Abstract
Background
Wilson disease requires lifelong therapy, currently given daily in multiple divided dosages.
Aim
To prospectively evaluate once-daily trientine as therapy for Wilson disease.
Methods
Study group: eight patients (seven males) aged 22–71 years with stable Wilson disease treated from 4 to 50 years. Patients were monitored for 3 months then for 12 months on a single daily dose of trientine (15 mg/kg).
Results
All patients remained clinically well. ALT and AST fluctuated in some, but none required treatment stoppages or side effects. Liver synthetic function was unchanged. Mean 24-h urine copper and zinc excretions at end of treatment were 313.4 ± 191.7 and 2,214 ± 1,346 μg, respectively.
Conclusions
Once-daily trientine should be explored further for possible maintenance therapy for WD. Single daily dose may improve adherence to therapy. Larger trials and longer-term follow-up will establish the safety and treatment efficacy of this once-daily treatment regimen for WD (registration: NCT01472874).
Keywords: Wilson disease, Treatment, Trientine, Adherence
Introduction
Wilson disease (WD) is an autosomal recessive disorder of copper metabolism affecting ~1:30,000 individuals [1, 2]. Liver and neurological injury may be prevented or treated by lifelong medical therapy with d-penicillamine or trientine, or zinc salts that inhibit copper absorption. Up to 50 % of WD patients have medication non-adherence [3]. Failure to adhere to treatment inevitably leads to neurological injury and psychiatric symptoms, hepatic failure, and ultimately death [4].
Trientine was approved for WD patients intolerant of d-penicillamine [5, 6]. Trientine is gaining popularity as a first-line agent due to its favorable safety profile. Trientine is conventionally dosed at 750–1,500 mg/day in 2–4 divided doses. Increases in urinary trientine excretion correlate with increased urinary copper excretion [7].
There have been very few prospective studies for treating WD. Weiss et al. [8] retrospectively reviewed treatments for WD, concluding that zinc was less effective than chelating agents for long-term use. They observed discontinuation for treatment failure for 14/88 on zinc compared to 4/213 on chelation therapy. Important factors such as having to interrupt daily routines to avoid taking medications with food is a significant lifestyle issue for some patients and important for maintaining medication adherence [9].
Once-daily dose yields the highest compliance for drugs with average tolerability; the more often a drug is taken, the less the adherence [10, 11]. Adherence is better for drugs perceived as having life and death consequence such as critical antiarrhythmic therapy [12]. Drug adherence is more problematic for asymptomatic or stable conditions such as hypertension or maintenance therapy for WD [13, 14]. Daily dosed medications available as long-acting preparations are frequently utilized in the treatment of chronic medical disorders such as hypertension [15]. These daily dosed medications had better adherence. There are currently no comparable extended release preparations for treatments for WD.
There are few investigations of available chelators for WD to inform about the frequency of their administration [16]. Conventional recommendations are multiple daily dosages administered apart from meals to enhance intestinal absorption and maximize copper excretion. The potential benefits of a once-daily trientine dose as maintenance therapy for WD were previously reported as a retrospective review of case reports [17]. Three patients took trientine once-daily for 2–15 years. Liver function and parameters of copper metabolism were normal, and examinations showed clinical stability. These data suggested the need for a prospective trial. To this end, we undertook a prospective study to determine the safety and effectiveness of a 12-month course of a once-daily weight-based dose of trientine for maintenance treatment for WD.
