducing further waves of gene expression or involved in synaptic adaptations (e.g., arc and homer). It is possible that only this small proportion of Fos-expressing neurons receive sufficiently high levels of integrated stimulatory input during learning and memory retrieval to undergo unique adaptations that may contribute to the development and consolidation of learned associations.
6. Electrophysiological alterations in activated neuronal ensembles
Alterations in synaptic strength are thought to be the main cellular mechanism underlying learning (Bowers et al., 2010; Bredt and Nicoll, 2003; Luscher and Malenka, 2011; Martin et al., 2000). However, these previous studies examined drug-induced global alterations in randomly selected neurons regardless of their activation state during drug-related behavior. As discussed above, alterations found in the general population do not have the resolution to encode specific learned behaviors. We had already shown that Fos-expressing accumbens neurons are necessary for context-specific sensitization of cocaine-induced locomotion while the majority of accumbens neurons are less directly involved (Koya et al., 2009). Thus, we assessed whether unique synaptic alterations were induced in Fos-expressing accumbens neurons that were strongly activated during cocaine-induced locomotion in sensitized animals and compared them to alterations in the surrounding non-activated accumbens neurons (Koya et al., 2012).
We used c-fos-GFP transgenic mice that were previously developed by Alison Barth (Barth et al., 2004; Barth, 2007; Benedetti et al., 2012; Clem and Barth, 2006; Yassin et al., 2010). The transgene contains a c-fos promoter that induces green fluorescent protein (GFP) in strongly activated Fos-expressing neurons in electrophysiological slice preparations. Cocaine injections on test day induced silent synapses only in GFP-expressing accumbens neurons in mice that were previously sensitized with 5 once daily injections of cocaine (Koya et al., 2012). Silent synapses contain functional NMDA receptors but do not have functional AMPA receptors (Brown et al., 2011; Huang et al., 2009; Lee and Dong, 2011; Lee et al., 2013). In contrast, cocaine injections on test day did not induce silent synapses in GFP-expressing neurons in mice that were not sensitized with repeated cocaine injections (Koya et al., 2012). Thus repeated cocaine injections during sensitization are necessary for silent synapse formation in behaviorally activated neuronal ensembles.
The silent synapses induced in our activated GFP-expressing neurons have different characteristics (Koya et al., 2012) than those induced in randomly selected accumbens neurons following repeated cocaine injections (Brown et al., 2011; Huang et al., 2009; Lee and Dong, 2011; Lee et al., 2013). NMDARs in silent synapses from these randomly selected neurons contain increased levels of GluN2B subunits that are thought to indicate the formation of new synapses with NMDA receptors but no functional AMPA receptors. In contrast silent synapses in our GFP-expressing neurons do not have GluN2B-containing NMDA receptors (Koya et al., 2012). We speculate that silent synapses on GFP-expressing neurons are due instead to AMPA receptor endocytosis, a commonly observed compensatory response to strong synaptic activation (Loweth et al., 2014).
Silent synapses may be just one stage in a dynamic regulation of nucleus accumbens synapses following psychostimulant injections, similar to that observed in the general population of accumbens neurons (Kourrich et al., 2007; Wolf and Ferrario, 2010b). Repeated cocaine injections to rats during sensitization enhance responsiveness of context-specific nucleus accumbens neurons to subsequent cocaine test injections resulting in enhanced Fos expression on test day (Crombag et al., 2002; Hope et al., 2006; Koya et al., 2009; Mattson et al., 2007). We hypothesize that repeated cocaine injections to mice during sensitization similarly enhance responsiveness of selectively activated synapses on accumbens neurons. Strong activation of these synapses on test day initially produces strong activation of these neurons and GFP expression while synapses on the surrounding neurons are less activated and do not induce GFP. AMPA receptors then undergo endocytosis as a compensatory response to stronger activation of the synapses on these GFP-expressing neurons (Bowers et al., 2010; Carroll et al., 2001; Huganir and Nicoll, 2013) that we detect as silent synapses. Previous work suggests that this compensatory response is temporary and that silent synapse will return to a heightened state of responsiveness following drug abstinence (Kourrich et al., 2007), perhaps due to incorporation of more highly conducting calcium-permeable GluR2-lacking AMPA receptors into these synapses (Boudreau and Wolf, 2005; Wolf and Ferrario, 2010a), so that they can induce Fos and GFP expression again. Unfortunately we cannot observe the entire time course of this process in selectively activated neuronal ensembles because they are not labeled with GFP prior to the cocaine injection on test day or for more than 4–6 hours after test day injections (Koya et al., 2012). Nevertheless enhanced neurotransmission at these synapses may play an important role in long-term enhanced responsiveness of neuronal ensembles to drug-paired contexts and cues.
