Abstract
Hyperthermia is one of the promising treatments for cancer therapy. However, the development of a magnetic fluid agent that can selectively target a tumor and efficiently elevate temperature while exhibiting excellent biocompatibility still remains challenging. Here a new core-shell nanostructure consisting of inorganic iron oxide (Fe3O4) nanoparticles as the core, organic alginate as the shell, and cell-targeting ligands (ie, D-galactosamine) decorated on the outer surface (denoted as Fe3O4@Alg-GA nanoparticles) was prepared using a combination of a pre-gel method and coprecipitation in aqueous solution. After treatment with an AC magnetic field, the results indicate that Fe3O4@Alg-GA nanoparticles had excellent hyperthermic efficacy in a human hepatocellular carcinoma cell line (HepG2) owing to enhanced cellular uptake, and show great potential as therapeutic agents for future in vivo drug delivery systems.
Keywords: hyperthermia, iron oxide, alginate, pre-gel, targeting
Introduction
Superparamagnetic iron oxide nanoparticles (SPIONs), including magnetite (Fe3O4) and maghemite (γ-Fe2O3), have been extensively utilized in biomedical applications, including as magnetic resonance imaging contrast agents,1 for biocatalysis,2 for biological separation,3 for biosensing,4 diagnostic medical devices,5 and hyperthermia. Among these applications, hyperthermia6 is a physical therapy in which SPIONs generate heat and thus kill cancer cells when they are exposed to an external magnetic field.7 The hyperthermia of SPIONs has been considered as one of the most promising cancer therapies because it can effectively destroy cancer cells while keeping normal cells intact. The reason is that cancer cells are more sensitive to heat than normal cells.8,9 When a high temperature (usually around 42.5°C) is produced locally, tumor tissue cannot release heat energy via the bloodstream due to dysfunction of blood vessels.10 In addition, studies have also shown that cancer cells produce heat shock proteins, resulting in an increase in major histocompatibility complex class I antigens. Thus, this increases the possibility of recognition of cancer cells by cytotoxic T lymphocytes.11 Consequently, hyperthermia is an effective and safe cancer therapy.
One of the challenges for effective cancer therapy is how to deliver therapeutic agents to tumor sites. Most anticancer drugs cannot distinguish between cancer cells and healthy cells, which results in undesirable side effects and low efficacy. The fabrication of nanoscale drug-loading carriers has been widely studied because these nanoparticles can accumulate in tumor sites via the enhanced permeability and retention effect.12 However, such passive targeting-based drug delivery systems sometimes cause unwanted accumulation of anticancer agents in the liver, kidney, and spleen.13,14 In contrast with passive targeting, active targeting has attracted more attention in terms of further enhancing delivery efficacy and cancer specificity. Specific ligands (eg, folate) that can be recognized by cancer cells have been widely studied and linked to the surfaces of anticancer agents so that receptor-mediated endocytosis can occur.15–17 For example, asialoglycoprotein receptors are located on liver cancer cells and can specifically bind with glycoprotein on/in galactose residues, leading to internalization and subsequent degradation of glycoprotein inside the cells.18 Such active targeting strategies can not only effectively deliver drugs into cancer cells, but also reduce the amounts of anticancer agents used.
The combination of hyperthermia and active targeting has been regarded as one of the most promising therapies for improving anticancer efficacy. Synthesis of SPIONs and further functionalization of SPIONs with specific targeting ligands is therefore of intense research interest. So far, SPIONs have been synthesized by a coprecipitation method,19 hydrothermal treatment,20 and an organic solvent-based method.21 For example, Hyeon et al synthesized uniform and well suspended SPIONs in organic solvent systems using Fe(CO)5 as the precursor.22 Daou et al reported synthesis of SPIONs with a uniform particle size of 39 nm by hydrothermal treatment.23 Despite these pioneering works, the coprecipitation method is more acceptable because of its simple synthesis (ie, relatively low temperature and a water system). However, this method usually results in aggregated SPIONs, which are difficult to use in biomedical applications. In addition, in order for SPIONs to be functionalized with targeting ligands, the synthesized SPIONs must be reacted with the targeting ligands under the desired conditions, which is usually a complicated and time-consuming process.24 Therefore, simultaneous synthesis and functionalization of SPIONs in aqueous systems is necessary.
In this study, we developed a new synthetic approach to prepare carboxylic acid-functionalized SPIONs (ie, Fe3O4) by combination of coprecipitation of iron oxides and a pre-gel method of alginate (denoted as Fe3O4@Alg nanoparticles). The COOH-functionalized Fe3O4 nanoparticles were further linked with a liver cancer cell-targeting ligand (ie, galactose), denoted as Fe3O4@Alg-GA nanoparticles, and used as targeted hyperthermia therapy. This new approach has several advantages: alginate has excellent biocompatibility and surface functionality (COOH group); coprecipitation of iron oxides takes place within the alginate polymer, preventing aggregation of the synthesized Fe3O4 nanoparticles; targeting ligands can be successfully linked on the external surface of Fe3O4@Alg nanoparticles, thereby ensuring targeting capability; and Fe3O4 nanoparticles with well dispersed, controllable particle sizes provide the capability for hyperthermia. We then characterized the synthesized Fe3O4@Alg nanoparticles with X-ray diffraction, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis. The Fe3O4@Alg-GA nanoparticles showed enhanced hyperthermic efficacy when compared with Fe3O4@Alg nanoparticles, which was confirmed to be the result of increased uptake of Fe3O4@Alg-GA by human liver cancer (HepG2) cells (ie, the amount of Fe3O4@Alg-GA nanoparticles taken up was 20-fold greater than the amount of Fe3O4@Alg nanoparticles).
Materials and methods
Chemicals
Iron(II) chloride tetrahydrate (FeCl2·4H2O, 98%) was purchased from Alfa Aesar (Ward Hill, MA, USA). Iron(III) chloride hexahydrate (FeCl3·6H2O), 1-(3-dimethylaminopropyl)-3-ethylcarbodimmide (EDC), N-hydroxysuccinimide (NHS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), potassium ferrocyanide (K4[Fe(CN)6]·3H2O), 10% neutral buffered formalin, nuclear fast red counterstain, and sodium alginate were purchased from Sigma-Aldrich (St Louis, MO, USA). D-(+)-galactosamine hydrochloride was provided by Acros Organics (Thermo Fisher Scientific, Bridgewater, NJ, USA). Phosphate-buffered saline (PBS, 10×), fetal bovine serum, sodium bicarbonate (7.5 wt%), L-glutamine (200 mM in saline), trypsin-ethylenediaminetetraacetic acid (10×), and Pen-Strep solution (penicillin 10,000 U/L and streptomycin 10 mg/mL) were provided by Biological Industries Israel Beit-Haemek Ltd (Beit-Haemek, Israel). CellTiter 96 Aqueous MTS Reagent Power was purchased from Promega (Madison, WI, USA). Phenazine methosulfate was purchased from AppliChem GmbH (Darmstadt, Germany).