Methods
We designed a prospective study of once-daily trientine for WD. The protocol was approved by the HIC committee at Yale University Medical Center. The trial was registered on www.clinicaltrials.gov (registration number NCT01472874, first received: November 11, 2011, last updated: July 17, 2012). Inclusion criteria required a diagnosis of WD, >1 year clinical stability on current therapy, and stable liver disease. The study group included eight patients (seven males) aged 22–71 years treated from 4 to 50 years (median of 8 years). Presentations of WD included asymptomatic (n = 1), incidental detection of KF rings (n = 1), neuropsychiatric then liver and stable neurological disease (n = 1), stable neurological with liver disease (n = 1) and liver disease alone (n = 4). Prior treatments were zinc acetate (n = 2), d-penicillamine (n = 1), and trientine (n = 5). Patients were consented before entry into the clinical trial. Three consecutive monthly clinical and laboratory evaluations were made before beginning the study treatment. The physical evaluations included assessments of the neurological and liver disease. Patients were asked to complete a pre- and post-study questionnaire. Serum and 24-h basal urinary studies prior to entry included blood counts with platelets, ALT, AST, ALP, total bilirubin, direct bilirubin, gamma glutamyl transpeptidase, albumin, international normalized ratio (INR), serum copper, serum ceruloplasmin, antinuclear antibody, erythrocyte sedimentation rate, 24-h urine for copper and zinc excretion and volume, urinalysis, AST-to-platelet ratio [18], and levels of several proteins important for liver fibrosis [19]. Statistical testing of data was performed using Student’s t test (two tailed) compared mean values obtained before and after treatment.
Treatment was with trientine, 15 mg/kg (rounded upward to the nearest 250 mg) once-daily for 12 months. Patients were monitored monthly for 3 months and at 6, 9, and 12 months, and study coordinators reviewed patient logs and conducted formal pill counts. Treatment dose was based on AASLD practice guidelines and represents the dosage used in children [1, 20, 21]. Subjects were to be removed from the study if the laboratory values were abnormal (ALT or AST > twice baseline values) for two sequential blood tests or for any decline in the patient’s health.
Results
After treatment with once-daily trientine, all were clinically well. Their physical examination remained unchanged, and no new neurological signs were detected. There were no stoppages of treatment or dropouts from the study.
Liver Function Tests, Copper and Zinc Studies
Results for ALT, AST, total bilirubin, INR, albumin, serum copper, 24-h urine copper and zinc are shown in Table 1. ALT and AST levels were not statistically changed by treatment in 6/8 patients, and mean values were 41.38 ± 31.81 and 28.22 ± 10.57 U/L, respectively, on their prior therapy, and 50.89 ± 32.29 and 33.53 ± 11.17 U/L at trial end. In two (patients 2 and 3), the differences in mean ALT before and after treatment did reach statistical significance, but end of treatment values were at the upper limit of normal in one and above normal but <2 times the upper limit of normal in the other. Neither had changes in hepatic synthetic function or bilirubin over the course of treatment. Total bilirubin, albumin, and INR were relatively unchanged for the cohort at the trial end. Urinary copper excretion increased from baseline values from 2.8 to 9.5 times baseline values in patients changed from pre-treatment zinc to trientine as expected, but was relatively unchanged for patients on d-penicillamine or trientine following their change to once-daily trientine. One patient, patient 4, had a 330 % increase in copper excretion, likely from better adherence. In line with the findings obtained from urinary copper, the patients all had non-ceruloplasmin copper that was below 15 mcg/dl prior to study entry and no patient exceeded this threshold during the study. Mean 24-h urine zinc excretion was not significantly changed after treatment for the cohort, but there were two patients on trientine that increased their zinc excretion by ~40 % (Fig. 1).
Table 1.