7. Summary
We provided correlational and causal role evidence for the hypothesis that Fos-expressing neuronal ensembles in the prefrontal cortex and nucleus accumbens mediate conditioned drug behaviors in rats. The specific pattern of Fos-expressing neurons is determined by the context and cues associated with the behavior. A critical question is whether these neurons are actually encoding the learned association or merely involved in retrieval of the memory. We do not have the answer to this question; however our working definition of ‘encoding’ neurons are neurons that have undergone long-lasting cue-specific adaptations that alter their activity response, as well as that of the neural network, to cue-related stimuli during learning. Neurons that do not undergo these long-lasting adaptations are not ‘encoding’ neurons; they can be involved in retrieval of the memory or have activity that merely correlates with behavior without playing a significant role in encoding the memory.
We hypothesize that these long-lasting cue-specific adaptations that encode memories can be found in Fos-expressing neuronal ensembles activated during conditioned drug behaviors. We have identified some candidate molecular and electrophysiological adaptations. However we cannot yet manipulate these adaptations selectively in the Fos-expressing neuronal ensembles to demonstrate that they play causal roles in altering the activity response of neurons in these ensembles, or altering the behavioral response, to cue-related stimuli during learning. Future experiments could use our recently developed c-fos-tetO-Cre transgenic rat system, or the Tet-tag mice from Mark Mayford’s laboratory (Garner et al., 2012; Matsuo et al., 2008; Reijmers et al., 2007), that can induce transgenes in Fos-expressing neurons that were activated during behavior. To understand addiction-related learning, future studies will also have to focus on Fos-expressing neuronal ensembles activated during the development of learning in addition to the current work that examined the expression of already learned behaviors.
In future experiments, we will use our c-fos-tetO-Cre transgenic rat system to investigate some of the hypotheses generated by in vivo electrophysiology and cellular imaging studies that suggest distinct ensembles encode drug reward versus non-drug rewards such as food (see above). It will be important to directly assess the degree of overlap between these ensembles in these rats as a way to test whether drugs use the same neuronal ensembles that are used in natural behaviors involving non-drug rewards. If these ensembles do not overlap, then it may be possible to selectively target the ensembles (or unique molecular or cellular alterations within them) that encode addiction-related memories in human addicts. We could then use these targets to ablate or attenuate the response of these neuronal ensembles to relapse-inducing cues. Our preclinical data indicate that this is at least theoretically possible with minimal effects on other memories.
Highlights.
Correlative evidence supports a role for neuronal ensembles in addiction.
Fos-expressing neuronal ensembles mediate conditioned drug behaviors.
Unique neuroadaptations are induced within these Fos-expressing ensembles.