Synthesis and functionalization of Fe3O4@ Alg nanoparticles
The synthesis process for Fe3O4@Alg nanoparticles is described as follows. Alginate 10 mg was dissolved in 55 mL of deionized (DI) water with stirring until the alginate was dissolved completely. An aqueous solution containing FeCl2·4H2O (50 mM, 10 mL) was added to this system at a rate of 0.5 mL per minute and kept under stirring for 1 hour. Subsequently, another solution containing FeCl3·6H2O (62.4 mM, 10 mL) was also added to this system by the same method, and the whole system was stirred for 1 hour. The Fe cations (Fe2+ and Fe3+) are selectively adsorbed onto the negatively charged COOH groups of the alginate polymer, which is a so-called pre-gel method. Next, we used an aqueous ammonia solution (1.5 M) to adjust the pH of the system to 10, in order to obtain magnetic Fe3O4 nanoparticles, which is a so-called coprecipitation method. After 30 minutes of stirring, the solid product was separated from the solution by a magnetic stone, washed with DI water several times, and then resuspended in DI water by sonication. We report for the first time simple and reproducible synthesis of polymer-coated magnetic nanoparticles by the combination of a pre-gel method and a coprecipitation method. Several factors, including the amount of alginate, pH value, and reaction time, were studied in order to control the particle size of the synthesized Fe3O4@Alg nanocomposites (see discussion below).
To functionalize Fe3O4@Alg nanoparticles with ligands that can specifically target cancer cells, we reacted the synthesized Fe3O4@Alg nanoparticles with galactosamine via the EDC/NHS reaction, because alginate has carboxylic acid groups and galactosamine has amine groups. Typically, the Fe3O4@Alg nanoparticle suspension was first diluted with PBS (pH 5) buffer to a concentration of 1.25 mg/mL. Next, EDC 50 mg and NHS 75 mg were separately dissolved in 1 mL of PBS (pH 5) and the two solutions were added into 8 mL of Fe3O4@Alg nanoparticle suspension. After the whole mixture had been stirred for 15 minutes, the EDC/NHS-functionalized Fe3O4@Alg nanoparticles were separated by a magnetic stone, washed with PBS buffer, and resuspended in 5 mL of PBS buffer (pH 7.5). A PBS (pH 7.5) solution containing galactosamine hydrochloride (52 mM, 5 mL) was added to this solution. After 4 hours of stirring, the galactosamine-functionalized Fe3O4@Alg nanoparticles (denoted as Fe3O4@Alg-GA nanoparticles) were separated by a magnetic stone, washed with PBS buffer (pH >9) several times (the basic buffer was used here to deprotonate the carboxylic acid groups of alginate), and resuspended in DI water by sonication.
Characterization
The hydrodynamic diameter and zeta potential of the synthesized Fe3O4@Alg nanoparticles were measured using a Zetasizer Nano ZS system (Malvern Instruments Ltd, Malvern, UK) at 25°C with DI H2O as the solvent. Samples were sonicated for 1 hour before measuring. The average particle size was evaluated based on dynamic light scattering. The morphology of the samples was observed using an SEM (Nova NanoSEM™, FEI, Hillsboro, NJ, USA). The Fe3O4@Alg nanoparticle suspension was sonicated for 1 hour, dropped onto copper grids (200 mesh, carbon-coated), dried under vacuum, and subjected to platinum coating before observation under SEM. Functional groups of the samples were examined by FTIR (Spectrum 100, PerkinElmer, Waltham, MA, USA) at a resolution of 4 cm−1. Samples used for FTIR measurements were prepared by mixing the vacuum-dried samples with KBr (KBr to sample, 100:1). The mixture was then ground extensively and pressed into a translucent disc. The structural properties of the samples were analyzed using an X-ray diffraction system (Ultima IV, Rigaku, Tokyo, Japan) with Cu Kα radiation (λ =1.5418 Å, 40 kV, 40 mA). Thermogravimetric analysis curves were recorded on a differential scanning calorimeter (Pyris 1, PerkinElmer). Samples (5 mg) were maintained at 50°C for 10 minutes, then heated to 800°C at 10°C per minute, and maintained at 800°C for 1 minute. Nitrogen flow was fixed at 20.0 mL per minute throughout the measurement process. The amount of iron element inside the cells was determined using an inductively coupled plasma mass spectrometer (ICP-MS, Elan-6000, PerkinElmer) after lysis of the cells. The cells were cultured as mentioned before. The cells were transplanted into 24 wells with 5×104 per well for 1 day. After washing with PBS several times, 0.5 mL of solution containing Fe3O4@Alg nanoparticles or Fe3O4@Alg-GA nanoparticles (1 mg/mL) was added into each well and the cells were cultured for another 4 hours. Each sample of HCl (12 M, 0.6 mL) was added into each well for 2 hours to dissolve the Fe3O4 nanoparticles. Next, 600 μL of HCl (6 M) was used to wash the wells. In total, 6 mL of solution (five wells for each sample) was collected into a vial for measurement; one well in which cells cultured without nanoparticles was used to count the number of cells. The weight of Fe3O4 in each well as measured by ICP-MS and was divided by the number of cells to get the average weight of Fe3O4 nanoparticles inside each cell.