Biochemical testing before and after trientine treatment
| Medication | Pre-treatment | Post-treatment | p value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Min–max | Mean ± SD | Min–max | |||
| ALT (0–35 U/L) | ||||||
| P1 | Trientine | 33.33 ± 4.04 | 31–38 | 58.66 ± 15.84 | 40–82 | 0.123 |
| P2 | d-Penicillamine | 16.75 ± 2.21 | 14–19 | 34 ± 4.77 | 28–41 | 0.006 |
| P3 | Zinc | 29.75 ± 5.56 | 26–38 | 52.67 ± 16.35 | 37–76 | 0.003 |
| P4 | Trientine | 17.75 ± 2.5 | 15–21 | 22.17 ± 4.44 | 19–31 | 0.242 |
| P5 | Zinc | 35.25 ± 23.64 | 17–69 | 19.2 ± 2.16 | 17–22 | 0.311 |
| P6 | Trientine | 100.75 ± 30.99 | 58–132 | 116.16 ± 12.73 | 102–139 | 0.483 |
| P7 | Trientine | 22.5 ± 2.08 | 20–25 | 26.67 ± 6.56 | 18–38 | 0.659 |
| P8 | Trientine | 73 ± 13.68 | 57–89 | 72.33 ± 6.37 | 64–80 | 0.955 |
| All | 41.38 ± 31.81 | 14–132 | 50.89 ± 32.29 | 17–139 | 0.448 | |
| INR (0.8–1.15) | ||||||
| P1 | Trientine | 0.95 ± 0.03 | 0.93–0.99 | 1.05 ± 0.05 | 0.99–1.13 | 0.129 |
| P2 | d-Penicillamine | 1.12 ± 0.05 | 1.05–1.17 | 1.24 ± 0.04 | 1.18–1.31 | 0.038 |
| P3 | Zinc | 0.94 ± 0.02 | 0.93–0.95 | 1.04 ± 0.03 | 1.01–1.09 | 0.019 |
| P4 | Trientine | 0.90 ± 0.02 | 0.88–0.94 | 0.96 ± 0.06 | 0.89–1.09 | 0.086 |
| P5 | Zinc | 1.01 ± 0.05 | 0.98–1.1 | 1.03 ± 0.02 | 0.99–1.07 | 0.706 |
| P6 | Trientine | 1 ± 0.04 | 0.96–1.05 | 1.03 ± 0.01 | 1.02–1.06 | 0.114 |
| P7 | Trientine | 0.99 ± 0.04 | 0.95–1.05 | 1.04 ± 0.04 | 0.99–1.12 | 0.071 |
| P8 | Trientine | 0.99 ± 0.02 | 0.95–1.01 | 1.02 ± 0.07 | 0.96–1.16 | 0.289 |
| All | 0.99 ± 0.07 | 0.93–1.17 | 1.05 ± 0.08 | 0.89–1.31 | 0.002 | |
| Cu serum (0.75–1.45 mcg/ml) | ||||||
| P1 | Trientine | 0.74 ± 0.07 | 0.66–0.81 | 0.79 ± 0.05 | 0.71–0.87 | 0.297 |
| P2 | d-Penicillamine | 0.29 ± 0.01 | 0.27–0.31 | 0.26 ± 0.02 | 0.24–0.3 | 0.048 |
| P3 | Zinc | 1.0 ± 0.07 | 0.94–1.1 | 0.88 ± 0.10 | 0.8–1.02 | 0.062 |
| P4 | Trientine | 0.65 ± 0.04 | 0.6–0.7 | 0.66 ± 0.01 | 0.64–0.69 | 0.533 |
| P5 | Zinc | 0.48 ± 0.59 | 0.4–0.53 | 0.43 ± 0.06 | 0.38–0.53 | 0.434 |
| P6 | Trientine | 0.11 ± 0.02 | <0.1–0.14 | 0.11 ± 0.008 | 0.11–0.13 | 0.634 |
| P7 | Trientine | 0.80 ± 0.06 | 0.74–0.9 | 0.84 ± 0.02 | 0.82–0.85 | 0.474 |
| P8 | Trientine | 0.16 ± 0.02 | 0.13–0.19 | 0.16 ± 0.009 | 0.15–0.18 | 0.717 |
| All | 0.54 ± 0.30 | <0.1–1.1 | 0.52 ± 0.30 | 0.11–1.02 | 0.964 | |
