Abstract
Anaplastic large cell lymphoma (ALCL) is one of the most common T-cell non-Hodgkin lymphomas and has two main subtypes: an ALK-positive subtype characterized by ALK gene rearrangements and an ALK-negative subtype that is poorly understood. We recently identified recurrent rearrangements of the DUSP22 locus on 6p25.3 in both primary cutaneous and systemic ALK-negative ALCLs. This study aimed to determine the relationship between these rearrangements and expression of the chemokine receptor gene, CCR8. CCR8 has skin-homing properties, and has been suggested to play a role in limiting extracutaneous spread of primary cutaneous ALCLs. However, overexpression of CCR8 also has been reported in systemic ALK-negative ALCLs. As available antibodies for CCR8 have shown lack of specificity, we examined CCR8 expression using quantitative real-time PCR in frozen tissue and RNA in situ hybridization (ISH) in paraffin tissue. Both approaches showed higher CCR8 expression in ALCLs with DUSP22 rearrangements than in non-rearranged cases (PCR: 19.5-fold increase, p=0.01; ISH: 3.3-fold increase, p=0.0008). CCR8 expression was not associated with cutaneous presentation, cutaneous biopsy site, or cutaneous involvement during the disease course. These findings suggest that CCR8 expression in ALCL is more closely related to the presence of DUSP22 rearrangements than to cutaneous involvement, and that the function of CCR8 may extend beyond its skin-homing properties in this disease. This study also underscores the utility of RNA-ISH as a paraffin-based method for investigating gene expression when reliable antibodies for immunohistochemistry are not available.
Keywords: anaplastic large cell lymphoma, T-cell lymphoma, CCR8, RNA in situ hybridization, chemokine receptor
INTRODUCTION
Anaplastic large cell lymphomas (ALCLs) are among the most common T-cell non-Hodgkin lymphomas, and comprise several subtypes that demonstrate overlapping morphologic and immunophenotypic characteristics but vary in clinical and genetic features. The World Health Organization (WHO) classification of hematopoietic neoplasms recognizes three distinct entities: primary cutaneous ALCL (pcALCL); anaplastic lymphoma kinase- (ALK-) positive ALCL; and, provisionally, ALK-negative ALCL.1–3 The latter two entities are systemic diseases and are distinguished from each other only by the presence or absence, respectively, of chromosomal rearrangements involving the ALK gene on 2p23.2. In contrast, pcALCL arises in the skin, typically does not disseminate systemically, and is ALK-negative in most cases. While ALK rearrangements play a key role in the pathogenesis of ALK-positive ALCL,4 little has been known about the genetics and pathogenesis of systemic ALK-negative ALCL and pcALCL.
Using next-generation sequencing and complementary approaches, we recently discovered and characterized recurrent chromosomal rearrangements involving the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangements) that occur almost exclusively in ALK-negative ALCLs, including both pcALCLs and systemic ALK-negative ALCLs.5–7 Although the biologic consequences of these rearrangements have not been established, preliminary data using gene expression profiling (GEP) suggested that cases with DUSP22 rearrangements demonstrated increased expression of the chemokine (C-C motif) receptor 8 (CCR8) gene.8 CCR8 also has been reported to be overexpressed in pcALCL compared to cases of primary cutaneous peripheral T-cell lymphoma, not otherwise specified (pcPTCL, NOS).9 It was proposed that, because of the skin-homing properties of CCR8, this finding might explain the decreased tendency for pcALCL to spread extracutaneously compared to pcPTCL, NOS. Interestingly, however, CCR8 expression also has been reported to be elevated in systemic ALK-negative ALCLs.10 Because both pcALCL and systemic ALK-negative ALCL may carry DUSP22 rearrangements (which we did not identify in a study of 25 cases of pcPTCL, NOS6), we sought to determine whether the cutaneous involvement or the DUSP22 rearrangement status was more closely associated with increased CCR8 expression in ALCLs.
MATERIALS AND METHODS
Patients
Patients were identified from the hematopathology practices at Mayo Clinic, Rochester, MN (n=44) and United Health Services Hospitals, Johnson City/Binghamton, NY (n=6). All patients had ALCL as classified by WHO criteria,11 had sufficient clinical data available for subclassification as pcALCL or systemic ALCL, and had formalin-fixed, paraffin-embedded tissue and/or frozen tissue available. Follow-up data were obtained when possible from the medical record to identify potential secondary skin involvement in patients with systemic disease. Research was conducted under protocols approved by the Institutional Review Boards of Mayo Clinic and United Health Services Hospitals.