Cell culture, viability test, and observation
The liver cancer (HepG2) cell line was purchased from the National Health Research Institutes, Miaoli, Taiwan. HepG2 cells were incubated in flasks with Dulbecco’s Modified Eagle’s Medium at 37°C and 5% CO2, in a 95% humidified atmosphere, and were subcultured every 3 days. Every 100 mL of Dulbecco’s Modified Eagle’s Medium was supplemented with 10 mL of fetal bovine serum, 2 mL of NaHCO3, 1 mL of L-glutamine, and 1 mL of Pen–Strep solution (penicillin 10,000 U/mL and streptomycin 10 mg/mL). Cell viability was measured using an MTT assay. HepG2 cells were seeded onto 96-well plates at a density of 2×104 cells per well and allowed to attach overnight. The medium was then removed, and each well was washed twice with 200 μL of PBS. Media containing samples with various concentrations were added to each well, and the cells were incubated at 37°C for 24 hours. The medium was then removed, and the wells were washed twice with 0.2 mL of PBS. To each well, 20 μL of MTT solution (5 mg/mL in the DI water) was added, and the cells were incubated for an additional 4 hours. The medium was then replaced with 150 μL of dimethyl sulfoxide. The plates were left stationary for 4 hours to dissolve the blue crystals, and the absorbance was recorded by a microplate reader at a wavelength of 570 nm. Cell viability was expressed as the average absorbance of treated samples relative to untreated ones. HepG2 cells were seeded onto four-well Lab-Tek slides at a density of 1×105 cells per well. After incubation at 37°C with 5% CO2 overnight, the medium was removed, and the slides were washed twice with PBS. To each well, 0.25 mL of DI water containing Fe3O4@Alg samples (1 mg/mL) and 0.25 mL of Dulbecco’s Modified Eagle’s Medium were added. After incubation for an additional 4 hours, the supernatant was removed, and the slides were washed extensively with PBS. Next, 1 mL of neutral buffered formalin (10%) was added to each well. After 12 hours, the neutral buffered formalin was replaced with Prussian Blue staining reagent [20% HCl(aq) and 10% K4Fe(CN)6(aq)]. After reaction for 20 minutes, the reagent was removed, and the slides were washed with DI water three times. After removal of DI water, 500 μL of nuclear fast red counter stain was added and reacted for 5 minutes. Finally, the slides were washed completely with DI water and placed on an optical microscope (Eclipse-80i, Nikon, Tokyo, Japan). The red and pink colors were produced from nuclei and cytoplasm, respectively, and the blue color was emitted from samples containing Fe3O4.
Magnetic thermal properties and in vitro hyperthermia
Room temperature magnetization curves of the samples were measured as a function of the applied magnetic field (H) with a superconducting quantum interference device (SQUID, MPMS7, Quantum Design, Tokyo, Japan). The hysteresis loop of the magnetization was obtained by changing the magnetic field between +20,000 and −20,000 gauss at 37°C. Magnetic thermal properties were examined with an alternating magnetic field heating system. The experiment was performed inside a copper coil providing an alternating magnetic field at a fixed frequency of 780 kHz and a fixed amplitude of 19 kA/m. Samples containing Fe3O4@Alg nanoparticles were first diluted to a final concentration of 0.5 mg Fe3O4@Alg nanoparticles per mL with DI water. One milliliter of the sample was then added to a centrifuge tube that was then placed in the copper coil. The temperature of the sample was kept at 37°C±0.5°C by using an isothermal pad and measured by a thermocouple fiber. The increased temperature versus time dependence was measured for all samples. The specific absorption rate (SAR) was defined as follows:
where Cwater is the specific heat capacity of water and has a numeric value corresponding to 4,185 J L/K and c is the sample concentration in g/L.
The in vitro effects of the hyperthermia treatments were evaluated by a proliferation assay. HepG2 cells were first cultured as described in the section on cell culture, viability test, and observation. A total of 1×105 cells were harvested in 24 wells for 1 day; 500 μL of suspension containing Fe3O4@Alg nanoparticles or Fe3O4@Alg-GA nanoparticles at a concentration of 0.5 mg/mL was then added to each well. The cells were cultured for another 4 hours, and then trypsinized and moved to a centrifuge tube. Fresh culture medium was added into the centrifuge tube to achieve 0.4 mL as the total volume. Three centrifuge tubes, including one control sample (without Fe3O4@Alg nanoparticles) and two experimental samples (with Fe3O4@Alg and Fe3O4@Alg-GA nanoparticles), were put in the copper coil at the same time, and the heating time was set to 20 minutes. In order to compare the effects of the magnetic field, cells (both with and without Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles) without treatment by a magnetic field were also prepared. After treatment, 100 μL of each sample was taken from the centrifuge tube and added to 96-well plates. The cells were further cultured at 37°C with 5% CO2 for 18 hours. Next, 20 μL of MTS solution was added to each well, and the cells were incubated for an additional 4 hours. Absorbance at 450 nm was recorded using an enzyme-linked immunosorbent assay plate reader. Cell viability was expressed as the average absorbance of treated samples relative to untreated ones.
Plasma clotting time and red blood cell hemolysis
The anticoagulant activity of the synthesized Fe3O4@Alg was evaluated by testing clotting time in human plasma. A 160 μL volume of 100% platelet-poor plasma solution was mixed with each solution containing Fe3O4@Alg nanoparticles (0.5 mg/mL, 46 μL) in a 96-well plate. The solution was then recalcified by addition of calcium chloride solution (1 M, 4 μL) and shaken for 30 seconds at 37°C. Plasma clotting time was determined as the time at which the onset of the absorbance transition occurred by reading the absorbance at 660 nm using a PowerWave™ microplate spectrophotometer (Bio-Tek, Winooski, VT, USA). Each clotting time is reported as the average value of repeated measurements of six samples.
Disruption of red blood cell membranes was estimated to determine the non-fouling nature of the synthesized Fe3O4@ Alg nanoparticles using a hemolysis assay. Red blood cells were isolated by blood centrifugation and washed three times with a 0.15 M saline solution. In each hemolysis experiment, 108 red blood cells were suspended in 500 μL of PBS. Preparation of 500 μL of red blood cell solution was followed by addition of 500 μL of solution containing Fe3O4@Alg, which was prepared in PBS at a concentration of 20 mg/mL. The Fe3O4@Alg nanoparticles and red blood cell mixtures were incubated in a 37°C water bath for 1 hour. The mixed solution was then centrifuged for 5 minutes at 2,000 rpm to separate intact red blood cells and disrupted membranes from the solution. Absorbance of the supernatant containing the released hemoglobin was then measured at 541 nm using the PowerWave microplate spectrophotometer. One hundred percent hemolysis was determined by measuring the absorbance of 1×108 red blood cells with complete lysis by suspending them in DI water. The control was 1×108 red blood cells in PBS. Each hemolysis is reported as the average value of three repeated measurements with 18 samples.