| Cu urine (15–60 mcg/24 h) | ||||||
| P1 | Trientine | 144.3 ± 9 | 135–154 | 239.8 ± 90.3 | 150–411 | 0.301 |
| P2 | d-Penicillamine | 843.5 ± 35.9 | 793–877 | 485.6 ± 276.4 | 131–833 | 0.084 |
| P3 | Zinc | 181.7 ± 34.8 | 144–224 | 513.5 ± 166.4 | 267–684 | 0.033 |
| P4 | Trientine | 60.2 ± 40.7 | 18–116 | 200 ± 33.5 | 155–234 | 0.028 |
| P5 | Zinc | 10 ± 1.4 | 9 and 11 | 95.2 ± 20.5 | 66–122 | 0.052 |
| P6 | Trientine | 327 ± 27.3 | 303–363 | 374 ± 113.9 | 203–476 | 0.625 |
| P7 | Trientine | 227.6 ± 33.7 | 189–251 | 370.8 ± 86.6 | 227– 450 | 0.104 |
| P8 | Trientine | 318.7 ± 154.4 | 135–507 | 211.8 ± 144.5 | 110–500 | 0.445 |
| All | 287.9 ± 259.1 | 9–877 | 313.4 ± 191.7 | 66–833 | 0.643 | |
| Zn urine (300–600 mcg/24) | ||||||
| P1 | Trientine | 1,109 ± 86 | 944–1,311 | 1,944 ± 1,110 | 820–2,917 | 0.442 |
| P2 | d-Penicillamine | 3,297 ± 106 | 3,192–3,439 | 3,441 ± 1,645 | 1,481–5,228 | 0.855 |
| P3 | Zinc | 1,990 ± 493 | 1,475–2,535 | 1,718 ± 799 | 727–3,091 | 0.969 |
| P4 | Trientine | 1,598 ± 566 | 912–2,297 | 2,669 ± 642 | 1,986–3,671 | 0.155 |
| P5 | Zinc | 656 ± 186 | 503–864 | 1,316 ± 209 | 963–1,461 | 0.113 |
| P6 | Trientine | 1,482 ± 322 | 1,002–1,698 | 1,231 ± 461 | 498–1,759 | 0.432 |
| P7 | Trientine | 3,607 ± 377 | 3,172–3,844 | 4,251 ± 784 | 2,892–4,837 | 0.405 |
| P8 | Trientine | 1,805 ± 737 | 764–2,503 | 1,336 ± 1,054 | 691–3,453 | 0.644 |
| All | 1,959 ± 1,003 | 503–3,844 | 2,214 ± 1,346 | 498–5,228 | 0.428 | |
| Albumin (3.5–5.0 g/dL) | ||||||
| P1 | Trientine | 4.4 ± 0.2 | 4.2–4.6 | 4.53 ± 0.18 | 4.3–4.8 | 0.789 |
| P2 | d-Penicillamine | 0.29 ± 0.01 | 0.27–0.31 | 0.26 ± 0.02 | 0.24–0.3 | 0.048 |
| P3 | Zinc | 1.0 ± 0.07 | 0.94–1.1 | 0.88 ± 0.10 | 0.8–1.02 | 0.062 |
| P4 | Trientine | 0.65 ± 0.04 | 0.6–0.7 | 0.66 ± 0.01 | 0.64–0.69 | 0.533 |
| P5 | Zinc | 0.48 ± 0.59 | 0.4–0.53 | 0.43 ± 0.06 | 0.38–0.53 | 0.434 |
| P6 | Trientine | 0.11 ± 0.02 | 0.1–0.14 | 0.11 ± 0.008 | 0.11–0.13 | 0.634 |
| P7 | Trientine | 0.80 ± 0.06 | 0.74–0.9 | 0.84 ± 0.02 | 0.82–0.85 | 0.474 |
| P8 | Trientine | 0.16 ± 0.02 | 0.13–0.19 | 0.16 ± 0.009 | 0.15–0.18 | 0.717 |
| All | 0.54 ± 0.30 | 0.1–1.1 | 0.52 ± 0.30 | 0.11–1.02 | 0.964 | |
Bold values are statistically significant (p < 0.05)
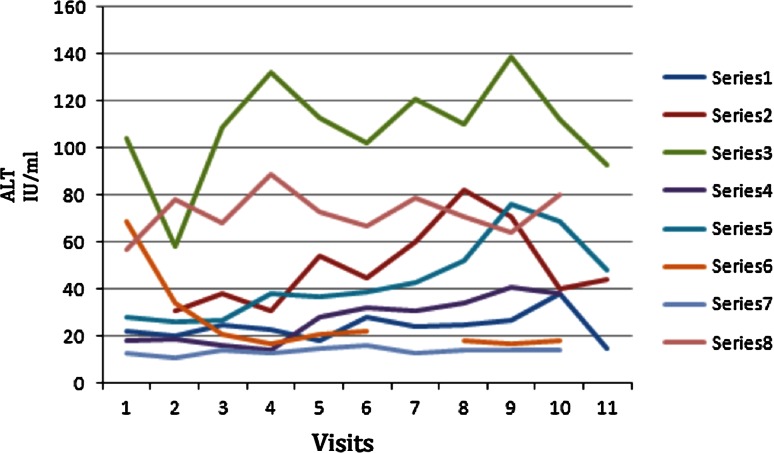
Fig. 1.