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agell N, et al. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behavioral neuroscience. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Badiani A, et al. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, et al. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behavioural pharmacology. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Bading H, et al. Gene regulation by nuclear and cytoplasmic calcium signals. Biochemical and biophysical research communications. 1997;236:541–543. doi: 10.1006/bbrc.1997.7037. [DOI] [PubMed] [Google Scholar]
- Bakina E, Farquhar D. Intensely cytotoxic anthracycline prodrugs: galactosides. Anti-cancer drug design. 1999;14:507–515. [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL. Visualizing circuits and systems using transgenic reporters of neural activity. Current opinion in neurobiology. 2007;17:567–571. doi: 10.1016/j.conb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle RM, et al. Novel cues reinstate cocaine-seeking behavior and induce Fos protein expression as effectively as conditioned cues. Neuropsychopharmacology. 2012;37:2109–2120. doi: 10.1038/npp.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, et al. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Benedetti BL, et al. Differential Wiring of Layer 2/3 Neurons Drives Sparse and Reliable Firing During Neocortical Development. Cerebral cortex. 2012 doi: 10.1093/cercor/bhs257. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. Journal of neurophysiology. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Berretta S, Robertson HA, Graybiel AM. Neurochemically specialized projection neurons of the striatum respond differentially to psychomotor stimulants. Progress in brain research. 1993;99:201–205. doi: 10.1016/s0079-6123(08)61347-3. [DOI] [PubMed] [Google Scholar]
- Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Sachs Z, Graybiel AM. Cortically driven Fos induction in the striatum is amplified by local dopamine D2-class receptor blockade. The European journal of neuroscience. 1999;11:4309–4319. doi: 10.1046/j.1460-9568.1999.00866.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A, et al. Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14296–14307. doi: 10.1523/JNEUROSCI.2890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best PJ, White AM, Minai A. Spatial processing in the brain: the activity of hippocampal place cells. Annual review of neuroscience. 2001;24:459–486. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Current opinion in neurobiology. 1997;7:419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- Blank T, et al. Small conductance Ca2+-activated K+ channels as targets of CNS drug development. Current drug targets. CNS and neurological disorders. 2004;3:161–167. doi: 10.2174/1568007043337472. [DOI] [PubMed] [Google Scholar]
- Bossert JM, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature neuroscience. 2011 doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of experimental psychology. Animal behavior processes. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, et al. Parsing molecular and behavioral effects of cocaine in mitogen-and stress-activated protein kinase-1-deficient mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, et al. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. Journal of neurochemistry. 2009;108:1323–1335. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Stellar JR. c-Fos and deltaFosB expression are differentially altered in distinct subregions of the nucleus accumbens shell in cocaine-sensitized rats. Neuroscience. 2006;137:773–780. doi: 10.1016/j.neuroscience.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill E, et al. Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse. Front Pharmacol. 2014;4:172. doi: 10.3389/fphar.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. The European journal of neuroscience. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, et al. Shifts in striatal responsivity evoked by chronic stimulation of dopamine and glutamate systems. Brain : a journal of neurology. 2002;125:2353–2363. doi: 10.1093/brain/awf239. [DOI] [PubMed] [Google Scholar]
- Carelli RM, et al. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dual factors controlling activity of nucleus accumbens cell-firing during cocaine self-administration. Synapse. 1996;24:308–311. doi: 10.1002/(SICI)1098-2396(199611)24:3<308::AID-SYN14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Cellular mechanisms underlying reinforcement-related processing in the nucleus accumbens: electrophysiological studies in behaving animals. Pharmacology, biochemistry, and behavior. 1997;57:495–504. doi: 10.1016/s0091-3057(96)00442-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, et al. Examination of factors mediating the transition to behaviorally correlated nucleus accumbens cell firing during cocaine self-administration sessions in rats. Behavioural brain research. 1999;104:127–139. doi: 10.1016/s0166-4328(99)00064-9. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain research. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behavioral and cognitive neuroscience reviews. 2002a;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiology & behavior. 2002b;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion AM, et al. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- Carroll RC, et al. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci. 2007;27:8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano M, et al. Drug context differently regulates cocaine versus heroin self-administration and cocaine- versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology (Berl) 2009;204:349–360. doi: 10.1007/s00213-009-1467-x. [DOI] [PubMed] [Google Scholar]
- Chang JY, et al. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, et al. Neuronal spike activity in rat nucleus accumbens during cocaine self-administration under different fixed-ratio schedules. Neuroscience. 1996;74:483–497. doi: 10.1016/0306-4522(96)00144-3. [DOI] [PubMed] [Google Scholar]