Immunohistochemistry and Fluorescence In Situ Hybridization (FISH)
Immunohistochemistry was performed on paraffin tissue sections as part of the diagnostic evaluation or subsequently using methods, antibodies, and conditions described previously.12,13 We were unable to evaluate CCR8 expression by immunohistochemistry due to lack of specificity of available antibodies in our experience and as previously published by others.14–16 DUSP22 rearrangement status based on breakapart FISH for the DUSP22-IRF4 locus was available from previous studies in a subset of the cases and was determined in additional cases as previously described.5–7
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR was performed in samples for which frozen tumor tissue was available using previously described methods with minor modifications.7 Briefly, total RNA was extracted from frozen tumor tissue specimens using PerfectPure (5 PRIME, Gaithersburg, MD) and converted to cDNA using the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The CCR8 primer/probe set was obtained from Applied Biosystems (Assay ID, Hs00174764_m1). The GAPDH primer/probe set was described previously.7 qRT-PCR was performed in duplicate on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Expression levels for each gene were compared to standard curves obtained from serially diluted reference standards, and CCR8 expression was normalized to GAPDH expression.
Ribonucleic Acid In Situ Hybridization (RNA-ISH)
For ISH, RNA probes (RNAscope; Advanced Cell Diagnostics, Hayward, CA) were obtained for CCR8, UBC (ubiquitin C; positive control), and dapB (negative control). Four micron thick paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated in graded ethanol concentrations, and pretreated in citrate buffer (pH 8.0) for 15 min at 98°C. Sections then were treated with protease for 5 min at room temperature and incubated at 40°C with probe for 2 hours. Signal was visualized by incubating sections sequentially with pre-amplifier, amplifier, and horseradish peroxidase-labeled probes, followed by color development with diaminobenzidine, using RNAscope 2.0 Brown Reagent Kit (Advanced Cell Diagnostics) according to the manufacturer’s instructions.
Digital Image Analysis (DIA)
For each sample, corresponding areas were circled on the CCR8 and UBC slides that contained predominantly tumor cells, had visible signal on the UBC positive control, and had minimal or no signal on the dapB negative control. Slides were scanned digitally at 40x magnification and 4096 x 64 pixel resolution using the NanoZoomer Digital Pathology System (Hamamatsu, Bridgewater, NJ). The circled areas of the CCR8 and UBC slides were used to select regions of interest (ROIs) to be analyzed and ISH signals were quantified using an algorithm for threshold detection from commercially available Tissue Studio software (Definiens, Munich, Germany). Signal then was expressed as the percentage of total area thresholded by calculating the ratio of pixels thresholded to total pixels in each ROI.
Statistical Analysis
Values were expressed as means ± standard deviations. Significance levels were analyzed using the Wilcoxon test, Spearman correlation, or chi-square test, as appropriate, using JMP Pro 9.0.1 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
There were 50 patients in the study, including 29 men and 21 women with a mean age of 60 years (range, 12 – 89 years; Table 1). Diagnoses included systemic ALK-positive ALCL in 6 patients, systemic ALK-negative ALCL in 28, and pcALCL in 16. DUSP22 rearrangements were present in 24 patients and absent in 26. DUSP22 rearrangement status was not significantly associated with gender. Of the 24 cases with DUSP22 rearrangements, 12 were systemic ALK-negative ALCLs and 12 were pc ALCLs; as previously reported, DUSP22 rearrangements were not seen in ALK-positive ALCLs.5,6 The specimens tested were obtained from a non-cutaneous site in 32/33 systemic ALCLs and from a cutaneous site in 13/16 pcALCLs.
TABLE 1.
Demographic, Pathologic, and Genetic Data of ALCLs Studied by qRT-PCR and/or RNA-ISH for CCR8 Expression
| qRT-PCR | RNA-ISH | All patients* | |
|---|---|---|---|
|
|
|||
| Total patients, n | 27 | 38 | 50 |
| Age, years | |||
| Mean | 56 | 60 | 60 |
| Range | 12–81 | 18–89 | 12–89 |
| Gender | |||
| Males | 17 | 20 | 29 |
| Females | 10 | 18 | 21 |
| Subtype | |||
| ALCL, ALK positive | 4 | 3 | 6 |
| ALCL, ALK negative | 17 | 23 | 28 |
| ALCL, primary cutaneous | 6 | 12 | 16 |
| DUSP22 rearrangement status | |||
| Present | 6 | 22 | 24 |
| Absent | 21 | 16 | 26 |
Abbreviations: ALCL, anaplastic large cell lymphoma; qRT-PCR, quantitative real-time polymerase chain reaction; RNA-ISH, ribonucleic acid in situ hybridization.