The graph demonstrates the variation of ALT during the period of the experimental study. Visits 1–4 were the pre-treatment and baseline visits. With upper limit of normal at 40, only 3 were above at the start. All the patients had end values below the 0 time point at the end of the study except series 3 but that patient had a value below 100 a year later
Full Blood Count, ANA, and Dropout from the Study
Blood counts remained stable after therapy (data not shown), and all had negative screens for antinuclear antibodies before and after treatment. There was no patient dropout from the trial, and no patients were removed from the study for abnormal laboratory values or clinical evidence of worsening disease.
Indirect Measures of Hepatic Fibrosis
The AST-to-platelet ratio did not change at treatment end (data not shown), and similarly, serum markers associated with fibrosis (that are also components of the Fibrotest™) remained similar or improved from before to end of treatment (see Table 2).
Table 2.
Serum fibrosis markers
| Medication | Alpha-2-macroglobulin | Haptoglobin | Apolipoprotein A1 | GGT | Total bilirubin | ALT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | Start | End | Start | End | Start | End | ||
| P1 | Trientine | 23 | 159 | 156 | 167 | 168 | 36 | 52 | 0.62 | 0.3 | 31 | 40 | |
| P2 | d-Penicillamine | 269 | 264 | <6.0 | <6.0 | 161 | 178 | 42 | 48 | 1.06 | 1.47 | 14 | 38 |
| P3 | Zinc | 565 | 443 | 89.9 | 60 | 145 | 180 | 17 | 32 | 0.38 | 0.47 | 38 | 69 |
| P4 | Trientine | 177 | 191 | 86 | 95.9 | 169 | 196 | 13 | 14 | 0.32 | 0.34 | 17 | 22 |
| P5 | Zinc | 208 | 181 | 155 | 271 | 171 | 134 | 27 | 18 | 0.38 | 0.32 | 17 | 18 |
| P6 | Trientine | 353 | 397 | 12.6 | 7.5 | 174 | 157 | 66 | 100 | 0.8 | 0.65 | 132 | 112 |
| P7 | Trientine | 208 | 188 | 117 | 126 | 130 | 103 | 27 | 31 | 0.92 | 0.47 | 23 | 27 |
| P8 | Trientine | 149 | 143 | 128 | 121 | 111 | 108 | 90 | 105 | 0.96 | 0.58 | 89 | 80 |
Units and ranges of normal for tests are as follows: alpha-2-macroglobulin: 131–293 mg/dL; haptogloblin: 20–200 mg/dL; apolipoprotein A1: 94–178 mg/dL; GGT: 11–55 U/L; total bilirubin: <1.2 mg/dL; ALT: 0–34 U/L
Questionnaires
Questionnaire results are shown in Table 3. Before the study, three patients missed one dose/week; one each missed 2, 4, and 5 doses/week, and two missed >5 doses/week. At study end, all patients reported that the once-daily dose of the trientine improved their ability to avoid missing medication dosages and that it was easier and preferable to take the medication once-daily.
Table 3.