- Chang JY, et al. Single neuronal responses in medial prefrontal cortex during cocaine self-administration in freely moving rats. Synapse. 1997a;26:22–35. doi: 10.1002/(SICI)1098-2396(199705)26:1<22::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chang JY, et al. Neuronal responses in prefrontal cortex and nucleus accumbens during heroin self-administration in freely moving rats. Brain Res. 1997b;754:12–20. doi: 10.1016/s0006-8993(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J. Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Chawla MK, et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Molecular and cellular biology. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, et al. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annual review of cell and developmental biology. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, et al. Context-induced relapse to drug seeking: a review. Trans. R. Soc. Lond. B: Biol. Sci. 2008a;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, et al. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behavioural brain research. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behavioral neuroscience. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Crombag HS, et al. Review. Context-induced relapse to drug seeking: a review. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008b;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, et al. New technologies for examining neuronal ensembles in drug addiciton and fear. Nature Reviews Neuroscience. 2013 doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Barium modulates c-fos expression and post-translational modification. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:8521–8524. doi: 10.1073/pnas.83.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Short-term cocaine self administration alters striatal gene expression. Brain Res Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- David O, et al. Dopamine-induced tyrosine phosphorylation of NR2B (Tyr1472) is essential for ERK1/2 activation and processing of novel taste information. Front Mol Neurosci. 2014;7:66. doi: 10.3389/fnmol.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Ensemble activity and behavior: what’s the code? Science. 1995;270:1316–1318. doi: 10.1126/science.270.5240.1316. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. The significance of neural ensemble codes during behavior and cognition. Annual review of neuroscience. 1997;20:217–244. doi: 10.1146/annurev.neuro.20.1.217. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, et al. Reward, memory and substance abuse: functional neuronal circuits in the nucleus accumbens. Neurosci Biobehav Rev. 2004;27:703–711. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Tsien RW. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron. 2002;34:179–182. doi: 10.1016/s0896-6273(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, et al. Signaling from synapse to nucleus: the logic behind the mechanisms. Current opinion in neurobiology. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Doetsch GS. Patterns in the brain. Neuronal population coding in the somatosensory system. Physiology & behavior. 2000;69:187–201. doi: 10.1016/s0031-9384(00)00201-8. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Cooke BM, Frantz KJ. A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacology. 2013;38:446–454. doi: 10.1038/npp.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Peterson MR, Robertson HA. Presence of c-fos-like immunoreactivity in the adult rat brain. European journal of pharmacology. 1987;135:113–114. doi: 10.1016/0014-2999(87)90767-9. [DOI] [PubMed] [Google Scholar]
- Fanous S, et al. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, et al. Unique gene alterations are induced in FACS-purified Fos-positive neurons activated during cue-induced relapse to heroin seeking. J Neurochem. 2013;124:100–108. doi: 10.1111/jnc.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farivar R, Zangenehpour S, Chaudhuri A. Cellular-resolution activity mapping of the brain using immediate-early gene expression. Front Biosci. 2004;9:104–109. doi: 10.2741/1198. [DOI] [PubMed] [Google Scholar]
- Farquhar D, et al. Suicide gene therapy using E. coli beta-galactosidase. Cancer chemotherapy and pharmacology. 2002;50:65–70. doi: 10.1007/s00280-002-0438-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Robinson TE. Amphetamine-evoked gene expression in striatopallidal neurons: regulation by corticostriatal afferents and the ERK/MAPK signaling cascade. J Neurochem. 2004;91:337–348. doi: 10.1111/j.1471-4159.2004.02712.x. [DOI] [PubMed] [Google Scholar]
- Fu L, Beckstead RM. Cortical stimulation induces fos expression in striatal neurons. Neuroscience. 1992;46:329–334. doi: 10.1016/0306-4522(92)90055-7. [DOI] [PubMed] [Google Scholar]
- Garner AR, et al. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, et al. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, et al. Calcium regulation of gene expression in neuronal cells. Journal of neurobiology. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, et al. A daunorubicin b-galactoside prodrug for use in conjunction with gene-directed enzyme prodrug therapy. Tetrahedron Letters. 2000;41:4871–4874. [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol. Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, et al. FACS Identifies Unique Cocaine-Induced Gene Regulation in Selectively Activated Adult Striatal Neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, et al. FACS purification of immunolabeled cell types from adult rat brain. Journal of neuroscience methods. 2012;203:10–18. doi: 10.1016/j.jneumeth.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, et al. Orbitofrontal and insular cortex: neural responses to cocaine-associated cues and cocaine self-administration. Synapse. 2010;64:1–13. doi: 10.1002/syn.20698. [DOI] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–39. doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, et al. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Haracz JL, et al. Amphetamine effects on striatal neurons: implications for models of dopamine function. Neuroscience and biobehavioral reviews. 1998;22:613–622. doi: 10.1016/s0149-7634(97)00057-2. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nature neuroscience. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hearing MC, et al. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008a;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008b;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior, a neuropsychological theory., Vol. New York: Wiley Subscription Services, Inc., A Wiley Company; 1949. [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain research. Brain research reviews. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membranes, Vol. Sunderland, Mass: Sinauer Associates; 1992. [Google Scholar]
- Hope B, et al. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, et al. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. The European journal of neuroscience. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, et al. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Progress in neurobiology. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jaber M, et al. Acute and chronic amphetamine treatments differently regulate neuropeptide messenger RNA levels and Fos immunoreactivity in rat striatal neurons. Neuroscience. 1995;65:1041–1050. doi: 10.1016/0306-4522(94)00537-f. [DOI] [PubMed] [Google Scholar]
- Johnson A, et al. Looking for cognition in the structure within the noise. Trends in cognitive sciences. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews. Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kasof GM, et al. Spontaneous and evoked glutamate signalling influences Fos-lacZ expression and pyramidal cell death in hippocampal slice cultures from transgenic rats. Brain research. Molecular brain research. 1995a;34:197–208. doi: 10.1016/0169-328x(95)00158-o. [DOI] [PubMed] [Google Scholar]
- Kasof GM, et al. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995b;15:4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasof GM, et al. Developmental expression of Fos-lacZ in the brains of postnatal transgenic rats. Brain research. Developmental brain research. 1996;93:191–197. doi: 10.1016/0165-3806(96)00005-3. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. Journal of neurophysiology. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:3594–3609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–741. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Dopamine in the nucleus accumbens: cellular actions, drug- and behavior-associated fluctuations, and a possible role in an organism's adaptive activity. Behavioural brain research. 2002;137:27–46. doi: 10.1016/s0166-4328(02)00283-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. I.v. cocaine induces rapid, transient excitation of striatal neurons via its action on peripheral neural elements: single-cell, iontophoretic study in awake and anesthetized rats. Neuroscience. 2007;148:978–995. doi: 10.1016/j.neuroscience.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Zhang K. Attractor dynamics of spatially correlated neural activity in the limbic system. Annu Rev Neurosci. 2012;35:267–285. doi: 10.1146/annurev-neuro-062111-150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, et al. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:5623–34. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and biobehavioral reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Kourrich S, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, et al. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Koya E, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature neuroscience. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter JD, et al. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience. 2004;127:233–242. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, et al. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav. 2008;90:349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Expression of c-fos, NGFI-A and secretogranin II mRNA in brain regions during initiation of cocaine self-administration in mice. Eur J Neurosci. 1999;11:3694–3700. doi: 10.1046/j.1460-9568.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, et al. Time course of striatal changes induced by 6-hydroxydopamine lesion of the nigrostriatal pathway, as studied by combined evaluation of rotational behaviour and striatal Fos expression. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1996;108:69–84. doi: 10.1007/BF00242905. [DOI] [PubMed] [Google Scholar]
- Labiner DM, et al. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, et al. Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Liste I, et al. Cortical stimulation induces Fos expression in striatal neurons via NMDA glutamate and dopamine receptors. Brain research. 1995;700:1–12. doi: 10.1016/0006-8993(95)00958-s. [DOI] [PubMed] [Google Scholar]
- Liu QR, et al. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS) J Neurochem. 2014;128:173–185. doi: 10.1111/jnc.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, et al. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nature neuroscience. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lobo MK. Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. International review of neurobiology. 2009;89:1–35. doi: 10.1016/S0074-7742(09)89001-6. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, et al. Role of ERK in cocaine addiction. Trends in neurosciences. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Luscher C, et al. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Progress in neurobiology. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, et al. Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. J Physiol. 