Total for “all patients” is less then sum of qRT-PCR and RNA-ISH because 14 samples were analyzed by both methods.
qRT-PCR Evaluation of CCR8 Expression in ALCL
To validate previous GEP data, we performed qRT-PCR on cDNA prepared from whole sections of frozen ALCL tissues. Expression of CCR8 was normalized to that of GAPDH and expressed as fold change compared to the mean value of cases without DUSP22 rearrangements. CCR8 expression was 19.5 ± 16.1 in ALCLs with DUSP22 rearrangements compared to 1.0 ± 2.0 in non-rearranged cases (i.e. 19.5-fold higher, p=0.01; Figure 1a). No significant difference in CCR8 expression was observed when cases were stratified by ALCL subtype (Figure 1b). In keeping with previously published data, there was a trend toward lower expression in ALK-positive ALCL (0.2 ± 0.2) than in ALK-negative ALCL (3.9 ± 7.9) or pcALCL (11.4 ± 17.6); however, this trend was primarily attributable to the cases with DUSP22 rearrangements. Taken together, these findings were in concordance with previous GEP data and suggested a relationship between DUSP22 rearrangements and CCR8 expression; however, the sample size was small and it was not possible to determine whether the CCR8 expression detected was derived from tumor cells specifically. Thus, we examined CCR8 expression in tissue sections from additional cases.
FIGURE 1.
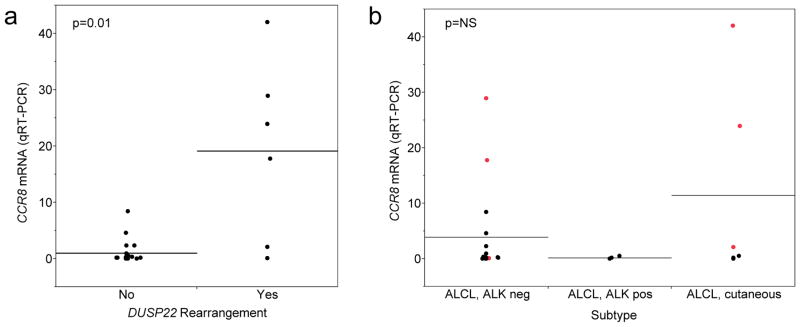
CCR8 mRNA levels as determined by quantitative real-time PCR (qRT-PCR) in whole sections from frozen tumor tissue. Values were normalized to GAPDH expression and expressed as fold change compared to the mean value of cases lacking DUSP22 rearrangements. Horizontal lines indicate means for each group and points indicate individual patients. (a) CCR8 expression was significantly higher in ALCLs with DUSP22 rearrangements than in non-rearranged cases. (b) No significant difference in CCR8 expression was observed among subtypes; though there was a trend toward higher expression in ALK-negative ALCL and pcALCL, this was mostly attributable to cases with DUSP22 rearrangements (red points). Black points are cases without DUSP22 rearrangements.
RNA-ISH Evaluation of CCR8 Expression in ALCL
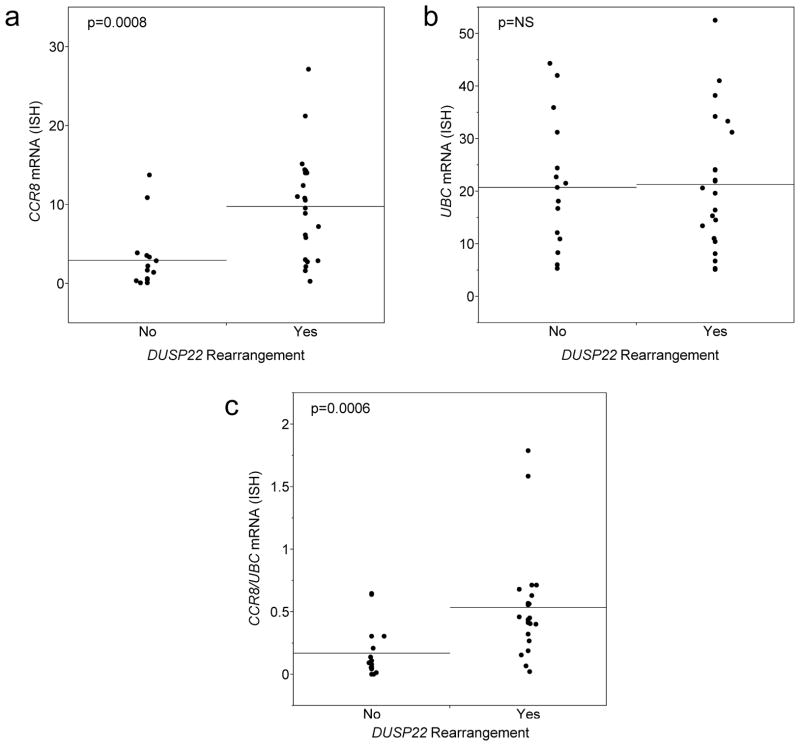
As discussed above, we were unable to assess CCR8 expression by immunohistochemistry due to lack of specific antibodies. Therefore, we examined CCR8 expression by RNA-ISH in paraffin tissue sections. We performed DIA of selected areas of the sections rich in tumor cells and showed appropriate staining for the positive and negative controls, UBC and dapB, respectively. CCR8 expression, as measured by the percent thresholded area in the selected portion of the tissue, was 9.8 ± 6.7 in ALCLs with DUSP22 rearrangements compared to 2.9 ± 3.9 in those without translocations (i.e. 3.3-fold higher, p=0.0003; Figure 2a). Although only a small number of patients had data for both qRT-PCR and RNA-ISH, there was a trend toward an association between these values, despite the differences in specimen type (frozen vs. paraffin) and sampling (whole section vs. selected area; Spearman ρ=0.47, p=0.09; data not shown).