Questionnaire responses at start and end of study
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Pre-treatment questionnaire | ||||||||
| What medication are you taking for Wilson’s disease? | Trientine | d-Pen | Zinc | Trientine | Zinc | Trientine | Trientine | Trientine |
| How long have you been on this medication? | 3 years | 40 years | 5 years | 5.5 years | 3 years | 4.3 years | 5 years | 5.25 years |
| How many times per day do you take your medication? | 2 | 1 | 2 | 2 | 3 | 2 | 2 | 2 |
| How many dosages of your medication are taken 1 h before or 2 h after meals? | All | All | All | All | 1 | All | All | All |
| How many dosages of your medication do you miss in a week’s time? | 1 | 1 | >5 | 1 | >5 | 2 | 5 | 4 |
| What is the longest time you have gone without your medication? | 1 day | 2 days | 4 days | 30 days | 4 days | 0 | 4 days | 300 days |
| Does having to take the medication 1 h before meals or 2 h after meals interfere with your daily schedule? | Yes | No | No | No | Yes | Yes | No | Yes |
| Do you think it will be easier for you to take your medication as a single dosage? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Post-treatment questionnaire | ||||||||
| Did taking your medication as a single daily dosage improve your ability to take the entire daily amount of medication needed? | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Was taking your medication as a single daily dosage less interfering with your daily schedule? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Would you prefer to take your medications as a single daily dosage if your testing showed that your Wilson disease was well controlled during this trial? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Which medication would you prefer to take for your Wilson disease? | Zinc* | Trientine | Trientine | Trientine | Trientine | Trientine | Trientine** | Trientine |
* Financial consideration; ** whichever is “cheapest”
Discussion
WD is treated with chelators to promote copper excretion, or with zinc to reduce copper absorption. There is no preferred treatment regimen given the lack of prospectively controlled studies to estimate the relative treatment efficacy or adherence to the available treatments for WD. Unfortunately, the lifelong need for therapy and requirement for multiple daily dose of medications has led to non-adherence to treatment and clinical deterioration and death for some WD patients, leading us to look for alternatives.
Trientine is increasingly recognized as first-line treatment for WD due to fewer unwanted effects than d-penicillamine. Pancytopenia occurs rarely, and hypersensitivity reactions and renal effects have not been reported [22]. Neurological deterioration during initiation of therapy is infrequent but recognized [23]. Therefore, we focused our attention on this agent.
In this prospective pilot study, we evaluated once-daily trientine for WD maintenance therapy. Our expectations of continued clinical and laboratory stability were mostly achieved. There were mild changes in serum aminotransferases in the group overall as reflected by the ranges noted in Table 1, but liver function (INR, and albumin and total bilirubin) remained mostly unchanged (Table 1). In two (patients 2 and 3, Table 1) in whom ALT levels increased slightly at study end, we found no obvious reason for this observation. Their exams, weight, and hepatic synthetic function remained intact. Patient 3 missed >5 doses a week prior to the study, and his increased urine copper excretion probably reflected consistent medication usage. In the study group, there were two patients in whom past symptoms included neurological and psychiatric symptomatology, but in one of these individuals the psychiatric symptoms were resolved, and in both neurological symptoms were stable. Both had been maintained on therapy with stable examinations prior to the study, as was a requirement for inclusion in the study, and remained stable throughout the study.
Urine copper excretion and serum copper were stable for patients before and during the study (Table 1), including the “free” non-ceruloplasmin copper and urinary copper. The patients all had non-ceruloplasmin copper that was below 15 mcg/dl prior to entry into the study, and no patient exceeded this threshold during the study (data not shown). Liver biopsies were not obtained, as they are not routinely used to follow treatment in WD. However, AST-to-platelet ratio and serum markers of fibrosis were not changed at study end, indirectly suggesting no change in hepatic fibrosis.
Zinc must be taken apart from meals for WD treatment and is often administered thrice daily [24]. The normative requirement for absorbed zinc is set at 1.4 mg/day for men and 1.0 mg/day for women. All patients had elevated urine excretion of zinc while on trientine. To our knowledge, there is no evidence for zinc deficiency in WD patients on therapy with trientine or d-penicillamine, likely due to the ubiquitous presence of dietary zinc. Activity of alkaline phosphatase, a zinc dependent enzyme, was not altered in our patients who transitioned from zinc to trientine (data not shown).
Patient questionnaires revealed a perceived advantage of trientine given once-daily was the convenience of not having to time dosages to 1 h before or 2 h after meals. The once-daily regimen seemed to improve patient adherence to treatment, in particularly in the two patients who missed >5 doses/week before the study. Though we cannot negate the effect of study enrollment on adherence since patients had medication checks and periodic pill counts, we hoped that once-daily treatment would improve adherence beyond the study period.