2012;590:2427–2442. doi: 10.1113/jphysiol.2011.225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Luscher C. Synaptic plasticity and addiction: Learning mechanisms gone awry. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Mao L, et al. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MT, et al. Context-specific modulation of cocaine-induced locomotor sensitization and ERK and CREB phosphorylation in the rat nucleus accumbens. The European journal of neuroscience. 2009;30:1931–1940. doi: 10.1111/j.1460-9568.2009.06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annual review of neuroscience. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, et al. Frequency of Cocaine Self-Administration Influences Drug Seeking in the Rat: Optogenetic Evidence for a Role of the Prelimbic Cortex. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M. Neuronal activity-regulated gene transcription: how are distant synaptic signals conveyed to the nucleus? F1000Res. 2012;1:69. doi: 10.12688/f1000research.1-69.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. Journal of neurochemistry. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, et al. Repeated amphetamine administration outside the home cage enhances drug-induced Fos expression in rat nucleus accumbens. Behavioural brain research. 2007;185:88–98. doi: 10.1016/j.bbr.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. The European journal of neuroscience. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, et al. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mittler T, et al. Representation of the body in the lateral striatum of the freely moving rat: single neurons related to licking. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1994;98:163–167. doi: 10.1007/BF00229122. [DOI] [PubMed] [Google Scholar]
- Moratalla R, et al. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:423–433. doi: 10.1523/JNEUROSCI.13-02-00423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Morgan JI, et al. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Calcium as a modulator of the immediate-early gene cascade in neurons. Cell calcium. 1988;9:303–311. doi: 10.1016/0143-4160(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Calcium and proto-oncogene involvement in the immediate-early response in the nervous system. Annals of the New York Academy of Sciences. 1989;568:283–290. doi: 10.1111/j.1749-6632.1989.tb12518.x. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annual review of neuroscience. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Murphy LO, et al. Molecular interpretation of ERK signal duration by immediate early gene products. Nature cell biology. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annual review of neuroscience. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Fanselow EE, Ghazanfar AA. Hebb’s dream: the resurgence of cell assemblies. Neuron. 1997;19:219–221. doi: 10.1016/s0896-6273(00)80932-0. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Ehrman RN, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg S, Stolerman I, editors. Behavioral analysis of drug dependence. Vol. Orlando: Academic Press; 1986. pp. 329–356. [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. The European journal of neuroscience. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yoshida H, Watanabe S. NMDA receptor-mediated expression of Fos protein in the rat striatum following methamphetamine administration: relation to behavioral sensitization. Brain research. 1994;665:135–140. doi: 10.1016/0006-8993(94)91163-0. [DOI] [PubMed] [Google Scholar]
- Olive MF, et al. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacology, biochemistry, and behavior. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends in neurosciences. 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations. Neuroscience. 2009;163:40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ML, Currie RW, Robertson HA. Priming of a D1 dopamine receptor behavioural response is dissociated from striatal immediate-early gene activity. Neuroscience. 1995;66:347–359. doi: 10.1016/0306-4522(94)00582-p. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, et al. Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner MR, Mizumori SJ. Neural systems analysis of decision making during goal-directed navigation. Progress in neurobiology. 2012;96:96–135. doi: 10.1016/j.pneurobio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Peoples LL, et al. Operant behavior during sessions of intravenous cocaine infusion is necessary and sufficient for phasic firing of single nucleus accumbens neurons. Brain research. 1997;757:280–284. doi: 10.1016/s0006-8993(97)00299-0. [DOI] [PubMed] [Google Scholar]
- Peoples LL, et al. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998a;18:7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, et al. Tonic inhibition of single nucleus accumbens neurons in the rat: a predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience. 1998b;86:13–22. doi: 10.1016/s0306-4522(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Peoples LL, et al. Tonic firing of rat nucleus accumbens neurons: changes during the first 2 weeks of daily cocaine self-administration sessions. Brain research. 1999;822:231–236. doi: 10.1016/s0006-8993(98)01271-2. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Cavanaugh D. Differential changes in signal and background firing of accumbal neurons during cocaine self-administration. Journal of neurophysiology. 2003;90:993–1010. doi: 10.1152/jn.00849.2002. [DOI] [PubMed] [Google Scholar]