FIGURE 2.
CCR8 mRNA levels as determined by RNA in situ hybridization (ISH) performed on paraffin tissue sections. CCR8 expression was calculated as percent thresholded area in a selected tumor-rich area of the slide with adequate UBC control staining. (a) CCR8 expression was significantly higher in ALCLs with DUSP22 rearrangements than in non-rearranged cases. (b) UBC expression was similar in both groups. (c) Using the CCR8/UBC ratio yielded results similar to using CCR8 expression alone.
Since all tumors were subjected to RNA-ISH for the UBC positive control, we also performed DIA of the corresponding areas on these slides to investigate potential contributions of variation in UBC staining to the results. UBC expression was similar in ALCLs with DUSP22 rearrangements (21.3 ± 12.7) and those without rearrangements (20.8 ± 12.2; p=0.99; Figure 2b). Using the CCR8/UBC expression ratio yielded results similar to those obtained with using CCR8 expression alone. This ratio was 0.54 ± 0.42 in ALCLs with DUSP22 rearrangements compared to 0.17 ± 0.21 in those without rearrangements (i.e. 3.2-fold higher, p=0.0006; Figure 2c).
CCR8 Expression and Clinicopathologic Features of ALCL
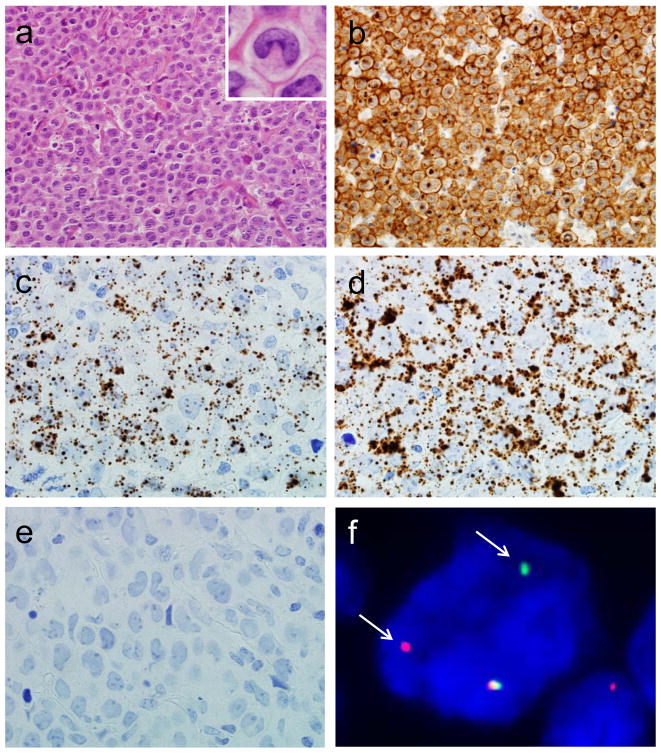
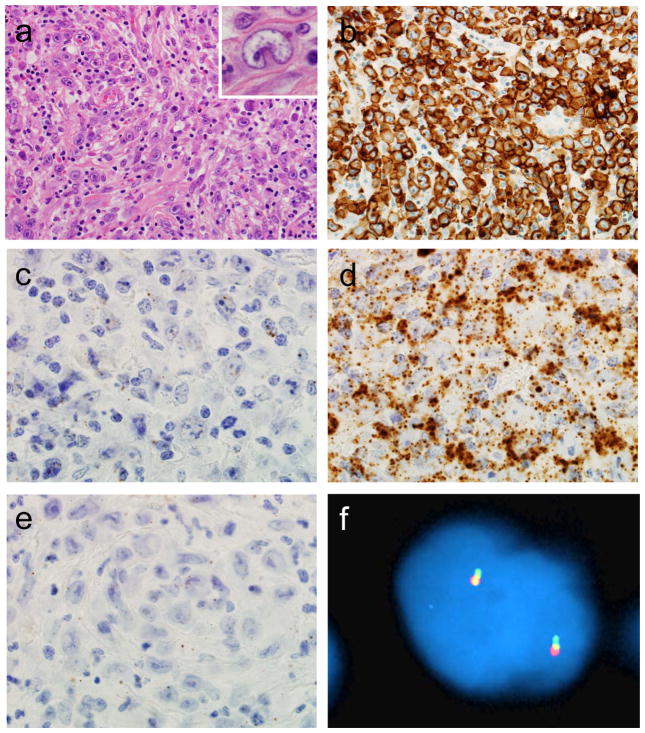
ALCLs in this study had pathologic features typical for this entity (Figures 3 and 4). All had characteristic hallmark cells noted on H&E stains and demonstrated uniform staining for CD30. CCR8 expression as visualized by RNA-ISH was almost entirely limited to tumor cells, and prominent staining was not observed in other areas of the sections, suggesting that differences observed by GEP and qRT-PCR were due to expression of CCR8 by tumor cells specifically. Breakapart FISH for the DUSP22-IRF4 locus on 6p25.3 was successful in determining DUSP22 rearrangement status in all cases (Table 1).
FIGURE 3.
ALK-negative ALCL with DUSP22 rearrangement. (a) H&E stain demonstrating hallmark cells (400x; inset, 1000x). (b) The tumor cells are uniformly positive for CD30 by immunohistochemistry (400x). RNA ISH shows substantial positivity for CCR8 (c) and the positive control, UBC (d), but is essentially negative for the negative control, dapB (e; all 1000x). (f) Fluorescence in situ hybridization (FISH) using a breakapart probe for the 6p25.3 locus demonstrates abnormal separation of the red and green signals (arrows), indicating a DUSP22 rearrangement.