We believe that once-daily weight-based dose of trientine merits further study for long-term maintenance therapy for WD. Of note, the pediatric weight-based dosage often exceeds what is given to adults on a daily basis. The choice of dose in adults is empiric and based on treatment experience of experts as there is a lack of evidence-based comparative data on this subject. The weight-based dose used in this study compared to the usual one-size fits all dose deserves particular mention and highlights the need for more formal studies to determine the proper dose of these medications. In our cohort, the patients previously on chelation therapy maintained similar 24-h urine copper excretions in the study, suggesting the 15 mg/kg yields adequate daily copper excretion. We do recognize the limitation of having not studied more than one dosage, and this can be addressed in future studies.
Since the study group was small in this pilot study, a larger study group would be required to achieve statistical significance to prove efficacy and bioequivalence. We believe that the time to detect signal for treatment failure or non-adherence is within 6 months based on prior studies of adolescents who were non-adherent with treatment [8], and is consistent with our personal experience with a large number of patients with Wilson disease over the years. Based on this, current recommendations are for bi-annual monitoring of patients in maintenance therapy in both AASLD and EASL guidelines [1, 26]. If we had seen lower urine copper excretion, then this might lend credence to the suggestion for potential for slow copper accumulation, but it is important that we did not observe this at all. Therefore, while longer periods of study are always wished for, we believe our period of observation exceeded the minimum needed to detect signal for treatment failure or non-adherence.
Our observations suggest that a once-daily weight-based dosage of trientine is an attractive regimen for WD patients for maintenance therapy. A recent publication of from Dzieżyc et al. [25] reported improved survival rates for asymptomatic patients adherent to therapy compared to non-compliant patients, further strengthening the argument that a simpler treatment regimen will benefit adherence. Longer-term follow-up of our patients and others on once-daily therapy is needed to establish its safety and treatment efficacy, with promising implications for the future management of patients with WD.
Acknowledgments
Aftab Ala was supported by the Royal College of Physicians, London Sheila Sherlock Travel Fellowship in Hepatology and the Surrey and Sussex National Institute for Health and Research (NIHR). The project was funded by Aton and Valeant Pharmaceuticals with recruitment assistance from the Wilson Disease Association, USA.
Conflict of interest
None.
Abbreviations
- ALT
Alananine aminotransferase
- AST
Aspartate aminotransferase
- INR
International normalized ratio
- WD
Wilson disease
References
- 1.Roberts E, Schilsky ML. A practice guideline on Wilson disease. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 2.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 3.Masełbas W, Chabik G, Członkowska A. Persistence with treatment in patients with Wilson disease. Neurol Neurochir Pol. 2010;44:260–263. doi: 10.1016/s0028-3843(14)60040-2. [DOI] [PubMed] [Google Scholar]
- 4.Schilsky ML, Scheinberg IH, Sternlieb I. Hepatic transplantation for Wilson’s disease: indication and outcome. Hepatology. 1994;19:583–587. doi: 10.1002/hep.1840190307. [DOI] [PubMed] [Google Scholar]
- 5.Walshe JM. Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet. 1982;1:643–647. doi: 10.1016/S0140-6736(82)92201-2. [DOI] [PubMed] [Google Scholar]
- 6.Scheinberg IH, Jaffe ME, Sternlieb I. The use of trientine in preventing the effects of interrupting penicillamine therapy in Wilson’s disease. N Engl J Med. 1987;317:209–213. doi: 10.1056/NEJM198707233170405. [DOI] [PubMed] [Google Scholar]