- Peoples LL, et al. Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. Journal of neurophysiology. 2004;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Persico AM, et al. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain research. Molecular brain research. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Pich EM, et al. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends in neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Caboche J, Kaczmarek L. Extracellular signal-regulated kinases (ERKs) modulate cocaine-induced gene expression in the mouse amygdala. Eur J Neurosci. 2005;22:939–948. doi: 10.1111/j.1460-9568.2005.04286.x. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, et al. L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, et al. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Robertson HA, et al. D1-dopamine receptor agonists selectively activate striatal c-fos independent of rotational behaviour. Brain research. 1989;503:346–349. doi: 10.1016/0006-8993(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, et al. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neuroscience and biobehavioral reviews. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse. 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Marshall JF. Amphetamine- and cocaine-induced fos in the rat striatum depends on D2 dopamine receptor activation. Synapse. 1994;18:233–240. doi: 10.1002/syn.890180309. [DOI] [PubMed] [Google Scholar]
- Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, et al. Cocaine- and alcohol-mediated expression of inducible transcription factors is blocked by pentobarbital anesthesia. Brain Res. 2000;877:251–261. doi: 10.1016/s0006-8993(00)02681-0. [DOI] [PubMed] [Google Scholar]
- Schwindel CD, McNaughton BL. Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Progress in brain research. 2011;193:163–177. doi: 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- Sgambato V, et al. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience. 1997;81:93–112. doi: 10.1016/s0306-4522(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Sgambato V, et al. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998a;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato V, et al. In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998b;18:214–226. doi: 10.1523/JNEUROSCI.18-01-00214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, et al. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav Brain Res. 2003;145:79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Siegel S. Drug anticipation and drug addiction. The 1998 H. David Archibald Lecture. Addiction. 1999;94:1113–1124. doi: 10.1046/j.1360-0443.1999.94811132.x. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller AM. Striatal c-fos induction by drugs and stress in neonatally dopamine-depleted rats given nigral transplants: importance of NMDA activation and relevance to sensitization phenomena. Experimental neurology. 1991;113:155–165. doi: 10.1016/0014-4886(91)90171-8. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological review. 1984;91:251–268. [PubMed] [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behavioural pharmacology. 1991;2:65–71. [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, et al. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, et al. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171:1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nature reviews. Neuroscience. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists. Brain research bulletin. 1993;30:173–176. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, et al. Bilateral cortical ablations attenuate amphetamine-induced excitations of neostriatal motor-related neurons in freely moving rats. Neuroscience letters. 1991;134:127–130. doi: 10.1016/0304-3940(91)90523-v. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, et al. Cortical lesions attenuate the opposing effects of amphetamine and haloperidol on neostriatal neurons in freely moving rats. European journal of pharmacology. 1994;257:161–167. doi: 10.1016/0014-2999(94)90708-0. [DOI] [PubMed] [Google Scholar]
- Uslaner J, et al. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. The European journal of neuroscience. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, et al. Amphetamine-induced c-fos mRNA expression in the caudate-putamen and subthalamic nucleus: interactions between dose, environment, and neuronal phenotype. Journal of neurochemistry. 2003;85:105–114. doi: 10.1046/j.1471-4159.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, et al. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Molecular and cellular biology. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioning and place-specific sensitization of increases in activity induced by morphine in the VTA. Pharmacology, biochemistry, and behavior. 1984;20:925–934. doi: 10.1016/0091-3057(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Vezina P, et al. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacology, biochemistry, and behavior. 1989;32:581–584. doi: 10.1016/0091-3057(89)90201-3. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Critical role of peripheral drug actions in experience-dependent changes in nucleus accumbens glutamate release induced by intravenous cocaine. J Neurochem. 2014;128:672–685. doi: 10.1111/jnc.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- West EA, et al. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, et al. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug and alcohol dependence. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49:296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010a;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neuroscience and biobehavioral reviews. 2010b;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DJ, et al. Activity patterns in mesolimbic regions in rats during operant tasks for reward. Progress in brain research. 2000;126:303–322. doi: 10.1016/S0079-6123(00)26021-4. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin L, et al. An embedded subnetwork of highly active neurons in the neocortex. Neuron. 2010;68:1043–1050. doi: 10.1016/j.neuron.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, et al. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology. 2010;35:445–463. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, et al. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, et al. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, et al. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]