Abstract
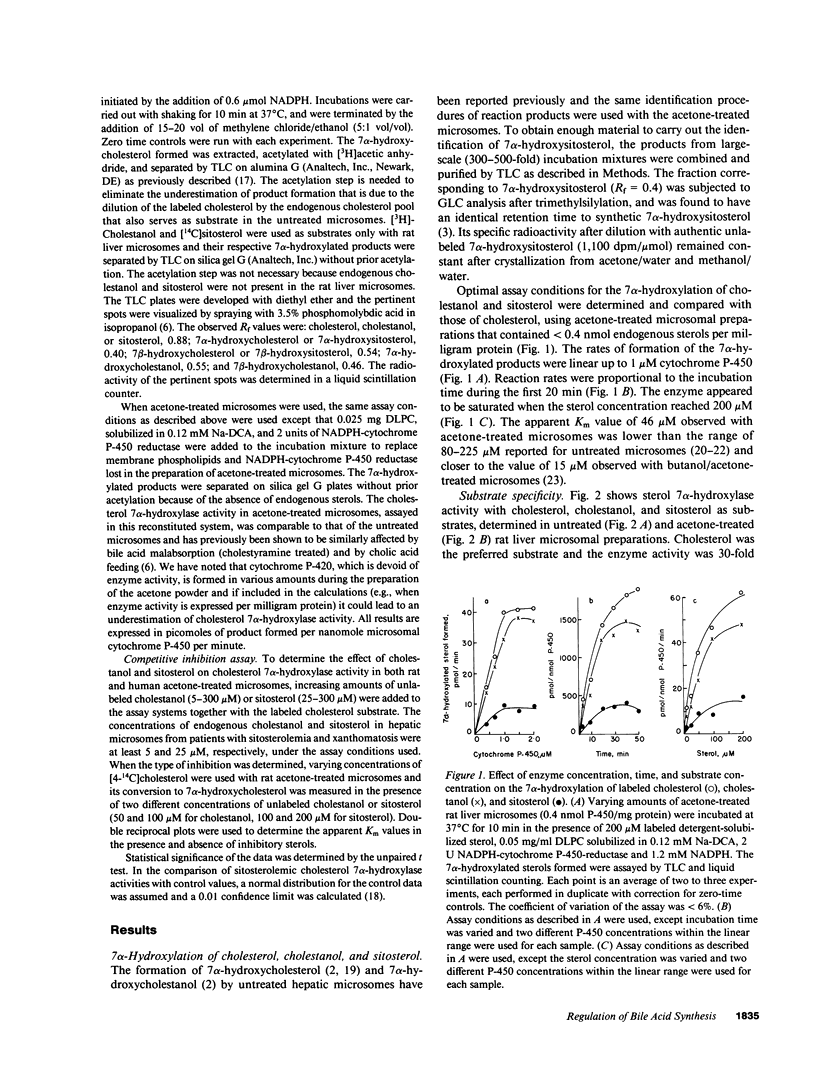
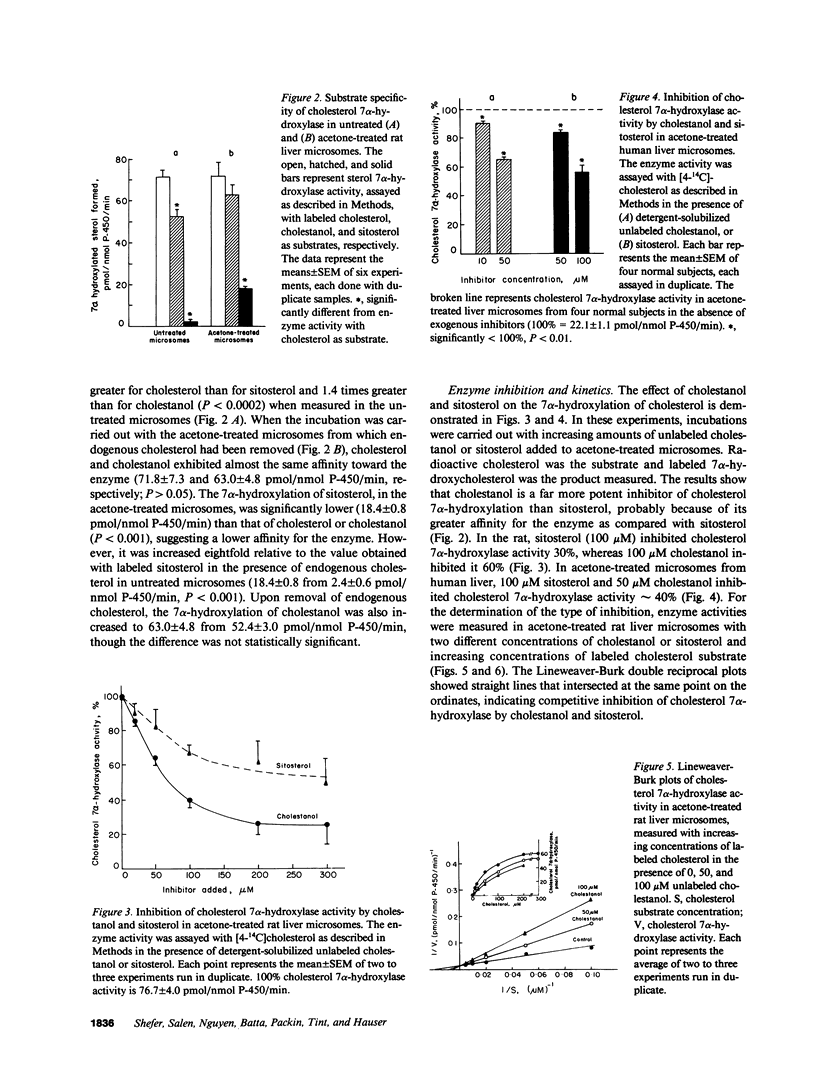
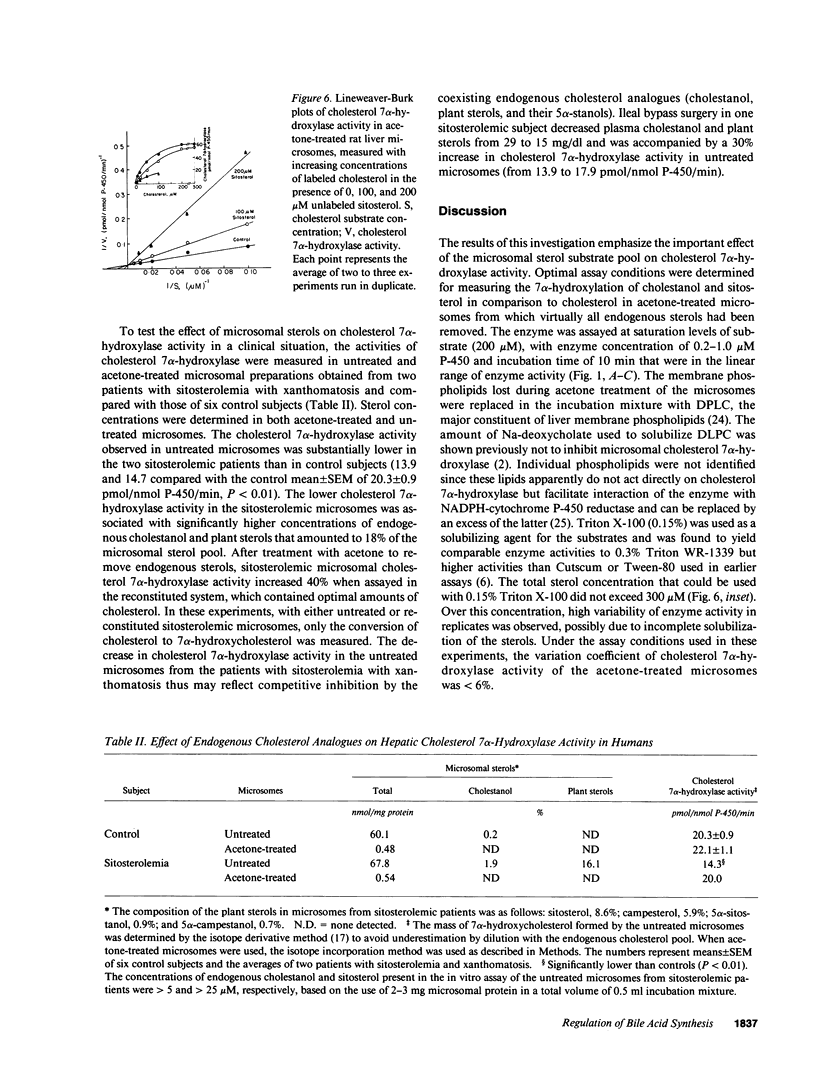
The 7 alpha-hydroxylation of two cholesterol analogues, sitosterol and cholestanol, and their effect on the 7 alpha-hydroxylation of cholesterol were measured in rat and human hepatic microsomes. In untreated rat liver microsomes, the 7 alpha-hydroxylation of cholesterol was higher than that of cholestanol (1.4-fold) and sitosterol (30-fold). After removal of endogenous sterols from the microsomes by acetone treatment, the 7 alpha-hydroxylation of cholesterol was similar to that of cholestanol and only fourfold higher than that of sitosterol. Cholestanol and sitosterol competitively inhibited cholesterol 7 alpha-hydroxylase in both rat and human liver microsomes, with cholestanol the more potent inhibitor. Patients with sitosterolemia with xanthomatosis, who have elevated microsomal cholestanol and sitosterol, showed reduced cholesterol 7 alpha-hydroxylase activity relative to the activity in control subjects (13.9 and 14.7 vs. 20.3 +/- 0.9 pmol/nmol P-450 per min, P less than 0.01). Enzyme activity in these patients was 40% higher when measured in microsomes from which competing sterols had been removed. Ileal bypass surgery in one sitosterolemic patient decreased plasma cholestanol and sitosterol concentrations and resulted in a 30% increase in hepatic microsomal cholesterol 7 alpha-hydroxylase activity. Cholesterol 7 alpha-hydroxylase appears to have a specific apolar binding site for the side chain of cholesterol and is affected by the presence of cholestanol and sitosterol in the microsomal substrate pool. Reduced bile acid synthesis in sitosterolemia with xanthomatosis may be related to the inhibition of cholesterol 7 alpha-hydroxylase activity by endogenous cholesterol analogues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond H. R., Vlahcevic Z. R., Bell C. C., Jr, Gregory D. H., Swell L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med. 1973 Dec 6;289(23):1213–1216. doi: 10.1056/NEJM197312062892302. [DOI] [PubMed] [Google Scholar]