- 7.Brewer GJ, Dick RD, Johnson VD, et al. Treatment of Wilson’s disease with zinc: XV long-term follow-up studies. J Lab Clin Med. 1998;132:264–278. doi: 10.1016/S0022-2143(98)90039-7. [DOI] [PubMed] [Google Scholar]
- 8.Weiss KH, Gotthardt DN, Klemm D, et al. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011;140:1189–1198. doi: 10.1053/j.gastro.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Walshe JM, Dixon AK. Dangers of non-compliance in Wilson’s disease. Lancet. 1986;1:845–847. doi: 10.1016/S0140-6736(86)90949-9. [DOI] [PubMed] [Google Scholar]
- 10.Kruse W, Rampmaier J, Ullrich G, Weber E. Patterns of drug compliance with medications to be taken once and twice daily assessed by continuous electronic monitoring in primary care. Int J Clin Pharmacol Ther. 1994;32:452–457. [PubMed] [Google Scholar]
- 11.Rosenbach KA, Allison R, Nadler JP. Daily dosing of highly active antiretroviral therapy. Clin Infect Dis. 2002;34:686–692. doi: 10.1086/338255. [DOI] [PubMed] [Google Scholar]
- 12.Laliberté F, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS. Impact of daily dosing frequency on adherence to chronic medications among nonvalvular atrial fibrillation patients. Adv Ther. 2012;29:675–690. doi: 10.1007/s12325-012-0040-x. [DOI] [PubMed] [Google Scholar]
- 13.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 14.Lowette KF, Desmet K, Witters P, Laleman W, Verslype C, Nevens F, Fevery J, Cassiman DM. Wilson’s disease: long-term follow-up of a cohort of 24 patients treated with d-penicillamine. Eur J Gastroenterol Hepatol. 2010;22:564–571. doi: 10.1097/MEG.0b013e3283353df8. [DOI] [PubMed] [Google Scholar]
- 15.Stroh M, Addy C, Wu Y, et al. Model-based decision making in early clinical development: minimizing the impact of a blood pressure adverse event. AAPS J. 2009;11:99–108. doi: 10.1208/s12248-009-9083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Poppitt SD, Othman AA, Sunderland T, Ruggiero K, Willett MS, Diamond LE, Garcia WD, Roesch BG, Cooper GJ. Pharmacokinetics, pharmacodynamics, and metabolism of triethylenetetramine in healthy human participants: an open-label trial. J Clin Pharmacol. 2010;50:647–658. doi: 10.1177/0091270009349379. [DOI] [PubMed] [Google Scholar]
- 17.Fox AN, Schilsky M. Once daily trientine for maintenance therapy of Wilson disease. Am J Gastroenterol. 2008;103:494–495. doi: 10.1111/j.1572-0241.2007.01646_15.x. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;2006:1887–1896. doi: 10.1373/clinchem.2006.070961. [DOI] [PubMed] [Google Scholar]
- 20.Arnon R, Calderon JF, Schilsky M, Emre S, Shneider BL. Wilson disease in children: serum aminotransferases and urinary copper on triethylene tetramine dihydrochloride (trientine) treatment. J Pediatr Gastroenterol Nutr. 2007;44:596–602. doi: 10.1097/MPG.0b013e3180467715. [DOI] [PubMed] [Google Scholar]
- 21.Masełbas W, Chabik G, Członkowska A. Persistence with treatment in patients with Wilson disease. Neurol Neurochir Pol. 2010;44:260–263. doi: 10.1016/s0028-3843(14)60040-2. [DOI] [PubMed] [Google Scholar]
- 22.Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007;56:115–120. doi: 10.1136/gut.2005.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suda M, Kubota J, Yamaguchi Y, Fujioka Y, Saito Y, Aoki T. A study of trientine therapy in Wilson’s disease with neurological symptoms. No To Hattatsu. 1993;25:429–434. [PubMed] [Google Scholar]
- 24.Brewer GJ, Askari F, Lorincz MT, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch Neurol. 2006;63:521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 25.Dzieżyc K, Karliński M, Litwin T, Członkowska A. Compliant treatment with anti-copper agents prevents clinically overt Wilson’s disease in pre-symptomatic patients. Eur J Neurol. 2014;21:332–337. doi: 10.1111/ene.12320. [DOI] [PubMed] [Google Scholar]
- 26.Ferenci P, Czlonkowska A, Stremmel W, Houwen R, Rosenberg W, Schilsky M, Jansen P, Moradpour D, Gitlin J. EASL clinical practice guidelines: Wilson’s disease. European Association for Study of Liver. J Hepatol. 2012;56:671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]