FIGURE 4.
ALK-negative ALCL without DUSP22 rearrangement. (a) H&E stain demonstrating hallmark cells (400x; inset, 1000x). (b) The tumor cells are uniformly positive for CD30 by immunohistochemistry (400x). RNA ISH is essentially negative for CCR8 (c), shows substantial expression of UBC (d), and is essentially negative for dapB (e; all 1000x). (f) Breakapart FISH shows a normal signal pattern, consisting of 2 fusion signals, indicating absence of a DUSP22 rearrangement.
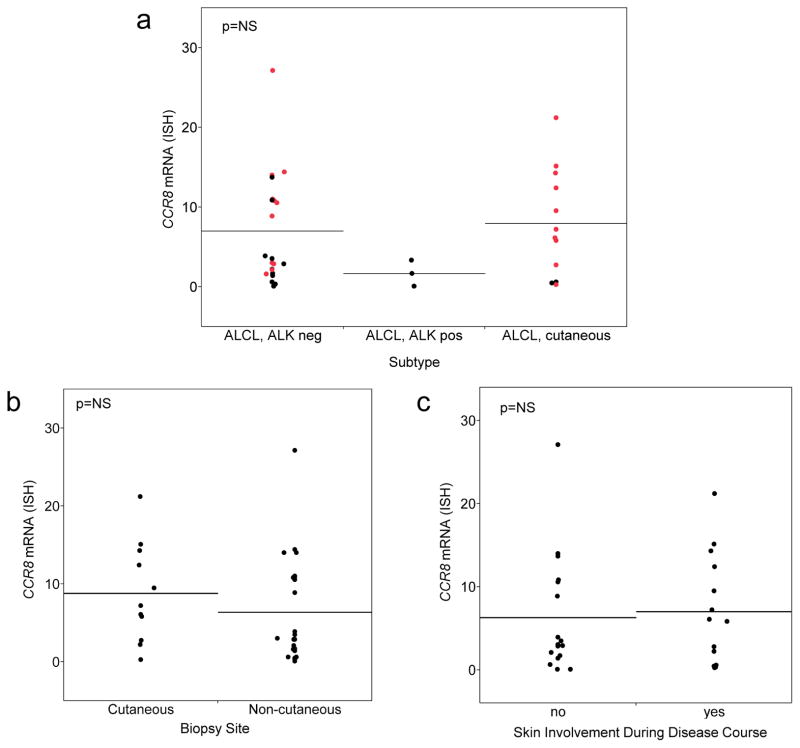
Because CCR8 is a chemokine receptor that has been implicated in skin homing, we investigated whether either CCR8 expression was associated with ALCL subtype; biopsy site (cutaneous vs. extracutaneous); or presence of skin involvement at any time in the patient’s disease course. Although these analyses are related, they are not identical, since systemic ALCL can secondarily involve the skin and primary cutaneous ALCL can secondarily involve extracutaneous sites.17 None of these analyses demonstrated statistically significant differences (Figure 5). Again, a trend toward lower CCR8 expression in ALK-positive ALCLs was observed Figure 5a). CCR8 expression was 7.0 ± 6.8 in ALK-negative ALCL, 1.7 ± 1.6 in ALK-positive ALCL, and 8.0 ± 6.7 in pcALCL. CCR8 expression was 8.8 ± 6.4 in skin biopsy specimens of ALCLs and 6.4 ± 6.8 in extracutaneous biopsy specimens of ALCL (Figure 5b). By definition, all 12 patients with pcALCL had skin involvement. Among 26 ALCLs presenting with systemic disease, follow-up data were available in 19, with a median follow-up time of 28 months (range, 1–207 months). Of these 19 patients, 2 developed secondary cutaneous disease (at 1 and 19 months after diagnosis, respectively), both of which were ALK-negative ALCLs lacking DUSP22 rearrangements. Considering pcALCLs and systemic ALCLs together, CCR8 expression was 7.0 ± 6.6 in ALCLs with skin involvement at any time in their disease course and 6.3 ± 7.1 in ALCLs without skin involvement (Figure 5c).
FIGURE 5.