Results and discussion
Synthesis and characterization of Fe3O4@ Alg nanoparticles
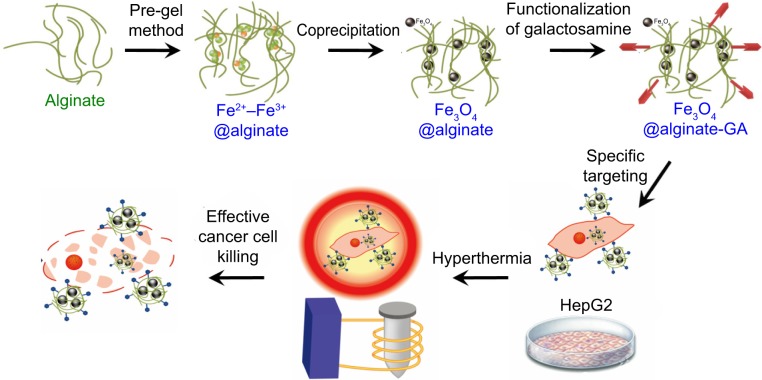
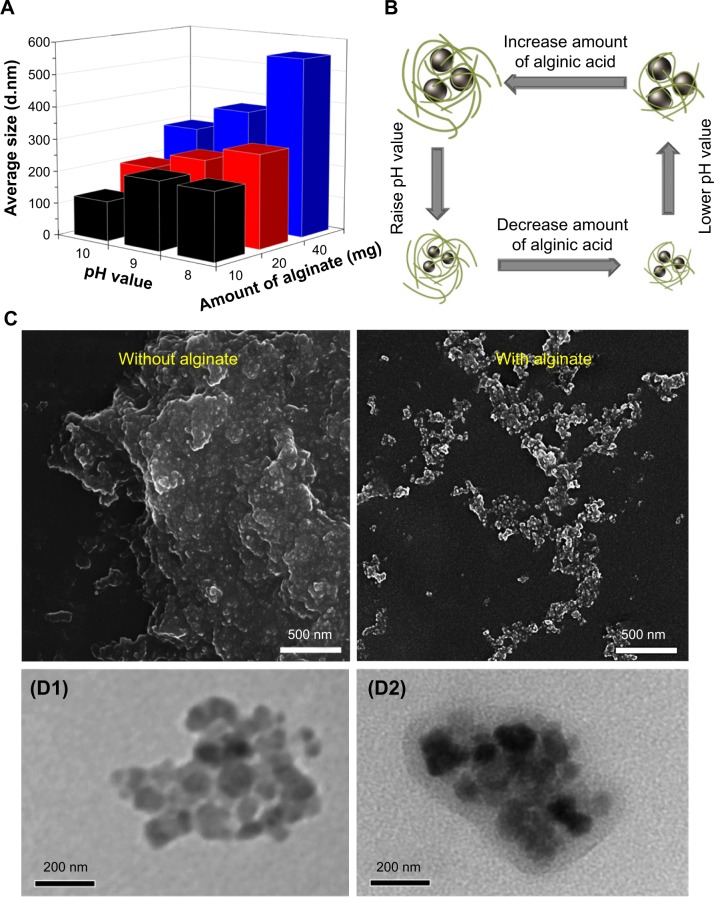
The synthesis process is shown in Figure 1. Both FeCl2 and FeCl3 solutions were added slowly to an alginate solution at various concentrations to induce the pre-gel state of alginate (ie, Fe2+ and Fe3+ ions interact with the carboxylate groups of alginate). An aqueous ammonia solution was then added to increase the pH value for formation of iron oxides (ie, coprecipitation) in alginate polymer (denoted as Fe3O4@Alg nanoparticles). Because alginate consists of d-mannuronic acid (M) and l-guluronic acid (G) with highly negative charges that can interact strongly with cations such as Fe2+ and Fe3+, the pre-gel state of alginate represents the egg-box structure of alginate-Fe2+/Fe3+,25 resulting in formation of nanoparticles. Complete precipitation of Fe3O4 is expected at basic pH values.26,27 Here we studied the effects of different pH values and amounts of alginate on the hydrodynamic diameter of the synthesized Fe3O4@Alg nanoparticles. As shown in Figure 2A, Figure S1, and Table S1, as the amount of alginate increased (from 10 to 40 mg), the hydrodynamic diameter of the synthesized Fe3O4@Alg nanoparticles also increased for all three different pH conditions. In addition, the pH in the final solution also influenced the hydrodynamic diameter, ie, particle sizes were decreased when pH increased. This is because Fe3O4 with smaller particle sizes can be synthesized at higher pH values, as shown in Figure 2B. Here we chose the experiment parameters of 10 mg of alginate and pH 10 for obtaining the optimal particle size for intravenous injection. The role of alginate is to homogeneously distribute the synthesized Fe3O4 nanoparticles. The SEM images in Figure 2C clearly show the different morphology of samples prepared without (left) and with (right) alginate. In the presence of alginate 10 mg, Fe3O4 displayed excellent separation owing to the abundant COOH groups of alginate, in contrast with the marked aggregation of Fe3O4 nanoparticles when they were synthesized in the absence of alginate. In Table 1, the sizes of Fe3O4 with and without alginate were 5.629±2.257 nm and 122±29.5 nm, respectively, which again indicates that alginate can prevent aggregation of Fe3O4. On the other hand, body temperature is about 37°C and the temperature of hyperthermia is 42.5°C. The hydrodynamic diameters of nanoparticles at both body temperature and hyperthermia temperature are also important. The hydrodynamic diameters of Fe3O4@Alg nanoparticles are 129±31.5 nm at 37°C and 275±77.2 nm at 42.5°C. It seems that Fe3O4@Alg nanoparticles would remain at a similar particle size at 37°C but aggregate slightly at 42.5°C. Slight aggregation of particles could be accepted for the in vitro experiment; however, the problem of aggregation at 42.5°C should be overcome in order to further apply our samples for in vivo experiment. TEM images clearly show successful alginate coating on Fe3O4 nanoparticles (Figure 2D). After coating with alginate, the surface charge of the samples was changed. For Fe3O4 nanoparticles, the surface potential was 16.1 mV and for Fe3O4@Alg nanoparticles was −60.1 mV. The negative charge on the Fe3O4@Alg nanoparticles resulted from the abundant COOH groups on alginate. The abundance of carboxylic groups provided a functional group for post-modification. Galactosamine is an asialoglycorprotein receptor containing an amine group which can react with the carboxylic group from alginate via EDC and NHS. At first the carboxylic group of alginate was reacted with EDC to form a highly unstable O-acylisourea, and then the NHS molecule was added to stabilize this compound. Finally, NHS-O-acylisourea, which has high reactivity toward primary amines, reacted with the amine group from galactosamine forming an amide bond between alginate and galactosamine. Post-modification of Fe3O4@Alg nanoparticles with galactosamine reduced the negative potential significantly; however, the particle sizes of Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles were similar with each other, meaning that the particle size of Fe3O4@Alg would not be affected by modification of galactosamine.