- Aringer L., Eneroth P. Studies on the formation of C7-oxygenated cholesterol and beta-sitosterol metabolites in cell-free preparations of rat liver. J Lipid Res. 1973 Sep;14(5):563–572. [PubMed] [Google Scholar]
- Boyd G. S., Brown M. J., Hattersley N. G., Suckling K. E. Studies on the specificity of the rat liver microsomal cholesterol 7alpha-hydroxylase. Biochim Biophys Acta. 1974 Jan 23;337(1):132–135. doi: 10.1016/0005-2760(74)90047-2. [DOI] [PubMed] [Google Scholar]
- Dayal B., Tint G. S., Batta A. K., Speck J., Khachadurian A. K., Shefer S., Salen G. Identification of 5 alpha-stanols in patients with sitosterolemia and xanthomatosis: stereochemistry of the protonolysis of steroidal organoboranes. Steroids. 1982 Aug;40(2):233–243. doi: 10.1016/0039-128x(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Ilias A. M., Connor W. E., Cory H. T., Lin D. S., Daves G. D., Jr, Krippaehne W. W. Sterols of human gallstones: the recent identification of eight different digitonin precipitable sterols. Gastroenterology. 1980 Sep;79(3):539–544. [PubMed] [Google Scholar]
- Koopman B. J., van der Molen J. C., Wolthers B. G., de Jager A. E., Waterreus R. J., Gips C. H. Capillary gas chromatographic determination of cholestanol/cholesterol ratio in biological fluids. Its potential usefulness for the follow-up of some liver diseases and its lack of specificity in diagnosing CTX (cerebrotendinous xanthomatosis). Clin Chim Acta. 1984 Mar 13;137(3):305–315. doi: 10.1016/0009-8981(84)90119-0. [DOI] [PubMed] [Google Scholar]