Relationships observed using RNA-ISH between CCR8 expression and ALCL subtype, biopsy site, and presence of skin involvement at any time during the disease course. (a) No significant difference in CCR8 expression was observed among subtypes. Red points represent cases with DUSP22 rearrangements; black points represent cases without DUSP22 rearrangements. (b) No significant difference was noted in CCR8 expression between biopsies obtained from cutaneous or non-cutaneous sites. (c) There was no significant difference in CCR8 expression between patients who did or did not have skin involvement during their disease course.
DISCUSSION
In the present study, we have demonstrated that the chemokine receptor gene, CCR8, is overexpressed in ALCLs with DUSP22 rearrangements. This is the first tissue-based study to provide insight on biologic features associated with this rearrangement. Importantly, the findings suggest that CCR8 expression in ALCL is more closely related to the presence of DUSP22 rearrangements than to cutaneous involvement. These data raise the possibility that the function of CCR8 may extend beyond its skin-homing properties in this disease.
Chemokines and chemokine receptors play a crucial role in mediating the migration of target cells to specific tissues and are involved in a variety of biological processes under both physiologic and pathologic conditions.18,19 Dermal microvessels and epidermal antigen-presenting cells express the CCR8 ligand, CCL1, which plays a role in regulating cutaneous migration of CCR8-positive immune surveillance T cells.20 van Kester et al have shown that CCR8 expression is higher in pcALCLs than in pcPTCL, NOS, and suggested that this expression (along with that of the related chemokine receptor gene, CCR10) might explain the lower tendency of pcALCL to disseminate to extracutaneous sites.9 Interestingly, even when pcALCL does exhibit extracutaneous spread, dissemination tends to be limited. Typically, only locoregional lymph nodes are involved, and outcomes in these cases are similar to those of pcALCLs limited to the skin.17 Of note, lymphatic endothelial cells in lymph node sinuses express CCL1, and CCR8 expression is critical in lymph node migration of both monocyte-derived dendritic cells and melanoma cells.21,22 Finally, CCR8/CCL1 interactions, as well as newly described CCR8/CCL18 interactions, have been implicated in T-cell homing to several other extracutaneous sites, including the esophagus, pancreatobiliary system, and lung.15,23,24 Taken together, these findings suggest the possibility that the role of CCR8 in ALCL extends beyond the cutaneous microenvironment.