Figure 1.
Synthesis of Fe3O4@Alg-GA nanoparticles for magnetic fluid hyperthermia.
Abbreviations: Fe3O4, iron oxide; Alg, alginate; GA, galactosamine.
Figure 2.
(A, B) Effects of pH level and amount of alginate on particle size of the synthesized Fe3O4@Alg nanoparticles. (C) Scanning electron micrographs of Fe3O4 samples with and without the presence of alginate. (D) Transmission electron micrographs of Fe3O4 (D1) and Fe3O4@Alg (D2) nanoparticle samples.
Abbreviations: Fe3O4, iron oxide; Alg, alginate.
Table 1.
Summary of the properties of the synthesized Fe3O4, Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles
| Diameter (nm) | PDI | Surface potential (mV) | Saturation magnetization (emu/g) | Amount of Fe3O4 in cancer cell (pg/cell) | |
|---|---|---|---|---|---|
| Fe3O4 | 5,629±2,257 | 0.401 | +16.1 | 66.2 | – |
| Fe3O4@Alg | 122±29.5 | 0.242 | −60.1 | 69.2 | 18.5 |
| Fe3O4@Alg-GA | 128±18.9 | 0.148 | −29.7 | 71.6 | 364.4 |
Abbreviations: Alg, alginate; Fe3O4, iron oxide; GA, galactosamine; PDI, polydispersity index.
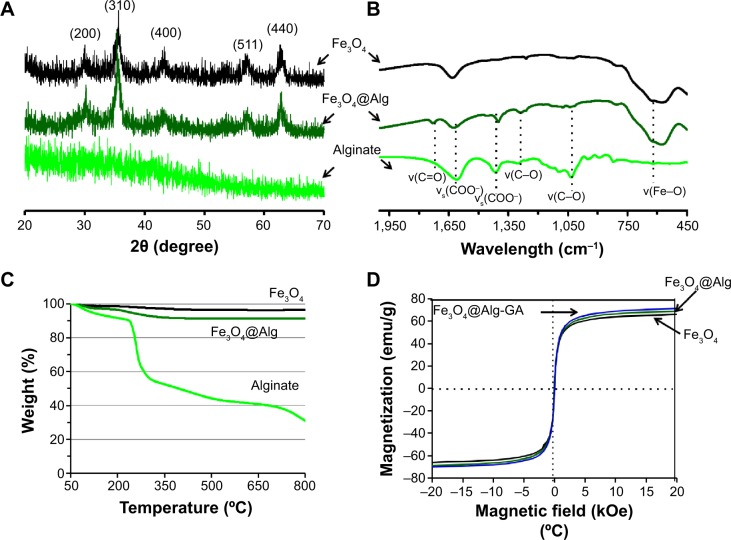
The synthesized Fe3O4, Fe3O4@Alg nanoparticles, and GA-functionalized Fe3O4@Alg nanoparticles were characterized with X-ray diffraction, FTIR, thermogravimetric analysis, and SQUID. As shown in Figure 3A, the X-ray diffraction patterns for Fe3O4 and alginate indicated a crystalline phase and an amorphous phase for Fe3O4 and alginate, respectively. The characteristic peaks at 2θ =20–70 were the (200), (310), (400), (511), and (440) of Fe3O4 cubic spinel structure phase. It is worth noting that the synthesized Fe3O4@Alg nanoparticles exhibited the same characteristic peaks as the above-mentioned Fe3O4, indicating that the synthetic method used in this study (ie, the combined coprecipitation and pre-gel methods) did not alter the crystalline phase of iron oxide. Further evidence of the formation of Fe3O4@Alg nanoparticles was provided by FTIR. As shown in Figure 3B, the FTIR spectrum of Fe3O4@Alg showed both of the characteristic bands from Fe3O4and alginate. The stretch vibrations of Fe–O at 577 cm−1 and 648 cm−1 were observed for Fe3O4, and the stretch vibrations of C–O at 1,046 cm−1 and 1,112 cm−1 were representative of C–O groups for alginate, the band at 1,316 cm−1 was the C–O stretch vibration of the COOH group, and the peaks at 1,628 cm−1 and 1,429 cm−1 were the asymmetry and symmetry stretching vibrations, respectively, of the COOH group for pure alginate.28 In order to calculate the amount of alginate in the Fe3O4@Alg nanoparticles, we used thermogravimetric analysis for all three samples. As shown in Figure 3C, samples were heated up to 800°C, and the remaining weight percentages of Fe3O4, alginate, and Fe3O4@Alg were 96.41%, 30.97%, and 91.45%, respectively. Thereafter, we calculated the percentage of alginate as 7.58 wt% per mg of Fe3O4@Alg nanoparticles. Finally, to ensure the efficiency of hyperthermia, the magnetic properties of the samples were analyzed with SQUID. As shown in Figure 3D, at a temperature of 310 K, the intensity of magnetization (emu) changes with the magnetic field (Oe). All three samples, ie, Fe3O4, Fe3O4@Alg nanoparticles, and Fe3O4@Alg-GA nanoparticles, showed similar saturation magnetization (emu/g), ie, 66.2, 69.2, and 71.6, respectively (Table 1), indicating their superparamagnetic properties.
Figure 3.
Characterization of alginate polymer, Fe3O4 nanoparticles, and Fe3O4@Alg nanoparticles.
Notes: (A) X-ray diffraction pattern, (B) Fourier transform infrared spectra, (C) thermogravimetric analysis profiles, and (D) superconducting quantum interference device measurement.
Abbreviations: Fe3O4, iron oxide; Alg, alginate.