- Kwok C. T., Burnett W., Hardie I. R. Regulation of rat liver microsomal cholesterol 7 alpha-hydroxylase: presence of a cytosolic activator. J Lipid Res. 1981 May;22(4):570–579. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miettinen T. A., Kesäniemi Y. A., Järvinen H., Hästbacka J. Cholesterol precursor sterols, plant sterols, and cholestanol in human bile and gallstones. Gastroenterology. 1986 Apr;90(4):858–864. doi: 10.1016/0016-5085(86)90861-9. [DOI] [PubMed] [Google Scholar]
- Miwa G. T., Lu A. Y. The association of cytochrome P-450 and NADPH-cytochrome P-450 reductase in phospholipid membranes. Arch Biochem Biophys. 1984 Oct;234(1):161–166. doi: 10.1016/0003-9861(84)90337-0. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ozasa S., Boyd G. S. Cholesterol 7 alpha-hydroxylase of rat liver. Studies on the solubilisation, resolution and reconstitution of the enzyme complex. Eur J Biochem. 1981 Oct;119(2):263–272. doi: 10.1111/j.1432-1033.1981.tb05603.x. [DOI] [PubMed] [Google Scholar]
- Salen G., Ahrens E. H., Jr, Grundy S. M. Metabolism of beta-sitosterol in man. J Clin Invest. 1970 May;49(5):952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Horak I., Rothkopf M., Cohen J. L., Speck J., Tint G. S., Shore V., Dayal B., Chen T., Shefer S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985 Sep;26(9):1126–1133. [PubMed] [Google Scholar]
- Salen G., Nicolau G., Shefer S., Mosbach E. H. Hepatic cholesterol metabolism in patients with gallstones. Gastroenterology. 1975 Sep;69(3):676–684. [PubMed] [Google Scholar]
- Schmitz F. J., McDonald F. J. Determination of hepatic cholesterol 7alpha-hydroxylase activity in man. J Lipid Res. 1974 Mar;15(2):146–151. [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Hauser S., Batta A. K., Salen G. Regulation of bile acid synthesis. Measurement of cholesterol 7 alpha-hydroxylase activity in rat liver microsomal preparations in the absence of endogenous cholesterol. J Lipid Res. 1981 Mar;22(3):532–536. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Feedback regulation of bile acid biosynthesis in the rat. J Lipid Res. 1969 Nov;10(6):646–655. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. 7-alpha-hydroxylation of cholestanol by rat liver microsomes. J Lipid Res. 1968 May;9(3):328–333. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. 7-alpha-hydroxylation of cholestanol by rat liver microsomes. J Lipid Res. 1968 May;9(3):328–333. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Salen G., Zaki F. G., Bullock J., Salgado E., Shevitz J. Comparative effects of cholestanol and cholesterol on hepatic sterol and bile acid metabolism in the rat. J Clin Invest. 1984 Nov;74(5):1773–1781. doi: 10.1172/JCI111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Nicolau G., Mosbach E. H. Isotope derivative assay of microsomal cholesterol 7 alpha-hydroxylase. J Lipid Res. 1975 Mar;16(2):92–96. [PubMed] [Google Scholar]
- Van Cantfort J., Renson J., Gielen J. Rat-liver cholesterol 7 alpha-hydroxylase. 1. Development of a new assay based on the enzymic exchange of the tritium located on the 7 alpha position of the substrate. Eur J Biochem. 1975 Jun 16;55(1):23–31. doi: 10.1111/j.1432-1033.1975.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Waskell L., Koblin D., Canova-Davis E. The lipid composition of human liver microsomes. Lipids. 1982 Apr;17(4):317–320. doi: 10.1007/BF02534948. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]
- Yokohama H., Ohtsuka I., Shiojiri H., Katayama K., Ishikawa S. A high-performance liquid chromatographic method for the assay of cholesterol 7 alpha-hydroxylase activity. Anal Biochem. 1986 Aug 15;157(1):186–190. doi: 10.1016/0003-2697(86)90212-5. [DOI] [PubMed] [Google Scholar]