DUSP22 rearrangements and ALK rearrangements are the two most common chromosomal rearrangements reported in ALCL, and studies to date have found them to be mutually exclusive.5–7,25 The breakpoints within the DUSP22-IRF4 locus on 6p25.3 may vary; regardless of breakpoint, however, these cases share the feature of marked down-regulation of DUSP22 expression without alterations in IRF4 expression.7 DUSP22 is a dual-specificity phosphatase involved in modulating mitogen-activated protein kinase (MAPK) activity, and specifically has been shown to inhibit T-cell receptor signaling.26,27 Though early data suggested that DUSP22 rearrangements were seen mostly in pcALCL, our subsequent studies have shown nearly the same frequency in systemic ALK-negative ALCLs.5–7 Interestingly, increased CCR8 expression has been reported not only in pcALCL but also in systemic ALK-negative ALCL.10 The additional finding that ALCLs with DUSP22 rearrangements showed increased CCR8 expression in a small pilot study using GEP prompted us to perform the current study.8 Our findings conclusively demonstrate a significant association between CCR8 expression and DUSP22 rearrangements. No significant association was found between CCR8 and cutaneous presentation, cutaneous site of biopsy, or cutaneous involvement at any time in the disease course. These findings raise the possibility that the previously reported increases in CCR8 expression in pcALCL or systemic ALK-negative ALCL may have been due in part to the presence of DUSP22 rearrangements in subsets of the cases analyzed; however, the DUSP22 rearrangement status of those cases is unknown. Also unknown is whether our findings have implications for the rare reported cases of ALK-positive pcALCL. Although not included in the present series, we have examined two such cases by FISH and both lacked DUSP22 rearrangements (ALF, unpublished data).
The finding that DUSP22 rearrangement is more strongly associated with CCR8 expression than cutaneous site should not be taken as evidence against the proposal by van Kester et al that CCR8 contributes to the more limited dissemination of pcALCL than pcPTCL, NOS. Rather, the possibility exists that the role of CCR8 in regulating migration of ALCL cells may extend beyond cutaneous sites. Indeed, systemic ALK-negative also has a clinical course significantly more favorable than systemic PTCL, NOS, and the biologic basis for this difference remains unclear.28 Furthermore, early data have suggested that DUSP22 rearrangements identify a group of patients with prognosis even better than that of other systemic ALK-negative ALCLs.29 Finally, we also recently identified DUSP22 rearrangements in a subset of patients with lymphomatoid papulosis (LyP), an indolent cutaneous T-cell neoplasm with excellent prognosis (although, to our knowledge, expression of CCR8 in this disease is unknown).30 Taken together, these findings suggest an association among DUSP22 rearrangements, CCR8 expression, and relatively favorable clinical outcomes. This clinical behavior might be related to homing signals, including expression of CCR8, that prevent more aggressive dissemination. It should be noted, however, that the presence of a DUSP22 rearrangements was neither entirely sensitive nor entirely specific for high CCR8 expression; thus, the mechanism responsible for the association between DUSP22 rearrangements and CCR8 expression merits further study.
The use of RNA-ISH in this study deserves mention, since analysis of protein expression would be preferable for proposing a hypothesis regarding the potential biologic function of CCR8 in ALCL. However, we and others have found the available CCR8 antibodies to lack specificity and reliability.14–16 GEP has identified expression signatures that have played a transformative role in advancing biologic understanding of lymphomas and other diseases.31 At the individual gene level, however, GEP findings typically have required validation by another method, preferably in tissue sections that allow identification of the cell type with which gene expression is associated. Some gene expression signatures can be examined effectively using immunohistochemical stains,32 but validated antibodies for immunohistochemistry are not available for the majority of genes assayed by GEP. Although RNA-ISH cannot assess protein expression, it shares features with immunohistochemistry in that it can be performed in paraffin tissue sections and can be interpreted in the context of tissue architecture and cell type. In addition, it offers the advantage of being relatively easy to develop highly specific probes for virtually any target. Thus, this study underscores the utility of this approach in situations where reliable antibodies are not available.
Footnotes
Disclosure of Funding Sources: Supported by the Center for Individualized Medicine and the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN; and by Award Numbers R01 CA177734 (ALF) and P50 CA97274 (University of Iowa/Mayo Clinic Lymphoma SPORE), National Cancer Institute. XX was supported by a scholarship award from the China Scholarship Council. ALF is a Damon Runyon Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI-48-09).