Magnetic thermal properties and cytotoxicity of Fe3O4@Alg nanoparticles
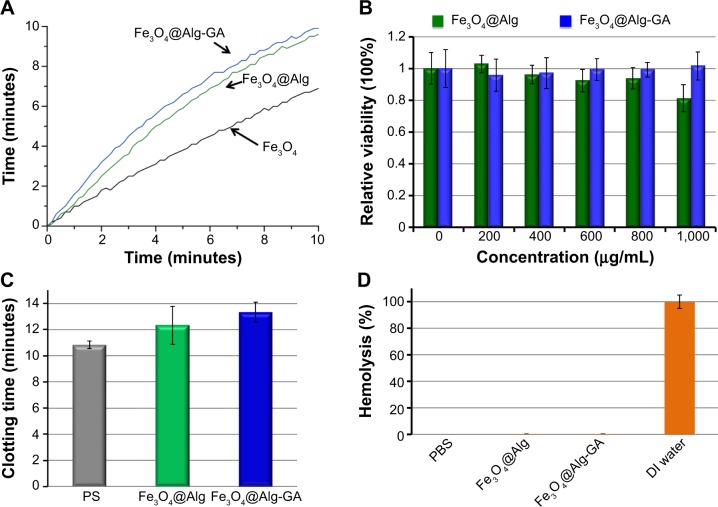
The heating properties of the synthesized Fe3O4@Alg nanoparticles were examined by measuring the increased temperature upon applying a high-frequency heating instrument (780 kHz, 19 kA/m). As shown in Figure 4A, Fe3O4@Alg nanoparticles could increase the temperature of water up to 10°C when they were exposed to a high frequency for 10 minutes. For a single-domain particle, such as the superparamagnetic particle, the external magnetic field would induce Brown relaxation and Neel relaxation to warm up the surrounding environment. Brown relaxation is described as the random rotation of the momentum through the movement of particles and Neel relaxation is described as the flipping motion of the magnetic moment relative to the particle.29 Enpuku et al30 showed that Brown relaxation would be affected by aggregation of nanoparticles. The Fe3O4 nanoparticles (without alginate) had worse heating efficiency because they aggregated easily, thereby reducing the Brownian motion.31 The SAR equation was used to determine the heat efficiency of the samples, and the SAR value for Fe3O4, Fe3O4@Alg, and Fe3O4@Alg-GA was 192.8, 212.0, and 308.4 W/g Fe, respectively. Ma et al32 showed that SAR values of magnetite particles were size-dependent. The SAR values increased as particle size decreased. It is always important to ensure biocompatibility of newly synthesized nanomaterials. Therefore, we examined the cytotoxicity of Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles by MTT assay. As shown in Figure 4B, different concentrations of Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles were cocultured with HepG2 cells for 4 hours and cell viability was measured using an ultraviolet spectrometer at 570 nm. Cell viability was not significantly decreased even when the concentration was up to 1,000 μg/mL, indicating excellent biocompatibility of the synthesized Fe3O4@Alg-based nanoparticles and implying that they are good candidates for intracellular drug delivery. It is well known that plasma membranes have a large negative charge, meaning that the membranes would repel a nanoparticle with a negative charge. Although cationic sites are scarcer on the plasma membrane, many researchers have pointed out that the cationic sites can be used for interacting with anionic nanoparticles.33–35 The process of cell uptake could be separated into two steps, ie, binding on the membrane and internalization. Wilhelm et al36 hypothesized that electrostatic interactions governed the adsorption of anionic nanoparticles onto the membrane, and interacted strongly and nonspecifically with the plasma membrane. This adsorption step precedes the internalization step and governs the overall cell uptake.
Figure 4.
Hyperthermic and cytotoxic properties of the synthesized Fe3O4 nanoparticles, Fe3O4@Alg nanoparticles, and Fe3O4@Alg-GA nanoparticles.
Notes: (A) Heating properties of these three samples. (B) MTT assays of HepG2 cells treated with different concentrations of Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles. (C) Plasma clotting time of Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles. (D) Hemolytic activity of Fe3O4@Alg nanoparticles and Fe3O4@ Alg-GA nanoparticles.
Abbreviations: Alg, alginate; DI, deionized; Fe3O4, iron oxide; GA, galactosamine; PBS, phosphate-buffered saline; PS, polystyrene.
We consider that the synthesized Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles could be applied for in vivo hyperthermia through intravenous injection, so the hemocompatibility of the samples, including anticoagulant activity and red blood cell hemolysis, was also examined. When nanomaterials interact with plasma, plasma proteins such as fibrinogen would adsorb onto the surface of the materials, resulting in plasma clotting.1 Measurement of anticoagulant activity based on plasma clotting has already become a recognized test to estimate the compatibility between blood and a nanomaterial. The synthesized Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles were directly incubated with 100% human plasma to assess the effects of direct-contact activation on plasma clotting induced by the nanomaterials as evaluated by their recalcified plasma clotting time. As shown in Figure 4C, plasma clotting time for the recalcified plasma solutions in blank polystyrene (PS) wells was determined to have an upper limit of around 11 minutes at 37°C. The Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles showed slightly prolonged plasma clotting times as well as anticoagulant activity in comparison with PS. The results indicate that the synthesized materials do not activate plasma clotting through the intrinsic coagulation pathway. A red blood cell hemolysis assay was performed to evaluate the antihemolytic activity and blood compatibility of the synthesized Fe3O4@Alg-based nanoparticles. The hemolysis values for red blood cells obtained in DI water and PBS solution at 37°C were used as positive and negative controls, respectively. Released hemoglobin that was destroyed by the nanomaterials could be detected by measuring absorbance of 541 nm. As shown in Figure 4D, both Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles showed excellent blood compatibility like the PBS solution, indicating that our Fe3O4@Alg and Fe3O4@Alg-GA nanoparticles did not have any hemolytic activity (ie, they did not cause disruption of biological membranes). The results obtained here indicate that the synthesized Fe3O4@Alg and Fe3O4@Alg-GA nanoparticles are suitable for in vivo intravenous injection.
In vitro hyperthermia
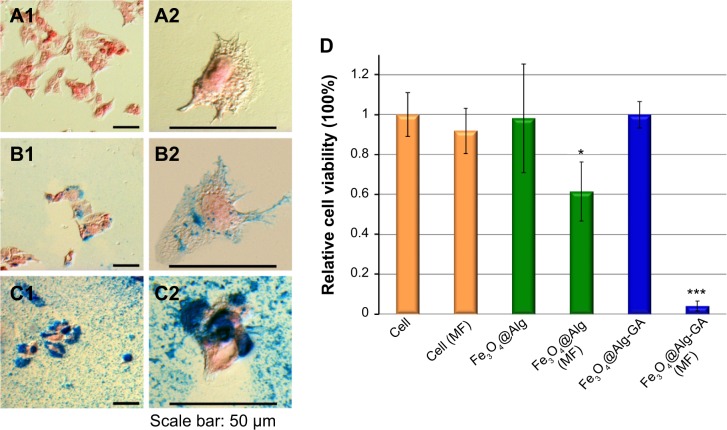
The morphology and viability of HepG2 cells with and without treatment with Fe3O4@Alg nanoparticles and Fe3O4@Alg-GA nanoparticles are shown in Figure 5A and D. Prussian blue and nuclear fast red were used to investigate the interaction between the Fe3O4@Alg samples and HepG2 cells, where Prussian blue can interact with iron oxide, resulting in blue precipitates, while nuclear fast red can stain the cytoplasm and cell nucleus. As shown in Figure 5A1 and A2, intact HepG2 cells appeared to have a clear cytoplasm and a red cell nucleus. In contrast, the HepG2 cells cultured with Fe3O4@Alg contained several blue precipitates in the cytoplasm, as shown in Figure 5B1 and B2. When HepG2 cells were treated with Fe3O4@Alg nanoparticles bearing a HepG2-targeting ligand (ie, Fe3O4@Alg-GA), there were numerous blue precipitates present in whole cells, indicating that the accumulation of Fe3O4@Alg nanoparticles could be greatly enhanced by functionalization of D-galactosamine on the surface of the Fe3O4@Alg nanoparticles, as shown in Figure 5C1 and C2. However, cells that had taken up Fe3O4@Alg nanoparticles still showed an elongated shape, indicating that the Fe3O4@Alg nanoparticles did not harm cells in the absence of a magnetic field. This conclusion can be further supported by the high cell viability in the case of Fe3O4@Alg and Fe3O4@Alg-GA nanoparticles, as shown in Figure 5D. The efficacy of hyperthermia with Fe3O4@Alg nanoparticles was evaluated by comparing cell viability with and without magnetic field treatment, as shown in Figure 5D. First, when applying a magnetic field to intact cells, cell viability only decreased slightly, as compared with the case without applying a magnetic field (ie, cell [MF]). We suggest that the anticancer effect from the magnetic field can be ignored. For the case of Fe3O4@Alg nanoparticles, cell viability decreased to around 60% after the magnetic field treatment (ie, Fe3O4@Alg [MF]), successfully demonstrating the hyperthermia effect. This effect was significantly enhanced when the cells were treated with Fe3O4@Alg-GA nanoparticles. Almost all the cells were killed (viability was only around 5% for the case of Fe3O4@Alg-GA [MF]). We propose that the hyperthermia effect results from the appearance of Fe3O4 nanoparticles inside the cells, and its efficacy should be proportional to the amount of Fe3O4 nanoparticles inside the cells. To verify this, we collected the cells that had taken up Fe3O4@Alg nanoparticles or Fe3O4@Alg-GA nanoparticles, lysed these cells, and then analyzed the amount of Fe3O4 inside them by ICP-MS. As shown in Table 1, the amounts of Fe3O4 inside the cells were 18.5 pg and 364.43 pg for the Fe3O4@Alg nanoparticle and Fe3O4@Alg-GA nanoparticle groups, respectively. This result indeed supports our hypothesis, and indicates that efficient hyperthermia could be achieved by enhanced internalization of Fe3O4 nanoparticles.
Figure 5.
(A–C) Cell morphology of HepG2 cells (A1, A2) treated with Fe3O4@Alg nanoparticles (B1, B2) and Fe3O4@Alg-GA nanoparticles (C1, C2). (D) Viability of HepG2 cancer cells incubated with Fe3O4@Alg and Fe3O4@Alg-GA nanoparticles with and without a magnetic field. The * and *** indicate the apparent difference of the results.
Abbreviations: Alg, alginate; DI, deionized; Fe3O4, iron oxide; GA, galactosamine; MF, magnetic field.
Conclusion
This study reports the synthesis of Fe3O4@Alg nanoparticles with controllable particle sizes through the combination of coprecipitation and pre-gel methods under desired synthesis conditions. The synthesized Fe3O4@Alg nanoparticles demonstrated excellent cell and blood compatibility, along with hyperthermic activity toward HepG2 cells. The abundant COOH groups on the external surface afford the ability to functionalize Fe3O4@Alg nanoparticles with a liver cancer cell-targeting ligand (ie, d-galactosamine). Fe3O4@Alg-GA nanoparticles show enhanced hyperthermia efficacy owing to their increased internalization, which shows significant potential as effective and visually observable transmembrane heat nanogenerator. Further development of these Fe3O4@Alg nanoparticles with controlled drug delivery can lead to a new generation of nanodevices for biomedical applications.
Supplementary materials
Size distribution of Fe3O4 (A), Fe3O4@Alg (B), Fe3O4@Alg-GA (C).
Table S1.