References
- 1.Delsol G, Falini B, Muller-Hermelink HK, et al. Anaplastic large cell lymphoma, ALK-positive. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. pp. 312–316. [Google Scholar]
- 2.Mason DY, Harris NL, Delsol G, et al. Anaplastic large cell lymphoma, ALK-negative. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. pp. 317–319. [Google Scholar]
- 3.Ralfkiaer E, Willemze R, Paulli M, Kadin ME. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. pp. 300–301. [Google Scholar]
- 4.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110(7):2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman AL, Law M, Remstein ED, et al. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia. 2009;23(3):574–580. doi: 10.1038/leu.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24(4):596–605. doi: 10.1038/modpathol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively-parallel genomic sequencing. Blood. 2011;117(3):915–919. doi: 10.1182/blood-2010-08-303305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman AL, Huang G, Porcher JC, et al. ALK-Negative Anaplastic Large Cell Lymphomas with 6p25.3 Translocations Show a Histone-Modifying Gene Expression Signature (abstract) Blood. 2011;118:88. [Google Scholar]
- 9.van Kester MS, Tensen CP, Vermeer MH, et al. Cutaneous anaplastic large cell lymphoma and peripheral T-cell lymphoma NOS show distinct chromosomal alterations and differential expression of chemokine receptors and apoptosis regulators. J Invest Dermatol. 2010;130(2):563–575. doi: 10.1038/jid.2009.270. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115(5):1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. In: Bosman F, Jaffe E, Lakhani S, Ohgaki H, editors. World Health Organization Classification of Tumours. 4. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 12.Kurtin PJ, Hobday KS, Ziesmer S, Caron BL. Demonstration of distinct antigenic profiles of small B-cell lymphomas by paraffin section immunohistochemistry. Am J Clin Pathol. 1999;112(3):319–329. doi: 10.1093/ajcp/112.3.319. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AL, Law ME, Inwards DJ, Dogan A, McClure RF, Macon WR. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod Pathol. 2010;23(4):593–602. doi: 10.1038/modpathol.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pease JE. Targeting chemokine receptors in allergic disease. Biochem J. 2011;434(1):11–24. doi: 10.1042/BJ20101132. [DOI] [PubMed] [Google Scholar]
- 15.Zen Y, Liberal R, Nakanuma Y, Heaton N, Portmann B. Possible involvement of CCL1-CCR8 interaction in lymphocytic recruitment in IgG4-related sclerosing cholangitis. Journal of hepatology. 2013 doi: 10.1016/j.jhep.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Chenivesse C, Chang Y, Azzaoui I, et al. Pulmonary CCL18 recruits human regulatory T cells. J Immunol. 2012;189(1):128–137. doi: 10.4049/jimmunol.1003616. [DOI] [PubMed] [Google Scholar]
- 17.Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95(12):3653–3661. [PubMed] [Google Scholar]
- 18.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 20.Schaerli P, Ebert L, Willimann K, et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199(9):1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu C, Edwards EW, Tacke F, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200(10):1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Sarrou E, Podgrabinska S, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210(8):1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam SA, Ling MF, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med. 2013;210(10):1889–1898. doi: 10.1084/jem.20130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Macneil AJ, Junkins R, Li B, Berman JN, Lin TJ. Mast cell FcepsilonRI-induced early growth response 2 regulates CC chemokine ligand 1-dependent CD4+ T cell migration. Journal of immunology. 2013;190(9):4500–4507. doi: 10.4049/jimmunol.1203158. [DOI] [PubMed] [Google Scholar]
- 25.Vasmatzis G, Johnson SH, Knudson RA, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood. 2012;120:2280–2289. doi: 10.1182/blood-2012-03-419937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama K, Nagata M, Oshima K, Matsuda T, Aoki N. Molecular cloning and characterization of a novel dual specificity phosphatase, LMW-DSP2, that lacks the cdc25 homology domain. J Biol Chem. 2001;276(29):27575–27583. doi: 10.1074/jbc.M100408200. [DOI] [PubMed] [Google Scholar]
- 27.Li JP, Yang CY, Chuang HC, et al. The phosphatase JKAP/DUSP22 inhibits T-cell receptor signalling and autoimmunity by inactivating Lck. Nature communications. 2014;5:3618. doi: 10.1038/ncomms4618. [DOI] [PubMed] [Google Scholar]
- 28.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 29.Parrilla Castellar ER, Grogg KL, Law ME, et al. Rearrangements of the 6p25.3 Locus Identify a Subset of Systemic ALK-Negative Anaplastic Large Cell Lymphomas with Favorable Prognosis. Mod Pathol. 2012;25(suppl 2):359A, abst 1508. [Google Scholar]
- 30.Karai LJ, Kadin ME, Hsi ED, et al. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am J Surg Pathol. 2013;37(8):1173–1181. doi: 10.1097/PAS.0b013e318282d01e. [DOI] [PubMed] [Google Scholar]
- 31.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 32.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]