Size and PDI of Fe3O4@Alg nanoparticles according to pH value and amount of alginate included during synthesis
| Amount of alginate (mg)
|
10 | 20 | 40 | |
|---|---|---|---|---|
| pH value | ||||
| 10 | Size | 122±29.5 | 196.6±72.9 | 292.6±115.3 |
| PDI | 0.242 | 0.372 | 0.395 | |
| 9 | Size | 213.8±61.6 | 245.4±97 | 368.1±157.9 |
| PDI | 0.289 | 0.396 | 0.429 | |
| 8 | Size | 210.2±92.8 | 288.4±133.3 | 552±316.3 |
| PDI | 0.442 | 0.463 | 0.573 |
Abbreviation: PDI, polydispersity index
Acknowledgments
This research was supported by the Ministry of Science and Technology, Taiwan (101-2628-E-002-015-MY3), National Taiwan University (102R7842, 102R7740), Center of Strategic Materials Alliance for Research and Technology, National Taiwan University (102R104100), and National Health Research Institutes of Taiwan (03A1-BNMP14-014).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Frohlich E. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258(2–3):139–147. doi: 10.1016/j.tox.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Yu CH, Saadi AA, Shih SJ, Qiu L, Tam KY, Tsang SC. Immobilization of BSA on silica-coated magnetic iron oxide nanoparticle. J Phys Chem. 2009;113(2):537–543. [Google Scholar]
- 3.Yu X, Wan J, Shan Y, Chen K, Han X. A facile approach to fabrication of bifunctional magnetic-optical Fe3O4@ZnS microspheres. Chem Mater. 2009;21(20):4892–4898. [Google Scholar]
- 4.Liu Y, Yuan M, Qiao L, Guo R. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens Bioelectron. 2014;52:391–396. doi: 10.1016/j.bios.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Xie J, Ho D, et al. Au-Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew Chem Int Ed. 2008;47(1):173–176. doi: 10.1002/anie.200704392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Yi P, Zhang Y, Zhang L, Deng Z, Zhang Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl Mater. 2011;3(10):4085–4091. doi: 10.1021/am2009647. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Ono K, Suzuki H, et al. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl Mater. 2010;2(7):1903–1911. doi: 10.1021/am100237p. [DOI] [PubMed] [Google Scholar]
- 8.Fukao H, Ikeda M, Ichikawa T, et al. Effect of hyperthermia on the viability and the fibrinolytic potential of human cancer cell lines. Clin Chim Acta. 2000;296(1–2):17–33. doi: 10.1016/s0009-8981(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 9.Storm FK, Harrison WH, Elliott RS, Morton DL. Normal tissue and solid tumor effects of hyperthermia in animal-models and clinical-trials. Cancer Res. 1979;39(6):2245–2251. [PubMed] [Google Scholar]
- 10.Fleet A. Radiobiology for the Radiologist: 6th edition. J Radiother Pract. 2006;5(4):237–237. [Google Scholar]
- 11.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci U S A. 1997;94(24):13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H, Xue M, Xia T, et al. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5(5):4131–4144. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 15.Low PS. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41(1):120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 16.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 17.Parker N. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Park EI, Mi Y, Unverzagt C, Gabius HJ, Baenziger JU. The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid α2,6GalNAc. Proc Natl Acad Sci U S A. 2005;102(47):17125–17129. doi: 10.1073/pnas.0508537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu M, Diao G. Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J Phys Chem C. 2011;115(39):18923–18934. [Google Scholar]
- 20.Zhang H, Zhu G. One-step hydrothermal synthesis of magnetic Fe3O4 nanoparticles immobilized on polyamide fabric. Appl Surf Sci. 2012;258(11):4952–4959. [Google Scholar]
- 21.Bateer B, Tian C, Qu Y, et al. Facile synthesis and shape control of Fe3O4 nanocrystals with good dispersion and stabilization. Cryst Eng Comm. 2013;15(17):3366–3371. [Google Scholar]
- 22.Hyeon T, Lee SS, Park J, Chung Y, Na HB. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 2001;123(51):12798–12801. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- 23.Daou TJ, Pourroy G, Bégin-Colin S, et al. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem Mater. 2006;18(18):4399–4404. [Google Scholar]
- 24.Huang J, Li Q, Li D, et al. Fluxible nanoclusters of Fe3O4 nanocrystal-embedded polyaniline by macromolecule-induced self-assembly. Langmuir. 2013;29(32):10223–102238. doi: 10.1021/la402367c. [DOI] [PubMed] [Google Scholar]
- 25.Rees RA, Welsh EJ. Secondary and tertiary structure of polysaccharides in solutions and gels. Angew Chem Int Ed. 1977;16(4):214–224. [Google Scholar]
- 26.Lian S, Wang E, Kang Z, et al. Synthesis of magnetite nanorods and porous hematite nanorods. Solid State Commun. 2004;129(8):485–490. [Google Scholar]
- 27.Sun J, Zhou S, Hou P, et al. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J Biomed Mater Res Part A. 2007;80(2):333–341. doi: 10.1002/jbm.a.30909. [DOI] [PubMed] [Google Scholar]
- 28.Watthanaphanit A, Supaphol P, Furuike T, Tokura S, Tamura H, Rujiravanit R. Novel chitosan-spotted alginate fibers from wet-spinning of alginate solutions containing emulsified chitosan-citrate complex and their characterization. Biomacromolecules. 2009;10(2):320–327. doi: 10.1021/bm801043d. [DOI] [PubMed] [Google Scholar]
- 29.Sarangi S, Tan IC, Brazdeikis A. Brownian relaxation of interacting magnetic nanoparticles in a colloid subjected to a pulsatile magnetic field. J Nanosci Nanotechnol. 2011;11(5):4136–4141. doi: 10.1166/jnn.2011.4112. [DOI] [PubMed] [Google Scholar]
- 30.Enpuku K, Tanaka T, Matsuda T, et al. Properties of magnetic nanoparticles in the Brownian relaxation range for liquid phase immunoassays. J Appl Phys. 2007;102(5):054910. [Google Scholar]
- 31.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14(14):2161–2175. [Google Scholar]
- 32.Ma M, Wu Y, Zhou H, Sun YK, Zhang Y, Gu N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J Magn Magn Mater. 2004;268(1–2):33–39. [Google Scholar]
- 33.Farquhar MG. Recovery of surface-membrane in anterior-pituitary cells – variations in traffic detected with anionic and cationic ferritin. J Cell Biol. 1978;77(3):R35–R42. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutsaers SE, Papadimitriou JM. Surface-charge of macrophages and their interaction with charged-particles. J Leukoc Biol. 1988;44(1):17–26. doi: 10.1002/jlb.44.1.17. [DOI] [PubMed] [Google Scholar]
- 35.Ghinea N, Simionescu N. Anionized and cationized hemeundecapeptides as probes for cell-surface charge and permeability studies – differentiated labeling of endothelial plasmalemmal vesicles. J Cell Biol. 1985;100(2):606–612. doi: 10.1083/jcb.100.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm C, Billotey C, Roger J, Pons JN, Bacri JC, Gazeau F. Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials. 2003;24(6):1001–1011. doi: 10.1016/s0142-9612(02)00440-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Size distribution of Fe3O4 (A), Fe3O4@Alg (B), Fe3O4@Alg-GA (C).
Table S1.
Size and PDI of Fe3O4@Alg nanoparticles according to pH value and amount of alginate included during synthesis
| Amount of alginate (mg)
|
10 | 20 | 40 | |
|---|---|---|---|---|
| pH value | ||||
| 10 | Size | 122±29.5 | 196.6±72.9 | 292.6±115.3 |
| PDI | 0.242 | 0.372 | 0.395 | |
| 9 | Size | 213.8±61.6 | 245.4±97 | 368.1±157.9 |
| PDI | 0.289 | 0.396 | 0.429 | |
| 8 | Size | 210.2±92.8 | 288.4±133.3 | 552±316.3 |
| PDI | 0.442 | 0.463 | 0.573 |
Abbreviation: PDI, polydispersity index